Back to Journals » International Medical Case Reports Journal » Volume 7
Sudden and rapid progression of lung affectation but stability in kidney function: a case report of anti-neutrophil cytoplasmic antibody-associated vasculitis
Authors Chen C, Zhu Y, Qian H, Yang L, Huang J
Received 10 June 2013
Accepted for publication 12 August 2013
Published 31 December 2013 Volume 2014:7 Pages 7—10
DOI https://doi.org/10.2147/IMCRJ.S49674
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Cheng Chen,* Ye-Han Zhu,* Hong-Ying Qian,* Ling-Yi Yang, Jian-An Huang
Respiratory Division, The First Affiliated Hospital of Soochow University, Suzhou, People's Republic of China
*These authors contributed equally to this article
Abstract: We report the case of a patient with anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) who exhibited sudden progression of lung infiltration while maintaining stable kidney function. The 69-year-old man was diagnosed with AAV and renal insufficiency 4 years ago. Pulmonic affectation was detected in the right lower lobe of lung by a computed tomography (CT) scan. After beginning cyclophosphamide pulse therapy sequential therapy with low-dose prednisone, he underwent a 4-year follow-up to detect changes in hemoglobin levels and serum creatinine levels, and had chest CT examinations. The CT scan and creatinine assay showed stable pulmonic fibrosis and kidney function. Although there was no increase of creatinine and detectable perinuclear ANCA, the patient suffered a pulmonary hemorrhage and levels of hemoglobin became progressive lower; the lung infiltration was found to be enlarged last year. After immunosuppressive therapy for one week, the lung fibrosis was progressive, increased pulmonary hemorrhage occurred, and the patient died due to respiratory failure but not end-stage renal failure.
Keywords: vasculitis, ANCA, lung fibrosis, pulmonary hemorrhage, renal insufficiency
Introduction
The anti-neutrophil cytoplasmic autoantibodies (ANCA)-associated vasculitides (AAVs) comprise a group of diseases characterized by necrotizing vasculitis of small vessels, frequently with involvement of the kidneys and lung.1 Genetic and environmental factors are involved in their etiopathogenesis, with a possible role for silica exposure in AAVs and Staphylococcus aureus infection in granulomatosis with polyangiitis.2 In the clinic, alveolar hemorrhage (AH) is a major cause of early death in AAV; severe AH is strongly correlated with renal vasculitis. AAV patients with renal involvement who need renal replacement therapy have the worst survival prognosis.3,4 We report a case of AAV that remained stable over 4 years and then showed sudden activity as lung infiltration instead of end-stage renal failure (ESRF).
Case report
A 69-year-old male patient presented as an outpatient with increased creatinine levels (281 μmol/L) associated with proteinuria (2.5 g/24 h) and hematuria (110 g/L). His history was not unusual and he denied any abuse of alcohol, drugs, or over-the-counter stimulants. Review of his history was negative for any recent fever, rash, contact with sick persons, or acute illness involving the respiratory or gastrointestinal tract. The examination of ANCA and a chest radiograph were unusual. He was diagnosed with ANCA-associated vasculitis after findings of a perinuclear ANCA (p-ANCA) positive reaction (495 U/mL), urine examination, renal insufficiency by creatinine assay, and lung infiltration by CT scan. After starting treatment with cyclophosphamide pulse therapy, sequential therapy with low-dose prednisone (10 mg qid [every other day]), he underwent a 4-year follow-up. During this time, the level of serum creatinine was stable (Figure 1), and both proteinuria and hematuria improved (Figure 2). A chest CT scan was performed, which showed that the lung fibrosis remained unchanged (Figure 3).
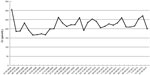  | Figure 1 The patient’s serum creatinine (Cr) level over a 4-year period. |
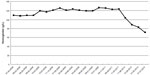  | Figure 2 The patient’s hemoglobin level over a 4-year period. |
  | Figure 3 The patient’s chest CTs over a 4-year period. The CT scan shows a progressive pulmonary affectation during the last 2 months. |
However, after the 4-year follow-up, the patient experienced a sudden and rapid deterioration in lung infiltration and exhibited pulmonary hemorrhage (PH) along with a progressively lower hemoglobin (Figure 3). Interestingly, there was not only no increase in creatinine levels (Figure 1), but also a negative conversion of p-ANCA. The galactomannan assay and Mycobacterium tuberculosis antigen-specific interferon (IFN)-gamma release assays (T-SPOT® -TB test; Oxford Immunotec Ltd, Oxford, UK) were both negative. After immunosuppressive therapy (methylprednisolone and cyclophosphamide pulse therapy for 1 week), and empirical widened coverage of anti-infective therapy, progressive lung fibrosis occurred, the PH worsened, and he died from respiratory failure.
Discussion
Vasculitides that cause granulomatous disease are characterized by the presence of ANCA-type antibodies. In most cases, the antibodies are directed against the proteinase 3-antineutrophil cytoplasmic antibody (PR3-ANCA). Such effects may well contribute to a proinflammatory environment in ANCA-associated small-vessel vasculitis and generalized vascular disease.5,6
Renal involvement is frequently present in AAV and is an important cause of ESRF. It has been reported that when patients with AAV progress to ESRF, they are less likely to experience relapse of their vasculitis.7 Furthermore, AAV is both a common cause of diffuse AH and a life-threatening disease.8,9 Although severe PH can occur in ESRD patients with AAV, disease activity and relapses of AAV should be monitored even before the patient’s disease progresses to PH.
In this case, we encountered a patient with AAV suffering from a fatal pulmonary affectation but presenting stable kidney function. Based on the patient’s progressively lower hemoglobin and CT results, AH might be involved in pulmonary affectation. It is suggested that hemorrhage may be a useful biomarker for evaluating the clinical status of AAV.
Induction therapy with oral cyclophosphamide has been a mainstay of treatment in patients with severe renal failure secondary to AAV. The role of plasmapheresis in the treatment of these diseases has also been studied retrospectively.10 Recently obtained insights into the pathogenesis of AAVs have led to a more targeted treatment of these life-threatening diseases. Furthermore, invasive pulmonary aspergillosis has been reported to be a severe opportunistic infection in immunocompromised patients.11,12 Patients with AAV undergoing immunosuppressive treatment are more prone to invasive pulmonary aspergillosis, but only a few cases have been reported in the literature. In this case, the serum galactomannan assay for invasive fungal infections was negative, and empirical antifungal therapy did not reverse his prognosis. Therefore, opportunistic infection was either not found or was not involved in acute exacerbations of the AAV in this patient.
ANCA-associated vasculitis and interstitial lung disease are uncommon conditions. The occurrence of both diseases in the same patient is increasingly recognized. As Arulkumaran et al13 have reported, interstitial lung disease was observed in 2.7% of patients with AAV. It is now recognized that a significant portion of patients with idiopathic pulmonary fibrosis can experience sudden and rapid deterioration in disease course that cannot be explained by infection, heart failure, or thromboembolic disease.14,15 These events are often fatal and have been termed acute exacerbations of underlying disease. Whereas they are most often described in patients with idiopathic pulmonary fibrosis, they have also been reported to be seen in patients with other forms of interstitial lung disease.16 Further investigation into similarities and common pathways in acute exacerbations of various fibrotic lung diseases (including pulmonary vasculitides) may yield additional insight into this recently recognized syndrome.
Diagnosis of AAV can be made according to clinical symptoms, laboratory test results, and image data; however, the gold standard remains the histological proof of a necrotizing, pauci-immune small vessel vasculitis. We report the case of a patient with AAV suffering fatal pulmonary affectation but presenting with almost normal kidney function, indicating that the lungs are possibly the only target organs in acute exacerbations of AAV. It is important to consider the progression of lung fibrosis as a possible complication in AAV patients who have an underlying abnormality of the lung. Even though the optimal strategies for AAV remain unclear, in the future, it might be possible to tailor the treatment modalities according to the risk factors. It is therefore necessary to establish an early diagnosis based on the symptoms. And thanks to new treatments, and despite AAVs being potentially serious diseases, their prognosis has considerably improved in recent years.17,18
Acknowledgments
This work was supported by Jiangsu Provincial Special Program of Medical Science (BL2012023) and Project of Department of Public Health of Jiangsu Province (H201208) and Natural Science Foundation of Jiangsu Province University (13KJB320021). We thank Dr S Puneeth Kumar (The First Affiliated Hospital of Soochow University) for reviewing this article.
Disclosure
The authors report no conflict of interest in this study.
References
Lee RW, D’Cruz DP. Pulmonary renal vasculitis syndromes. Autoimmun Rev. 2010;9(10):657–660. | |
Kallenberg CG, Stegeman CA, Abdulahad WH, Heeringa P. Pathogenesis of ANCA-associated vasculitis: new possibilities for intervention. Am J Kidney Dis. Epub June 27, 2013. | |
Fatma LB, El Ati Z, Lamia R, et al. Alveolar hemorrhage and kidney disease: characteristics and therapy. Saudi J Kidney Dis Transpl. 2013;24(4):743–750. | |
Hruskova Z, Casian AL, Konopasek P, et al. Long-term outcome of severe alveolar haemorrhage in ANCA-associated vasculitis: a retrospective cohort study. Scand J Rheumatol. 2013;42(3):211–214. | |
Sanders JS, Huitema MG, Hanemaaijer R, van Goor H, Kallenberg CG, Stegeman CA. Urinary matrix metalloproteinases reflect renal damage in anti-neutrophil cytoplasm autoantibody-associated vasculitis. Am J Physiol. 2007;293(6):F1927–F1934. | |
Heeringa P, Huugen D, Tervaert JW. Anti-neutrophil cytoplasmic autoantibodies and leukocyte-endothelial interactions: a sticky connection? Trends Immunol. 2005;26(11):561–564. | |
Seck SM, Dussol B, Brunet P, Burtey S. Clinical features and outcomes of ANCA-associated renal vasculitis. Saudi J Kidney Dis Transpl. 2012;23(2):301–305. | |
Chen M, Zhao MH. Severe pulmonary hemorrhage in patients with end-stage renal disease in antineutrophil cytoplasmic autoantibody-associated vasculitis. Am J Med Sci. 2009;337(6):411–414. | |
Cordire JF, Cottin V. Alveolar hemorrhage in vasculitis: primary and secondary. Semin Respir Crit Care Med. 2011;32(3):310–321. | |
Aydin Z, Gursu M, Karadag S, et al. Role of plasmapheresis performed in hemodialysis units for the treatment of anti-neutrophilic cytoplasmic antibody-associated systemic vasculitides. Ther Apher Dial. 2011;15(5):493–498. | |
Weidanz F, Day CJ, Hewins P, Savage CO, Harper L. Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis. Am J Kidney Dis. 2007;50(1):36–46. | |
Praprotnik S, Snezna S-S, Tomsic M, Shoenfeld Y. The curiously suspicious: infectious disease may ameliorate an ongoing autoimmune destruction in systemic lupus erythematosus patients. J Autoimmun. 2008;30(1–2):37–41. | |
Arulkumaran N, Periselneris N, Gaskin G, et al. Interstitial lung disease and ANCA-associated vasculitis: a retrospective observational cohort study. Rheumatology (Oxford). 2011;50(11):2035–2043. | |
Judge EP, Fabre A, Adamali HI, Egan JJ. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J. 2012;40(1):93–100. | |
Simon-Blancal V, Freynet O, Nunes H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration. 2012;83(1):28–35. | |
Wilcox BE, Ryu JH, Kalra S. Exacerbation of pre-existing interstitial lung disease after oxaliplatin therapy: A report of three cases. Respir Med. 2008;102(2):273–279. | |
Birck R, Schmitt WH, Kaelsch IA, van der Woude FJ. Serial ANCA determinations for monitoring disease activity in patients with ANCA-associated vasculitis: systematic review. Am J Kidney Dis. 2006; 47(1):15–23. | |
Sanders JS, Huitema MG, Kallenberg CG, Stegeman CA. Prediction of relapses in PR3-ANCA-associated vasculitis by assessing responses of ANCA titres to treatment. Rheumatology (Oxford). 2006;45(6):724–729. |
 © 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2013 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
