Back to Journals » International Journal of Nanomedicine » Volume 12
Surface-enhanced Raman spectroscopy of serum accurately detects prostate cancer in patients with prostate-specific antigen levels of 4–10 ng/mL
Authors Chen N , Rong M, Shao X, Zhang H, Liu S, Dong B, Xue W, Wang T, Li T, Pan J
Received 23 March 2017
Accepted for publication 23 May 2017
Published 27 July 2017 Volume 2017:12 Pages 5399—5407
DOI https://doi.org/10.2147/IJN.S137756
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Linlin Sun
Na Chen,1 Ming Rong,1 Xiaoguang Shao,2 Heng Zhang,1 Shupeng Liu,1,3 Baijun Dong,2 Wei Xue,2 Tingyun Wang,1 Taihao Li,3 Jiahua Pan2
1Key Laboratory of Specialty Fiber Optics and Optical Access Networks, School of Communication and Information Engineering, Shanghai University, 2Department of Urology, Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, 3Beijing Advanced Innovation Center for Imaging Technology, Capital Normal University, Beijing, People’s Republic of China
Abstract: The surface-enhanced Raman spectroscopy (SERS) of blood serum was investigated to differentiate between prostate cancer (PCa) and benign prostatic hyperplasia (BPH) in males with a prostate-specific antigen level of 4–10 ng/mL, so as to reduce unnecessary biopsies. A total of 240 SERS spectra from blood serum were acquired from 40 PCa subjects and 40 BPH subjects who had all received prostate biopsies and were given a pathological diagnosis. Multivariate statistical techniques, including principal component analysis (PCA) and linear discriminant analysis (LDA) diagnostic algorithms, were used to analyze the spectra data of serum from patients in control (CTR), PCa and BPH groups; results offered a sensitivity of 97.5%, a specificity of 100.0%, a precision of 100.0% and an accuracy of 99.2% for CTR; a sensitivity of 90.0%, a specificity of 97.5%, a precision of 94.7% and an accuracy of 98.3% for BPH; a sensitivity of 95.0%, a specificity of 93.8%, a precision of 88.4% and an accuracy of 94.2% for PCa. Similarly, this technique can significantly differentiate low- and high-risk PCa with an accuracy of 92.3%, a specificity of 95% and a sensitivity of 89.5%. The results suggest that analyzing blood serum using SERS combined with PCA–LDA diagnostic algorithms is a promising clinical tool for PCa diagnosis and assessment.
Keywords: Ag nanoparticles, linear discriminant analysis, gray zone, principle component analysis, benign prostatic hyperplasia, spectral classification
Introduction
Prostate cancer (PCa) is the most common malignancy in the male urinary system and is a major contributor to cancer-related deaths worldwide. While the incidence of PCa is highest in Western countries, in recent years, it has been increasing in Asian countries.1 According to 2016 statistics for Americans, ~180,890 new cases of PCa were diagnosed, accounting for 21% of all new cancer cases, and caused 26,120 deaths, accounting for 8% of all cancer-related deaths.2 In order to improve survival rates of patients with PCa, it is important to diagnose and treat it at an early stage. The most important method for PCa screening is the prostate-specific antigen (PSA) test; according to a European randomized study of screening for PCa, this test can dramatically lower associated mortality rates.3 Unfortunately, the PSA test has some limitations. PSA is not a specific biomarker of PCa; serum levels of PSA can also rise in response to another common disease in elderly men, benign prostatic hyperplasia (BPH). Therefore, because of its low specificity for PCa, positive PSA test generally leads to many unnecessary biopsies. In addition, overdiagnosis and overtreatment of indolent cancers reduce patients’ quality of life and increase their financial burden.4 The PSA test has even lower specificity when PSA levels are in the range of 4–10 ng/mL, which is conventionally defined as gray zone; in this PSA range, only 25% of men who undergo biopsy are actually diagnosed with cancer.5 Several PSA parameters have been reported to improve the specificity of detecting PCa early, including PSA density, PSA velocity, PSA doubling time, and f-PSA percentage. However, even with these extra parameters, the ability of the PSA test to discriminate between cancerous and noncancerous conditions is still unsatisfying.6,7 Therefore, in order to maintain a high sensitivity and avoid unnecessary biopsies of patients within the PSA “gray zone”, it is important to develop adjunct clinical tools to help decide which patients actually need biopsies. Various other biomarkers and protocols have been explored and have achieved some promising results.8–11 After diagnosis, a crucially important issue is to assess aggressiveness of cancer, so as to discriminate indolent PCa from aggressive ones, enabling the doctors to discriminate patients suitable for active surveillance from those who need radical prostatectomy.12 The Gleason score (GS) of prostate biopsy specimens diagnosed by pathologists is of crucial importance to assess aggressiveness of PCa; however, several studies revealed discrepancies of GS between biopsy specimens and the corresponding radical prostatectomy specimens.13 This leads to a large number of overtreatment of indolent PCa. Therefore, development of effective tools for diagnosis and assessment of PCa is quite urgent.
Blood is the most widely used biofluid for non-invasive disease assessment because of minimal invasiveness and convenient collection in clinical practice. During malignant transformation, the biomolecular components such as amino acid, peptide metabolites, lipids and nucleic acid in human blood alter due to apoptosis and necrosis of cells; as a result, blood can potentially provide biomarks for early diagnosis of cancer and monitoring prognosis.14 Recently, serum metabolites have been studied to analyze constituent difference in blood between PCa patients, BPH patients and healthy people and probing PCa with a high accuracy. Serum metabolomics revealed four main components differences (alanine, pyruvate, glycine and sarcosine) between PCa and healthy people, with a discriminating accuracy of 90.2%. In addition, three main component differences (alanine, pyruvate and glycine) were able to discriminate 92.9% of low-grade (LG; GS ≤7) PCa from high-grade (HG; GS >7) PCa with 92.5% sensitivity and 93.3% specificity.15 1H-nuclear magnetic resonance (NMR)-based metabolic profiling of filtered serum revealed that alanine, sarcosine, creatinine, glycine and citrate were able to discriminate PCa from BPH, exhibiting a high accuracy (88.3%) to differentiate PCa from BPH.16 Serum metabolites showed promising capacity of analyzing molecular composition, however, they are an expensive and complex technology, which may introduce challenges for clinical application.17
Numerous methods and techniques have been applied in PCa detection, such as computed tomography (CT)/positron emission tomography (PET) by 18F-choline18 and gallium-68 PSMA ligand Glu-urea-Lys(Ahx)-HBED-CC,19 biopsy20 and PSA screen.21 However, it is difficult for CT to detect PCa in early stage. Biopsy, as an accurate diagnostic method in invasive way, may bring patients more body damages and suffers. A recent study has shown great diagnostic specificity achieved in the detection of PCa by ELISA, in which TKTL1 in serum was taken as a special molecular marker, but it was some what time consuming in diagnosis.22 Pihikova et al23 proposed a new electrochemical lectin-based immunosensor, a construction with sandwich configuration, which could effectively discriminate serum samples from healthy individuals and patients with PCa, but the molecule preparation is complex. Similar to our study, there was another research that also used surface-enhanced Raman spectroscopy (SERS) to measure serum spectra for prostate detection and showed great advantage as an invasive method.24 This work involved normal and PCa participants with support vector machine (SVM) classification and lack of BPH discrimination.
Raman spectroscopy is a non-invasive technology that can provide fingerprint-type molecular and chemical information of biological samples. SERS is a special type of Raman spectroscopy that utilizes nanotechnology. By mixing the sample with silver nanoparticles (AgNPs) or gold nanoparticles (NPs), the Raman signal of the sample can achieve 106–1014 times signal enhancement and at the same time inhibit the autofluorescence background interference.25 As a result, SERS has been widely applied in medical science to detect proteins, nucleic acids, DNA and other biomolecules.26 In recent years, SERS has been used to diagnose various cancers with a high accuracy, such as breast cancer, colorectal cancer and bladder cancer.27–29 An investigation into diagnosing PCa in 2015 reported that by analyzing the Raman spectra of serum separated from a peripheral blood sample and applying SVM techniques, PCa could be diagnosed with an accuracy of up to 98.1%.24 While this study offered extremely promising results, it focused on developing the methodology for using SERS in PCa diagnosis and thus did not study the method in a clinically relevant situation. Thus, this method would become closer to clinical application if it was tested on clinically relevant subjects. According to pathological diagnosis results in males who have received prostate biopsies, most confirm the presence of PCa or BPH. Therefore, if SERS could be validated to distinguish between PCa and BPH in patients whose serum PSA levels are in the gray zone, this would represent a highly beneficial diagnostic tool that would reduce the number of unnecessary biopsies for PCa.
As a type of spectrum technology for characterizing molecular vibration, surface-enhanced Raman scattering (SERS) can recognize and identify substances at the molecular level, and it has several advantages: offers a quick response, has a high accuracy and strong sensitivity, is non-invasive and hard to quench and can provide a spectrum’s fingerprint in traces, through which detailed information, such as molecular vibration, rotation, crystal structure and phase transition, can be obtained on the molecular scale. Owing to these advantages, it has been applied in various human organs and shown promising results.30 Several studies have reported applications of Raman spectroscopy for PCa, from cell lines to prostate tissue. In 2003, Crow et al31 distinguished BPH from adenocarcinoma with sensitivities and specificities >90% and found that tissue of adenocarcinoma contained higher concentration of nucleic acids and lower concentration of glycogen compared with tissue of BPH. In 2007, they used Raman spectroscopy to investigate the biochemical changes in tissues from BPH, prostatitis, GS <7 PCa, GS =7 PCa, and GS >7 PCa, and they found that DNA concentration, choline and cholesterol increased with malignancy, which represented the biochemical alteration during malignant transformation that potentially arose because of increased proliferation and necrosis.32 In addition, Raman spectroscopy has been used to distinguish cell lines of PCa, including LNCap, PCa2b, DU145, and PC3, with a sensitivity of 96% and a specificity of 99%. In this study, the authors found that the poorly differentiated cell lines (DU145 and PC3) had lower concentrations of glycogen.33 However, using Raman spectroscopy as clinical tool has some drawbacks, such as light cannot penetrate the sample deeply enough, making the Raman signal too weak, which has hindered its clinical application. SERS can overcome the major defects of regular Raman spectroscopy: once AgNPs or gold NPs are added into the target sample, the strong surface plasmon resonance among the metal NPs can create a localized electromagnetic field and greatly increase the Raman signal by up to 106–1014 times.34
Therefore, this study investigated if the SERS spectra of serum could be used to detect PCa in males with PSA levels in the gray zone who had already received prostate biopsies in our medical center and were given a pathological diagnosis. Subjects are in good agreement with actual clinical situation, and they were diagnosed with either PCa or BPH. Our results clearly show that SERS can be used to highly accurately discriminate between PCa and BPH, suggesting that SERS is a promising tool that may help doctors make decisions about whether a patient with a PSA level in the gray zone needs a biopsy.
Materials and methods
Study population and collection of blood serum samples
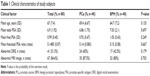
The experimental protocols were approved for the use of human subjects by the institution ethics committee of Shanghai Ren Ji Hospital affiliated with Shanghai Jiao Tong University and by the institution ethics committee of Shanghai University, and all methods were carried out in accordance with the approved guidelines. Patients who were suspected of PCa and were to receive ambulatory prostate biopsy were informed about the study in detail and gave their written informed consent. Patients with the following criteria were included in: patients who agreed to join this study, patients >65 years old and patients with total PSA within 4–10 ng/mL. The exclusion criteria were as follows: patients with acute prostatitis, patients who had previously regularly taken dutasteride or finasteride and patients who had cancers at other sites in the body. These exclusion criteria were chosen because the conditions may affect PSA levels or interfere in their measurement. The prostate biopsy was a transperineal 12-core biopsy performed by an experienced surgeon. According to the final pathology results of biopsy samples diagnosed by two experienced pathologists, patients were divided into two groups, those with PCa and those with BPH; both groups contained 40 patients. The detailed clinical information for the patients is shown in Table 1. After 12 hours of overnight fasting, all patients (who all received prostate biopsies) gave 3-mL blood samples. The samples were centrifuged with 3,000 rpm for 5 min. Subsequently, serum samples were collected and were then frozen at −80°C before the SERS analysis.
AgNP preparation
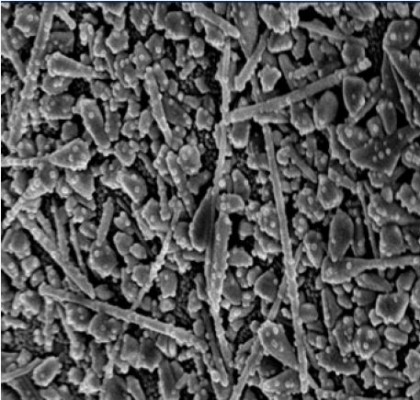
AgNPs were synthesized by the sodium citrate reduction method.35 Briefly, 1 mL of a 0.1 M AgNO3 solution was added into 100 mL deionized water and heated to boiling. In all, 1.8 mL of a 1% sodium citrate tribasic solution was added into the AgNO3 solution mentioned earlier. The solution was kept boiling until the color of solution turned celadon. The AgNPs were observed using a scanning electron microscope (SEM; Figure 1).
  | Figure 1 The SEM image of AgNPs (scale =100 nm). |
Sample preparation and SERS measurement
Before SERS measurement, 10 min centrifugation at 7,800 rpm was needed to obtain a silver colloid in a higher concentration. In all, 40 μL of silver colloidal NPs were homogenously mixed with 20 μL of serum. After incubating for 5 min at room temperature, a drop of the mixture was transferred onto a silicon plate for SERS measurement. A Raman microscopy (inVia Renishaw, London, UK) was used for the collection of SERS spectra with a spectral resolution of ~1 cm−1. The 633-nm He–Ne laser was focused on the sample surface with a spot diameter of 6 μm. The spectral data acquisition time was 10 s by a Leica DM2500 microscope (Leica Microsystems, Wetzlar, Germany) equipped with an objective lens of L50× (NA 0.5). We randomly measured three points on the sample. Data were acquired using the software WiRE 2.0 (Renishaw, London, UK), and the spectra preprocessing and analysis were performed using the software Origin (OriginLab Corporation, Northampton, MA, USA) and SPSS (IBM Corporation, Armonk, NY, USA).
Results
A total of 80 patients were selected to undergo prostate biopsy; their descriptive clinical characteristics are summarized in Table 1. According to the final pathology results of biopsy samples diagnosed by two experienced pathologists, patients were divided into two groups. The PCa group accounted for 50% of the patients, while the remaining 50% (40 patients) of the patients were in the BPH group. Comparing clinical characteristics of PCa and BPH patients, we can find that they have similar ages and PSA levels. In addition, there were no significant differences in abnormal magnetic resonance imaging (MRI) image ratio and abnormal digital rectal examination (DRE) ratio, and current clinical tools were not able to discriminate PCa from BPH without biopsy.
We measured 120 spectra from the PCa group, 120 spectra from the BPH group and 120 spectra from the control (CTR) group. All spectra were normalized to the integrated area under the curve in the range of 400–1,800 cm−1, and the fluorescence backgrounds were removed. In this study, we applied SERS to the serum of PCa and BPH patients with a PSA level in the gray zone to determine if this analysis could detect PCa. We measured the SERS spectra of blood serum from the three groups, as shown in Figure 2. Upon comparing the SERS spectra of serum among the PCa, BPH and CTR groups, there are some discernible differences found at peaks 492, 637, 727, 743, 808, 960, 1,134, 1,208, 1,326, 1,445, 1,573 and 1,655 cm−1, and these specific differences suggest a great potential for SERS in discriminating PCa from BPH in men with PSA of 4–10 ng/mL. The PCa samples show much higher intensities at peaks 637, 808 and 1,655 cm−1 but lower intensities at peaks 492, 727, 743, 960, 1,134, 1,208, 1,326, 1,445, 1,573 cm−1, indicating that these differences could potentially discriminate PCa from BPH. Raman spectra provide fingerprint information of biological molecules in samples.24,36,37 The tentative assignments of serum SERS spectra bands are listed in Table 2. The cancer group contains higher concentration of amide I and C=C lipid stretch (1,655 cm−1) in serum, while lower concentration of glycogen (492 cm−1), hypoxanthine (727 cm−1), D-mannose (1,134 cm−1), CH vibration in DNA/RNA (1,326 cm−1), and guanine in DNA (1,573 cm−1).
  | Table 2 Tentative assignments for SERS bands |
Principal component analysis (PCA) and linear discriminant analysis (LDA) were applied to discriminate among PCa, BPH and CTR groups. In total, 120 post-processed spectra of PCa, 120 spectra of BPH and 120 spectra of CTR were analyzed, giving nine PCs (PC1–PC9) that were significant (***P<0.001) for discriminating the three groups, accounting for 75% of the variance of the data. Then, using the leave-one-(spectrum)-out cross-validation, the three significant PCs were used to develop the LDA-discriminated model. By utilizing the PCA–LDA diagnostic model and the scatter plot of the linear discriminant scores of CTR, PCa, and BPH, the serum spectra were analyzed, as shown in Figure 3. The majority of PCa, BPH, and CTR plots can be separated by the separation line with a minimal overlap. Table 3 summarizes the confusion matrix of the PCA–LDA model based on the SERS spectra of serum. The second row indicates that 39 CTRs are recognized correctly, 0 CTR is recognized as BPH and 1 CTR is recognized as PCa. The third row indicates that 36 BPHs are recognized correctly, 0 BPH is recognized as CTR and 4 BPHs are recognized as PCa. The final row indicates that 38 PCas are recognized correctly, 0 PCa is recognized as CTR and 2 PCas are recognized as BPH. Table 4 lists the performances over each single group (ie, a single group versus the combination of other two groups), which can be directly calculated from Table 3.
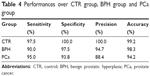  | Table 4 Performances over CTR group, BPH group and PCa group |
The SERS spectra were used to distinguish the high- and low-risk cancers in the PCa group using the same method mentioned earlier, as shown in Figure 4. The result also shows the obvious differences with an accuracy of 92.3%, a specificity of 95% and a sensitivity of 89.5%, which are summarized in Table 5.
To further evaluate the ability of the PCA–LDA model based on the SERS spectra of serum to discriminate between HG and LG in patients with a PSA level of 4–10 ng/mL, the receiver operating characteristic (ROC) curves were plotted with PCA–LDA, as shown in Figure 5. The integration of the area under the ROC curve (AUC) is 0.966.
Discussion
In our study, the Raman signal of blood serum was greatly enhanced when it was mixed with AgNPs. In addition, SERS can be used to investigate individual components in detail and provide ultrasensitive molecular and chemical information. SERS has been used to analyze biological molecules, from DNA, nucleotides to proteins.38,39 On this basis, SERS has been extensively used to detect various cancers with a high accuracy in recent years.27–29
In the present study, the SERS spectra of serum revealed significant differences between cancer patients and BPH patients, implying biomolecular component differences in blood. The Raman peak of 492 cm−1 is related to glycogen, while 1,445 cm−1 is related to CH2 bending, collagen/lipids. Because both peaks exhibited lower signal intensity, this may suggest some changes in glycogen and lipids in PCa blood serum. This probably results from the tumor’s vigorous metabolism and proliferation, which consume considerable glycogen and fat.40 Glycogen content was observed to decrease in esophageal and bladder cancer blood samples, compared to that in the blood samples of healthy subjects.29,41 There was also a relative intensity decrease in peak 1,208 cm−1, indicating a reduction of tryptophan in blood, which is in agreement with a report on the Raman signal change in malignant gastric tissues.42 The abnormal changes in the constitutes of PCa are in good agreement with biochemical analysis’ results of blood serum detection.
Recent studies have reported several peaks of 860, 941, 1,003/1,006, 1,445/1,455, and 1,659 cm−1 found in Raman spectra detected from prostate tissues.43,44 Compared with Raman peaks in serum (Table 2), the chemical components assigned tentatively by related peaks showed many differences. In detail, the lipid–phosphate group of PO4 corresponding to 860 cm−1 peak was present in prostate tissue but absent in serum, while the serum may contain hypoxanthine (in 727 cm−1) that lacked in tissue. Although higher diagnosis accuracy might be provided, the tissue Raman spectra detection, as a kind of direct but invasive methodology, could bring patients much more suffers. On the contrary, the serum spectra detection adopted in this study need no more than a wee bit of blood sampled from patients when taking routine body examination. For urine Raman spectra detection,45 a pilot trial for PCa diagnosis seemed to be much more invasive and safer than using serum; however, its accuracy and specificity were inferior to serum detection. This study shows a high diagnostic accuracy of serum SERS detection for PCa, and the accuracy is believed to be high enough to take the place of biopsy in PCa diagnosis to some extent, thus lessening much pain and suffering for patients. Among several common detection methods for PCa, SERS shows a relatively high sensitivity (Table 6). In addition, using high-concentration sliver colloid as enhancement substrate, serum containing some biomarkers and PCA–LDA analysis with a high accuracy may also contribute to the high accuracy of SERS detection as the potential factors.
The Raman shifts (cm−1) of the serum spectra were same using 514, 633, and 785 nm as the light source. The Raman signal using 514 nm as the light source were stronger than the signal using 633 and 784 nm as the light sources, but the higher fluorescent background make the Raman signal hard to be figured out for the biomolecular analysis.50,51 Raman spectra analysis of the biomedical samples always use 785 and 633 nm as light sources with a low fluorescent background. In this study, 633 nm source is suitable for the Raman signal measurement using AgNPs as the enhance substrates with a low fluorescent background by our experiments.
Spectra analysis can provide quantitative information about how biochemical constituents change with malignant transformation. However, between the PCa and BPH groups in this study, the variations in the spectra along with the overlapping peaks mean that quantitative analysis of peak intensities from SERS can only provide limited information for differentiation. In order to fully utilize the whole SERS spectrum and automatically determine the most meaningful spectral features that can be used to differentiate PCa from BPH, we used PCA–LDA to analyze the spectra data and built a diagnostic algorithm for differentiation, a technique that has been widely used in spectral data management in many studies and has shown promising results.27,42,52 In our study, by utilizing the PCA–LDA model, the PCa, BPH and CTR groups were separated significantly. Figure 3 shows the scatter plot of the linear discriminant scores of CTR, PCa and BPH serum spectra. These three groups can be differentiated significantly with only a little overlap, and the diagnostic sensitivity and specificity of each group are shown in Table 4. In addition, this technique can significantly differentiate low- and high-risk cancers with an accuracy of 92.3%, a specificity of 95% and a sensitivity of 89.5%.
Conclusion
Our study chose to evaluate a promising technique for detecting PCa using subjects who accurately reflect an actual clinical situation. Overall, the SERS diagnostic approach shows a great potential in characterizing the differences in biochemical constituents within the blood serum of PCa and BPH patients who had a PSA level in the gray zone. Combined with the PCA–LDA multivariate analysis, this diagnostic technique can attain high sensitivity, specificity and accuracy, thus demonstrating that the SERS could be a promising clinical tool for improving the rate of positive PCa results from biopsies in men who have a PSA in the gray zone, while only a small number of cancers are missed. Related closely to the actual conditions of clinical practice, our study applied SERS to the urgent issue of PCa detection and achieved promising results, which could be meaningful for promoting the clinical application of SERS.
Acknowledgments
We thank Dr Bo Lu for providing constructive suggestions in the Raman spectroscopy measurement. This study was funded by the Natural Science Foundation of China (NSFC; 61422507, 61575120, 61475095) and the Science and Technology Commission of Shanghai Municipality (STCSM; 16411969800, 14440500100) and supported by the Beijing Advanced Innovation Center for Imaging Technology. Thanks for the support of Renji Medical Research Seed Project (RJZZ13-016) and the Key Laboratory of Specialty Fiber Optics and Optical Access Networks (SKLSFO2015-06). Thanks also to Zhenyi Chen, Fufei Pang and Sujuan Huang for their support provided for the paper.
Author contributions
M Rong, and X Shao performed the experiments. H Zhang, and N Chen analyzed the data. T Wang, B Dong, W Xue and J Pan contributed the materials. T Li edited the manuscript. N Chen, S Liu and J Pan conceived and designed the experiments and wrote the paper. All authors have reviewed the manuscript. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Schröder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366(11):981–990. | ||
Heijnsdijk EA, De Carvalho TM, Auvinen A, et al. Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data. J Natl Cancer Inst. 2014;107(1):366. | ||
Barry MJ. Clinical practice. Prostate-specific-antigen testing for early diagnosis of prostate cancer. N Engl J Med. 2011;344(18):1373–1377. | ||
Partin AW, Brawer MK, Subong ENP, et al. Prospective evaluation of percent free-PSA and complexed-PSA for early detection of prostate cancer. Prostate Cancer Prostatic Dis. 1998;1(4):197–203. | ||
Kikuchi E, Nakashima J, Ishibashi M, et al. Prostate specific antigen adjusted for transition zone volume: the most powerful method for detecting prostate carcinoma. Cancer. 2000;89(4):842–849. | ||
Guazzoni G, Nava L, Lazzeri M, et al. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng/mL: results of a prospective study in a clinical setting. Eur Urol. 2011;60(2):214–222. | ||
Catalona WJ, Partin AW, Sanda MG, et al. A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/mL prostate specific antigen range. J Urol. 2011;185(5):1650–1655. | ||
Lazzeri M, Haese A, de la Taille A, et al. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2–10 ng/mL: a multicentric European study. Eur Urol. 2013;63(6):986–994. | ||
Gronberg H, Adolfsson J, Aly M, et al. Prostate cancer screening in men aged 50–69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol. 2015;16(16):1667–1676. | ||
Hoeks CM, Somford DM, Van Oort IM, et al. Value of 3-T multiparametric magnetic resonance imaging and magnetic resonance-guided biopsy for early risk restratification in active surveillance of low-risk prostate cancer: a prospective multicenter cohort study. Invest Radiol. 2014;49(3):165–172. | ||
Rajinikanth A, Manoharan M, Soloway CT, et al. Trends in Gleason score: concordance between biopsy and prostatectomy over 15 years. Urology. 2008;72(1):177–182. | ||
Wang GF, Lipert RJ, Jain M, et al. Detection of the potential pancreatic cancer marker MUC4 in serum using surface-enhanced Raman scattering. Anal Chem. 2011;83(7):2554–2561. | ||
Kumar D, Gupta A, Mandhani A, Sankhwar SN. Metabolomics-derived prostate cancer biomarkers: fact or fiction? J Proteome Res. 2015;14(3):1455–1464. | ||
Kumar D, Gupta A, Mandhani A, Sankhwar SN. NMR spectroscopy of filtered serum of prostate cancer: a new frontier in metabolomics. Prostate. 2016;76(12):1106–1119. | ||
Kast RE, Tucker SC, Killian K, Trexler M, Honn KV, Auner GW. Emerging technology: applications of Raman spectroscopy for prostate cancer. Cancer Metastasis Rev. 2014;33(2–3):673–693. | ||
Beheshti M, Imamovic L, Broinger G, et al. 18Fcholine PET/CT in the preoperative staging of prostate cancer in patients with intermediate or high risk of extracapsular disease: a prospective study of 130 patients. Radiology. 2010;254(3):925–933. | ||
Verburg FA, Pfister D, Heidenreich A, et al. Extent of disease in recurrent prostate cancer determined by [(68)Ga]PSMA-HBED-CC PET/CT in relation to PSA levels, PSA doubling time and Gleason score. Eur J Nucl Med Mol Imaging. 2016;43(3):397–403. | ||
Pepe P, Pennisi M, Fraggetta F. Anterior prostate biopsy at initial and repeat evaluation: is it useful to detect significant prostate cancer? Int Braz J Urol. 2015;41(5):844–848. | ||
Palma A, Lounsbury DW, Schlecht NF, Agalliu I. A system dynamics model of serum prostate-specific antigen screening for prostate cancer. Am J Epidemiol. 2016;183(3):227–236. | ||
Tsaur I, Thurn K, Juengel E, et al. Evaluation of TKTL1 as a biomarker in serum of prostate cancer patients. Cent Eur J Urol. 2016;69(3):247–251. | ||
Pihikova D, Kasak P, Kubanikova P, Sokol R, Tkac J. Aberrant sialylation of a prostate-specific antigen: electrochemical label-free glycoprofiling in prostate cancer serum samples. Anal Chim Acta. 2016;934:72–79. | ||
Li SX, Zhang YJ, Xu JF, et al. Noninvasive prostate cancer screening based on serum surface-enhanced Raman spectroscopy and support vector machine. Appl Phys Lett. 2014;105(9):091104. | ||
Jeong IG, Lim JH, Hwang SS, et al. Nomogram using transrectal ultrasound-derived information predicting the detection of high grade prostate cancer on initial biopsy. Prostate Int. 2013;1(2):69–75. | ||
Vendrell M, Maiti KK, Dhaliwal K, Chang YT. Surface-enhanced Raman scattering in cancer detection and imaging. Trends Biotechnol. 2013;31(4):249–257. | ||
Cervo S, Mansutti E, Del Mistro G, et al. SERS analysis of serum for detection of early and locally advanced breast cancer. Anal Bioanal Chem. 2015;407(24):7503–7509. | ||
Wang J, Lin D, Lin JQ, et al. Label-free detection of serum proteins using surface-enhanced Raman spectroscopy for colorectal cancer screening. J Biomed Opt. 2014;19(8):087003. | ||
Li S, Li L, Zeng Q, et al. Characterization and noninvasive diagnosis of bladder cancer with serum surface enhanced Raman spectroscopy and genetic algorithms. Sci Rep. 2015;5:9582. | ||
Tu Q, Chang C. Diagnostic applications of Raman spectroscopy. Nanomedicine. 2012;8(5):545–558. | ||
Crow P, Stone N, Kendall CA, et al. The use of Raman spectroscopy to identify and grade prostatic adenocarcinoma in vitro. Br J Cancer. 2003;89(1):106–108. | ||
Stone N, Prieto MCH, Crow P, Uff J, Ritchie AW. The use of Raman spectroscopy to provide an estimation of the gross biochemistry associated with urological pathologies. Anal Bioanal Chem. 2007;387(5):1657–1668. | ||
Crow P, Barrass B, Kendall C, et al. The use of Raman spectroscopy to differentiate between different prostatic adenocarcinoma cell lines. Br J Cancer. 2005;92(12):2166–2170. | ||
Kneipp K. Surface-enhanced Raman scattering. Phys Today. 2007;60(11):40–46. | ||
Lee PC, Melsel D. Absorption and surface-enhanced Raman of dyes on silver and gold sols. J Phys Chem. 1982;86(17):3391–3395. | ||
Stone N, Kendall C, Smith J, Crow P, Barr H. Raman spectroscopy for identification of epithelial cancers. Faraday Discuss. 2004;126:141–157. | ||
Yan B, Li B, Wen Z, Luo X, Xue L, Li L. Label-free blood serum detection by using surface-enhanced Raman spectroscopy and support vector machine for the preoperative diagnosis of parotid gland tumors. BMC Cancer. 2015;15:650. | ||
Harper MM, Dougan JA, Shand NC, Graham D, Faulds K. Detection of SERS active labelled DNA based on surface affinity to silver nanoparticles. Analyst. 2012;137(9):2063–2068. | ||
Han XX, Zhao B, Ozaki Y. Surface-enhanced Raman scattering for protein detection. Anal Bioanal Chem. 2009;394(7):1719–1727. | ||
Moazzami AA, Zhang JX, Kamal-Eldin A, et al. Nuclear magnetic resonance–based metabolomics enable detection of the effects of a whole grain rye and rye bran diet on the metabolic profile of plasma in prostate cancer patients. J Nutr. 2011;141(12):2126–2132. | ||
Li D, Feng SY, Huang H, et al. Label-free detection of blood plasma using silver nanoparticle based surface-enhanced Raman spectroscopy for esophageal cancer screening. J Biomed Nanotechol. 2014;10(3):478–484. | ||
Connolly JM, Davies K, Kazakeviciute A, et al. Non-invasive and label-free detection of oral squamous cell carcinoma using saliva surface-enhanced Raman spectroscopy and multivariate analysis. Nanomedicine. 2016;12(6):1593–1601. | ||
Silveira L, Leite KRM, Silveira FL, et al. Discrimination of prostate carcinoma from benign prostate tissue fragments in vitro by estimating the gross biochemical alterations through Raman spectroscopy. Lasers Med Sci. 2014;29(4):1469–1477. | ||
Patel II, Trevisan J, Singh PB, et al. Segregation of human prostate tissues classified high-risk (UK) versus low-risk (India) for adenocarcinoma using Fourier-transform infrared or Raman microspectroscopy coupled with discriminant analysis. Anal Bioanal Chem. 2011;401(3):969–982. | ||
Del Mistro G, Cervo S, Mansutti E, et al. Surface-enhanced Raman spectroscopy of urine for prostate cancer detection: a preliminary study. Anal Bioanal Chem. 2015;407(12):3271–3275. | ||
Boesen L. Multiparametric MRI in detection and staging of prostate cancer. Dan Med J. 2017;64(2):B5327. | ||
Zamboglou C, Drendel V, Jilg CA, et al. Comparison of 68Ga-HBED-CC PSMA-PET/CT and multiparametric MRI for gross tumour volume detection in patients with primary prostate cancer based on slice by slice comparison with histopathology. Theranostics. 2017;7(1):228–237. | ||
Abdellaoui Maane I, El Hadi H, Qmichou Z, et al. Evaluation of combined quantification of PCA3 and AMACR gene expression for molecular diagnosis of prostate cancer in Moroccan patients by RT-qPCR. Asian Pac J Cancer Prev. 2016;17(12):5229–5235. | ||
Guimarmes GC, Costa WH, Rosa RA, Zequi S, Favaretto R. Predictive role of Trimprob associated with multiparametric MRI in the diagnosis of prostate cancer. Int Braz J Urol. 2017;43(1):29–35. | ||
Lee LG, Woo SL, Head DF, Dubrow RS, Baer TM. Near-IR dyes in three-color volumetric capillary: cell analysis with 633- and 785-nm laser excitation. Cytometry. 1995;21(2):120–128. | ||
Swiech D, Ozaki Y, Kim Y, Proniewicz E. Surface- and tip-enhanced Raman scattering of bradykinin onto the colloidal suspended Ag surface. Phys Chem Chem Phys. 2015;17(26):17140–17149. | ||
Lin J, Chen R, Feng S, et al. A novel blood plasma analysis technique combining membrane electrophoresis with silver nanoparticle-based SERS spectroscopy for potential applications in noninvasive cancer detection. Nanomedicine. 2011;7(5):655–663. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.