Back to Journals » International Journal of Women's Health » Volume 8
Surgeons’ early experience with the Acessa™ procedure: gaining proficiency with new technology
Authors Braun KM, Sheridan M, Latif EZ, Regush L, Maksymowicz A, Weins L, Bedaiwy MA, Tyson N, Davidson MJ, Sanders BH
Received 8 August 2016
Accepted for publication 18 October 2016
Published 23 November 2016 Volume 2016:8 Pages 669—675
DOI https://doi.org/10.2147/IJWH.S119265
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Elie Al-Chaer
Kelli M Braun,1 Mark Sheridan,2 Erin Z Latif,1 Lexy Regush,3 Anet Maksymowicz,4 Laura Weins,5 Mohamed A Bedaiwy,6 Nerissa Tyson,5 Marilyn J Davidson,2 Barry H Sanders7
1Department of Obstetrics and Gynecology, Medical College of Georgia at Augusta University, Augusta, GA, USA; 2Department of Obstetrics and Gynecology, Saskatoon Obstetric and Gynecologic Consultants, 3Department of Obstetrics and Gynecology, Royal University Hospital, Saskatoon, 4Department of Obstetrics and Gynecology, Regina General Hospital, Regina, 5Department of Obstetrics and Gynecology, University of Saskatchewan, Saskatoon City Hospital, Saskatoon, SK, 6Department of Obstetrics and Gynecology, British Columbia Women’s Hospital, Vancouver, 7Department of Obstetrics and Gynecology, Division of General Gynaecology and Obstetrics and Division of Gynaecologic Specialties, Vancouver General Hospital, Vancouver, BC, Canada
Purpose: Successful adoption of a new surgical procedure varies among practicing surgeons, and skill acquisition depends on the surgeon’s innate ability, the complexity of the technique, and training. We report intraoperative and near-term postoperative outcomes from the Acessa procedure conducted by minimally invasive gynecologic surgeons new to Acessa, and report the surgeons’ experiences during the training period.
Patients and methods: The study was designed as a postmarket, prospective, single-arm, multicenter analysis of operative and early postoperative outcomes after proctored surgical training with the Acessa device and procedure (laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic fibroids) in premenopausal, menstruating women as conducted in community and university hospitals in the USA and Canada. Surgeons completed evaluation forms once they felt they could safely and comfortably conduct the operations.
Results: Ten gynecologic surgeons without prior Acessa experience completed 40 Acessa procedures – all on an outpatient basis. Mean procedure time was 1.9±1.0 hours and was similar to that reported in the pivotal premarket study (2.1±1.0 hours). Two intraoperative complications occurred: a 1 cm uterine serosal laceration due to uterine manipulation and blood loss from both the probe insertion site and the lysis of uterine-omental adhesions. No postoperative complications or reinterventions for fibroid symptoms were reported. The surgeons completed the evaluation forms after two to five cases, and none found any factors affecting procedure efficiency to be inferior or needing improvement.
Conclusion: Minimally invasive gynecologic surgeons new to Acessa can perform the procedure and provide acceptable outcomes after two to five proctored cases.
Keywords: myoma, fibroid, leiomyoma, laparoscopic ultrasound, education, radiofrequency ablation
Introduction
Gynecologic surgeons are often presented with new surgical techniques, procedures, and devices given the rapid advancement of science and technology for minimally invasive surgery. During residency training, new techniques are adopted under the watchful supervision of a faculty member. For practicing surgeons, however, acquiring new surgical skills outside of residency training can become a daunting task; ultimate skill acquisition is dependent upon the complexity of the surgical technique, the innate ability of the surgeon, and the training of the surgeon.1,2 Although medical device manufacturers enlist selected experienced surgeons to perform procedures using new surgical devices within pivotal clinical trials before the devices can be cleared by the US Food and Drug Administration (FDA) for clinical use, the successful adoption of the device or procedure by practicing surgeons – once the device and procedure are cleared for clinical use – may vary.
In November 2012, the Acessa™ System (Halt Medical, Inc., Brentwood, CA, USA) and procedure were cleared for commercialization in the USA for the treatment of uterine fibroids causing heavy menstrual bleeding, bulk, and pain symptoms. The procedure utilizes laparoscopic, intra-abdominal ultrasound to identify fibroids followed by directed radiofrequency ablation of each targeted fibroid. Clearance of the device was based on safety and efficacy results from a clinical trial conducted at carefully selected and monitored sites by 13 experienced minimally invasive gynecologic surgeons.3 In an effort to evaluate how easily minimally invasive gynecologic surgeons without prior Acessa experience can operate using the Acessa System in terms of surgical time, intraoperative blood loss, and adverse events, we conducted a prospective postmarket analysis of the first procedures performed by a group of community and university physicians during the training period for the Acessa technique.
The objective of this study was to evaluate the intraoperative and near-term postoperative outcomes from the Acessa procedure as conducted by minimally invasive gynecologic surgeons new to the Acessa procedure and to report our perspectives regarding our Acessa experience during the training period.
Materials and methods
The current trial is a postmarket, prospective, single-arm, multicenter analysis of operative and postoperative outcomes with 4–8 weeks follow-up after laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation (RFVTA, the Acessa procedure) of symptomatic fibroids in premenopausal, menstruating women. The study, which was developed in accordance with the US FDA, includes outcomes from 10 surgeons who are a subset of investigators and who learned the Acessa procedure during the run-in (training) phase (prior to the randomization phase) of an ongoing and much larger randomized controlled trial in the USA and Canada (TRUST, ClinicalTrials.gov identifiers: NCT02163525 for the US trial and NCT01563783 for the Canadian trial). None of the participating surgeons had prior experience with the Acessa ablation procedure and only one surgeon had prior experience with laparoscopic ultrasound. The surgeons were selected based on their interest in the trial, comfort with performing laparoscopic surgery, each site’s ability to perform the comparative procedures during the randomization phase, and having adequate research study support personnel who comply with the principles of Good Clinical Practice Guidelines.
The protocol for structured and consistent surgical training in Acessa follows that suggested by Sachdeva1 and Houck et al2 for the successful adoption of the surgical technique. First, new surgeons are oriented to the procedure through didactic instruction. This is followed by hands-on practice with both the intra-abdominal ultrasound and the Acessa device in a simulated laboratory environment. Once the surgeon is comfortable in the simulated environment, operative cases are then performed in the presence of a trained proctor until both the surgeon and the proctor deem that the surgeon is comfortable and competent to perform the procedure.
This analysis spans 17 months with patient treatment for the run-in phase from September 2014 through January 2016. All patients signed informed consent and were enrolled in four clinical sites. Forty-one women were recruited; however, one subject did not undergo a surgical procedure due to a positive pregnancy test within 24 hours prior to her surgery. The sites obtained prior local institutional review board approval (Table S1) of the TRUST protocol, which included the run-in (training/prerandomization) phase. The study was carried out according to the general ethical principles described in the Declaration of Helsinki and the US FDA regulations concerning the rights and welfare of human subjects taking part in medical research.
Enrolled women were aged ≥18 years, were menstruating, had symptomatic uterine fibroids <10 cm in greatest diameter (as assessed with transvaginal ultrasound), and desired uterine-conserving treatment for their fibroids. The maximum allowable uterine size was 16 gestational weeks as determined by pelvic examination. Each enrollee had a normal – no untreated cervical malignancy or dysplasia – Papanicolaou test within the past 36 months. Women were excluded from the trial if they were contraindicated for laparoscopic surgery and/or general anesthesia; were expected to be at high risk for, or known to have significant intra-abdominal adhesions; required major elective concomitant procedures; were pregnant or lactating; had taken any depot GnRH agonist within 3 months of screening; had an implanted intrauterine or fallopian tube contraceptive device not removed within 10 days of treatment; had chronic pelvic pain known not to be caused by uterine fibroids; had or were suspected of having adenomyosis (as suggested in ultrasound or magnetic resonance images) or endometriosis stage 3 or 4; had a history (within 5 years) or evidence of gynecologic malignancy or premalignancy; had previous pelvic radiation; had a cervical fibroid, or had ≥1 completely intracavitary fibroids (type 0) or only type 0/1 submucosal fibroids that are better treated via hysteroscopy.
RFVTA was performed on an outpatient basis using the Acessa System and involved the percutaneous, laparoscopic ultrasound-guided radiofrequency ablation of fibroids using a disposable 3.4 mm probe coupled to a dual-function monopolar radiofrequency generator. Detailed descriptions of the device and technology have been reported.3–10 Two minimally invasive gynecologic surgeons performed each procedure: one was primary with the second surgeon assisting. Patients were placed in supine position, and a laparoscope was introduced through a 5 or 10 mm umbilical trocar based on the surgeon’s standard practice. The primary surgeon then mapped the myomatous uterus using a laparoscopic ultrasound transducer placed through a standard 10 or 12 mm suprapubic trocar and identified the location, size, and number of all fibroids. The Acessa handpiece was inserted percutaneously under laparoscopic guidance and advanced into the target fibroid using laparoscopic ultrasound. Depending on the size and shape of the fibroid, the electrode array was deployed according to a proprietary treatment algorithm. After verifying in three dimensions with the ultrasound transducer, the correct position of the array within the fibroid capsule, the surgeon initiated ablative treatment. Current was delivered to the electrode tip and array (if the latter was deployed), permitting ablation of only the targeted and localized fibroid tissue.
A thermocouple in each electrode needle in the array allowed continuous real-time temperature feedback to the surgeon via the generator screen. Current was removed via large, temperature-monitored, dispersive pads that were placed on the patient’s thighs above the patella. After each ablation, the probe was withdrawn from the fibroid with concurrent coagulation of the probe track. Hemostasis was confirmed visually after each ablation. Depending on the size and location of the fibroids, multiple fibroids could be ablated through one serosal puncture. After completion of the ablative treatment, the trocar fascial and skin sites were repaired according to the surgeon’s standard surgical practice. No serosal or myometrial suturing was required or performed. Patients were followed postoperatively for safety and recovery at 24–72 hours, 1 week, and 4–8 weeks.
For the purposes of gathering surgeon feedback regarding the Acessa System training, surgeons were asked to complete an Acessa Procedure Evaluation Form (Figure 1) once they felt comfortable with the procedure and could perform it safely after at least two surgical cases as the primary surgeon. All evaluation forms were tabulated to provide an indication of the ease of performing the surgery. Exploratory analyses using SAS v9.3 (SAS Institute, Cary, NC, USA) were employed to describe patient outcomes and surgeon responses.
  | Figure 1 Acessa™ procedure evaluation form. |
Results
Ten practicing minimally invasive gynecologic surgeons, only one of whom was in fellowship training, completed a total of 40 Acessa procedures, with 37 cases proctored; all procedures were performed on an outpatient basis. Five (50%) surgeons completed evaluation forms after performing two to three cases and five (50%) after four to five cases (as the primary surgeon). In terms of laparoscopic experience (excluding that gained during residency or fellowship), three (30%) surgeons had 0–5 years of experience, three (30%) had 6–15 years of experience, and four (40%) had >15 years of experience. Surgeon responses to questions regarding factors affecting the efficiency of the Acessa procedure during the run-in cases are provided in Table 1. None of the surgeons found any of the factors inferior or needing improvement, and none experienced any problems with the Acessa device. Two surgeons provided feedback on possible enhancements to the laparoscopic ultrasound probe as they felt learning the correct orientation of the ultrasound probe was the more technically challenging aspect of the cases.
  | Table 1 Surgeons’ responses to questions regarding factors affecting the efficiency of the procedure |
Of the 40 patients receiving fibroid treatment, 38 were available for follow-up at 24–72 hours, 38 were available for follow-up at 1 week, and 36 patients were available for follow-up at 4–8 weeks postprocedure; the mean follow-up for the last visit was 46.4±21.0 days. Demographic characteristics of all patients are reported (Table 2). The five most predominant presenting complaints were heavy menstrual bleeding (n=34; 85.0%), pelvic discomfort/pain (n=30; 75.0%), dysmenorrhea (n=25; 62.5%), increased abdominal girth (n=25; 62.5%), and urinary frequency (n=22; 55.0%). At baseline, patients reported having fibroid symptoms for a mean of 26±22 days during the 3 months prior to surgery.
  | Table 2 Baseline demographics of study participants (n=40) |
The intraoperative, laparoscopic ultrasound assessment of fibroid number, type, and size are presented in Table 3. Mean length of procedure (the time from first incision to the time of skin closure) was 1.9±1.0 hours (n=36), which was similar to the reported procedure time in the pivotal trial (2.1±1.0 hours3). The mean largest fibroid diameter as found on laparoscopic ultrasound was 6.03±1.77 cm. Two patients underwent adhesiolysis, which added ~35 minutes to their procedures and increased blood loss. Estimated mean blood loss for all patients without missing data was 47±137 mL (median, 10 mL; range, 0–800 mL; n=35). Mean hospitalization time (the time from the start of anesthesia to the time of discharge from the hospital) was 6.8±3.2 hours (n=34).
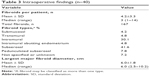  | Table 3 Intraoperative findings (n=40) |
There were two reported intraoperative complications during two proctored cases. One complication was minor and nondevice-related involving a 1 cm uterine serosal laceration resulting from uterine manipulation. The other complication was considered device related due to inadequate coagulation by the performing surgeon at one of the three probe insertion sites that continued to ooze, ultimately requiring two laparoscopic sutures to obtain hemostasis. The bleeding was compounded by extensive sharp (noncautery) adhesiolysis between the rectosigmoid and the posterior uterus in addition to adhesiolysis of the omentum at the anterior wall. An estimated total intraoperative blood loss of 800 mL occurred. The patient’s postoperative hemoglobin was 105 g/L, and she did not require transfusion. She was observed for 7 hours postsurgery and was discharged the same day.
Postprocedure observations at 1 week were typical of patients undergoing gynecologic laparoscopic surgery. Most women experienced some uterine bleeding (67.6%) and pelvic pain or cramping (67.6%). Patients experienced one or more of the following: bloating, gas, and general abdominal discomfort (45.9%); fatigue or general malaise (45.9%); shoulder pain (35.1%); and nausea and vomiting (35.1%). At the last follow-up visit between 4 and 8 weeks postprocedure, no patients reported complications, hospitalizations, or reinterventions for fibroid symptoms.
Discussion
Advances in minimally invasive surgery have provided practicing gynecologists with alternative surgical options for their patients; however, learning a new surgical technique outside of residency training can result in variable performance and affect ultimate acquisition of the technique. One promising advance in the minimally invasive treatment of symptomatic fibroids is RFVTA (the Acessa procedure).
Our study is the first to describe near-term results of the Acessa procedure as conducted by minimally invasive gynecologic surgeons practicing in university and community hospital settings who were new to the procedure and had varying surgical expertise. Near-term outcomes for this new group of Acessa surgeons during their procedure training phase are comparable to those in the pivotal trial: The mean procedure time (1.9±0.9 hours) tended to be less than that for the procedures in the pivotal trial (2.1±1.0 hours), and a similar number of fibroids were treated per patient (4.2±3.3 versus 5.0±4.4, respectively).3 We are encouraged by comparable outcomes in operative time and number of treated fibroids. Similar procedure times to the pivotal trial occurred despite two cases of concomitant adhesiolysis.
There was one intraoperative device-related event in this study (1/40, 2.5%), which compares with the 3.6% device-related adverse event rate in the pivotal study.3 Although this low rate of adverse events is encouraging given the training of 10 new surgeons, this study is not powered to detect a significant difference.
Strengths of our study include the patient, surgeon, institutional heterogeneity, and a method of surgical training that was consistent with training procedures defined in the literature.1,2 Studies have shown that participation in an animate laboratory and a preceptorship experience may determine the future performance of the surgeon in advanced laparoscopic surgery and that formal training can be a predictive factor for reduced complications and improved laparoscopic surgical skills.2 We feel that the Acessa training model contributes to successful acquisition and performance of the procedure. As reflected in the postprocedure evaluation forms, all of the surgeons participating in the study felt comfortable performing the Acessa procedure safely and effectively after two to five cases and were confident, in all instances, to discharge their patients the day of surgery. The mean duration of outpatient hospitalization was 6.8±3.2 hours. The principal limitations of the study are the small sample size and the lack of long-term follow-up data.
Conclusion
The Acessa procedure can be safely learned and adopted by minimally invasive gynecologic surgeons under the guidance of an experienced intraoperative proctor after two to five cases. The intraoperative and near-term results for surgeons new to the Acessa procedure compare well to those achieved by selected, experienced surgeons in the pivotal trial. Future postmarket studies will provide more information on long-term results and on device-related events.
Acknowledgments
The authors thank the following contributors: Halt Medical, Inc. (Brentwood, CA, USA) for surgical materials; Innovative Analytics (Kalamazoo, MI, USA) for data analysis support; and Wainwright Medical Communications (Los Gatos, CA, USA) for editorial support.
This paper was presented as a conference talk with interim findings at the 25th Annual Congress of the European Society of Gynaecological Endoscopy (ESGE); October 2–5, 2016; Square–Brussels–Belgium and the associated abstract was published in Gynecol Surg 2016;13 (Suppl 1):S126.
Disclosure
The authors report no conflicts of interest in this work.
References
Sachdeva AK. Acquiring skills in new procedures and technology: the challenge and the opportunity. Arch Surg. 2005;140(4):387–389. | ||
Houck J, Kopietz CM, Shah BC, Goede MR, McBride CL, Oleynikov D. Impact of advanced laparoscopy courses on present surgical practice. JSLS. 2013;17(2):174–177. | ||
Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082. | ||
Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371. | ||
Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric thermal ablation of uterine myomas with 12 months of follow-up. Int J Gynaecol Obstet. 2013;120(1):65–69. | ||
Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study. J Minim Invasive Gynecol. 2013;20(6):830–835. | ||
Brucker SY, Hahn M, Kraemer D, Taran FA, Isaacson KB, Krämer B. Laparoscopic radiofrequency volumetric thermal ablation of fibroids versus laparoscopic myomectomy. Int J Gynaecol Obstet. 2014;125(3):261–265. | ||
Hahn M, Brucker S, Kraemer D, et al. Radiofrequency volumetric thermal ablation of fibroids and laparoscopic myomectomy: long-term follow-up from a randomized trial. Geburtsh Frauenheilk. 2015;75(5):442–449. | ||
Lee BB, Isaacson KB, Diamond MP. Radiofrequency volumetric thermal ablation of symptomatic uterine fibroids: the Acessa procedure. In: Al-Hendy A, Salama S, editors. Etiology, Clinical Manifestations, Evaluation and Management of Uterine Leiomyoma. New York, NY, USA: Nova Science Publishers; 2015:175–193. | ||
Krämer B, Hahn M, Taran F-A, Kraemer D, Isaacson KB, Brucker SY. Interim analysis of a randomized controlled trial comparing laparoscopic radiofrequency volumetric thermal ablation of uterine fibroids with laparoscopic myomectomy. Int J Gynaecol Obstet. 2015;133(2):206–211. |
Supplementary material
  | Table S1 List of institutional review boards that approved this study |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
