Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Successful Therapy of Alopecia Universalis Using a Combination of Systemic Methotrexate and Corticosteroids and Topical 5% Minoxidil
Authors Ruchiatan K , Avriyanti E , Hindritiani R , Puspitosari D , Suwarsa O , Gunawan H
Received 16 November 2021
Accepted for publication 12 January 2022
Published 25 January 2022 Volume 2022:15 Pages 127—132
DOI https://doi.org/10.2147/CCID.S349683
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Jeffrey Weinberg
Kartika Ruchiatan, Erda Avriyanti, Reti Hindritiani, Diah Puspitosari, Oki Suwarsa, Hendra Gunawan
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin General Hospital, Bandung, West Java, Indonesia
Correspondence: Kartika Ruchiatan, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran – Dr. Hasan Sadikin General Hospital, Pasteur 38, Bandung, West Java, 40161, Indonesia, Tel +62222032426 ext. 3449; +62811247932, Email [email protected]
Abstract: Alopecia areata (AA) is an autoimmune disease-specific to specific organs mediated by T lymphocytes with hair follicles as targets. Severe AA could be in the form of alopecia universalis (AU). AU therapy is relatively difficult and challenging with varying outcomes. Herein, we reported a case of AU in a 19-year-old man with alopecia in the hairy scalp area, eyebrows, eyelashes, moustache, beard, and axillary hair since 2.5 years ago. The patient’s severity of alopecia tool (SALT) score was 100%. The patient was given a combination therapy of 15 mg methotrexate per week and 16 mg methylprednisolone per day orally and topical treatment with minoxidil 5%. Observations after nine months of treatment showed an improvement in the decrease in SALT scores to 41%. However, striae were found after 3rd month of therapy. Systemic combination therapy of methotrexate and low-dose corticosteroids and topical minoxidil 5% in this patient gave responsive results. Performed the hematological examination, liver function levels, blood glucose levels, and cortisol during long-term use of methotrexate and corticosteroids are necessary. The combination of systemic methotrexate and corticosteroids, and topical minoxidil showed promising results in AU. Nevertheless, long-term observation is still needed to monitor the side effects of therapy.
Keywords: alopecia areata, alopecia universalis, corticosteroid, methotrexate, minoxidil
Introduction
Alopecia areata (AA) is an organ-specific autoimmune disease mediated by T lymphocytes targeting hair follicles.1 AA often occurs on the scalp, although it could affect hair on other parts of the body.2 It is acute, characterized by oval or round bald patches, well-defined, and smooth skin surface.1 In addition, the characteristic sign of AA is exclamation mark hairs on the edge of baldness.3 AA can also extend to the entire hairy scalp and hair on the body surface.1,3 Based on the area of hair loss, AA can be divided into the patch, alopecia totalis (AT), and alopecia universalis (AU) types.4–6
Alopecia areata could reduce self-confidence and have a significant psychological impact that affects the patient’s social life, so the management of AA is a challenge for dermatologists.1 Various therapies are available for AA with varying success and safety rates. Topical therapy is used as first-line therapy in AA, followed by systemic therapy if AA progresses to AT or AU and does not respond to topical treatment.5 Corticosteroids (topical, oral, intralesional, or intravenous), methotrexate, topical minoxidil, and phototherapy are often used.5,6 Study on the effectiveness of treatment using systemic methotrexate alone or in combination with systemic oral corticosteroids for AT and AU has obtained good results.7 Methotrexate is thought to be effective in treating the autoimmune cell-mediated attack and the inflammatory reactions implicated in the pathogenesis of AA.5–7 The mechanism of action of corticosteroids in AA is to suppress the T-cell-mediated immune response against hair follicles.8 Methotrexate and corticosteroid have mechanisms to modulate the inflammatory process around the follicle.5–8 While minoxidil stimulates germ cells of hair follicles and accelerates the shift from the telogen phase to the anagen phase.9
This case report aims to report one case of AU that was responsive to combination therapy of systemic methotrexate and corticosteroids and topical 5% minoxidil.
Case Report
A 19-year-old man came to our department with the chief complaint of baldness on the scalp, hair, eyebrows, eyelashes, moustache, beard, and axillary hair without itch nor pain. Three years previous, there was a couple of coin-size baldness on the top and back of the head, with no itch and pain sensation. In one-year time, baldness on the scalp became widespread, accompanied by the loss of eyebrows, eyelashes, moustache, beard, and axillary hair. The patient went for treatment and was given topical and oral medication and injections at the scalp. There was a temporary improvement as the growth of small and fine hair at the injection site lasted about six months, but they fell out again. Furthermore, the patient did not continue the therapy. History of trichotillomania, psychological stress, infection, cicatricial alopecia, alopecia in other family members, lupus erythematosus, psoriasis, vitiligo, atopic dermatitis, thyroid disease, and promiscuity were denied by the patient.
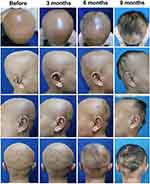
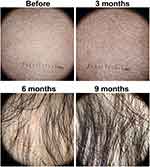
The vital signs and general health status were within normal limits. From the physical examination, no nail abnormalities were found. The distribution of baldness was generalized on the entire surface of the scalp (Figure 1). The skin surface in the alopecia area looked normal. The severity of the alopecia tool (SALT) score in this patient was 100%. On Wood’s lamp examination, the scalp area did not show any bright green fluorescence. The direct skin scrapings on the scalp using 10% potassium hydroxide solution revealed no fungal elements. Trichoscopic examination of the alopecia area on the scalp shows short vellus hairs in white colour (Figure 2). Haemoglobin, leukocytes, hematocrit, erythrocytes, platelets, examination of serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT), triiodothyronine (T3), free thyroxine (fT4), thyroid-stimulating hormone (TSH), serum Fe, total iron-binding capacity (TIBC), and chest radiological examination were performed. These laboratory investigations were all done for the patient, and all were within normal limits. Venereal disease research laboratory (VDRL) titer, Treponema pallidum hemagglutination assay (TPHA), and anti-nuclear antibody (ANA) were also nonreactive.
This patient was diagnosed as AU with unknown precipitating factors. As injections were painful and uncomfortable, we decided to give the topical minoxidil treatment and combined it with systemic therapy of methotrexate and corticosteroids. He was given combination therapy of oral 15 mg methotrexate per week, 16 mg methylprednisolone per day, and topical treatment with minoxidil 5%. Additionally, we gave a 5 mg folic acid supplementation on the off-days of oral methotrexate dose. Three months after treatment, we observed no significant improvement in hair growth. However, the trichoscopy examination revealed white and black short vellus hair growth. After six months of observations, there was an improvement marked with terminal hair growth. Then, we could gradually taper the methylprednisolone dose every two weeks until stopped.
Methotrexate and topical minoxidil were continued. After nine months of observations, there was improvement shown as a decrease in the SALT score to 41%, indicating high responsiveness to therapy marked with 59% hair growth compared to the pre-treatment state (Figure 1). Hair growth was also seen in the eyebrows, eyelashes, moustaches, and beards. The growth of terminal hair getting evident through the trichoscopy examination (Figure 2). Hematological, liver function levels, blood glucose levels, and cortisol examination during long-term use of methotrexate and corticosteroids were within normal limits. We observed side effects as striae which appeared on the back of the trunk after three months of therapy.
Discussion
Alopecia areata dramatically affects the patient’s appearance, self-confidence, and psychology, making its therapy a challenge for dermatologists.1 Clinical manifestation of AA is hair loss that causes well-defined patches of an oval or round shape until complete baldness with a smooth skin surface.1,3 Based on the area of baldness, AA is divided into patches if it is oval or round shape and affects a small area of the head, AT if total or near-total loss of hair on the scalp, and AU if total to near-total loss of hair on all haired surfaces of the body.6,10 Nail involvement can be found ranging from 7% to 66%, with pitting nails as the most common abnormality.11
The SALT score is a tool to quantitatively assess the severity of baldness and therapy evaluation.6,10,12 The scalp area is divided into four parts: vertex (upper) 40%, posterior 24%, right 18%, and left side of the head 18%. This score is calculated using the percentage of alopecia multiplied by the percentage of the scalp area, and then the sum of the four scalp areas is carried out.12
The diagnosis of AA is made based on clinical manifestations and other supporting examinations, such as hair pull tests.1,10,12 A positive result of a hair pull test in the form of six or more hairs apart from 50 to 60 hairs being drawn indicates that AA is active and progressive.10 Hair pull tests could not be performed on this patient.
Establishing the diagnosis of AA by physical examination is relatively easy. However, the management of AA patients tends to be complex, challenging, and often give unsatisfactory results. The main principle of AA treatment is to inhibit or alter the immune response by modulating the inflammatory process around the hair follicles.13 Topical therapies for AA include topical corticosteroids, intralesional corticosteroid injections, topical immunotherapy, and minoxidil, while systemic treatment includes systemic corticosteroids, methotrexate, and cyclosporine.1,5,6 Intralesional injection of corticosteroids with triamcinolone acetonide (TA) is the first treatment choice in adult AA patients.6
Minoxidil is one of AA’s most widely used drugs, but data on its efficacy and effectiveness in severe AA cases are unclear. Minoxidil stimulates germ cells in hair follicles and accelerates the shift from the telogen phase to the anagen phase. This effect increases hair growth and reduces hair loss in AA. Still, as monotherapy, topical minoxidil does not provide significant improvement in AA.9 Olsen et al suggested that topical minoxidil may help to reduce hair loss after tapering doses of corticosteroids for AA therapy.14 The adverse effects of topical minoxidil are contact dermatitis and facial hypertrichosis.5
Methotrexate and corticosteroids can be used in combination in systemic therapy of AA.7 Around 77.3% of AA patients who received a combination of systemic corticosteroid and methotrexate therapy experienced more than 50% hair regrowth. In comparison, only 44.4% of patients experienced hair regrowth with methotrexate monotherapy.15 The duration of using a combination of corticosteroids and methotrexate ranges from 1 to 12 months (±4 months) with steroid doses from 20 to 30 mg per day (±30 mg). The mean methotrexate dose was 10 mg once weekly (0–15 mg), and the mean daily dose of prednisone was 6 mg (0–20 mg).16 Joly used methotrexate alone or in combination with low-dose oral corticosteroids to treat AT and AU with a success rate of 64%.7 Adverse effects to methotrexate and corticosteroid systemic should be cautious and limited. Methotrexate could induce persistent nausea, transient elevation of hepatic enzymes, and leucopenia. Meanwhile, the corticosteroid can cause hyperglycemia, weight gain, hypertension, adrenal suppression, dysmenorrhea, immunosuppression, and acneiform eruption.5
The results of several studies regarding the effectiveness of AA therapy suggest that therapy should be appropriately evaluated.5,6 The patient in this case report used a combination therapy of systemic methotrexate, low-dose corticosteroids, and topical minoxidil 5% with responsive results gaining more than 50% hair growth within nine months. Hence, we suggested routine side effects monitoring due to the long-term use of methotrexate and corticosteroids.
Conclusion
This case report showed the effective and promising results with the use of combinations of systemic methotrexate, corticosteroids, and topical minoxidil. As alopecia treatment is challenging and often give unsatisfactory results. However, long-term monitoring of the treatment and its side effects are necessary.
Ethical Statement
Written informed consent was provided by the patient to have the case details and accompanying images published for scientific purposes. Institutional approval from The Research Ethics Committee of Dr. Hasan Sadikin General Hospital, Bandung, Indonesia has been obtained to publish the case details (approval number: LB.02.01/X.6.5/364/2021).
Acknowledgments
The authors would like to thank all of the Dermatology and Venereology Department staff, Faculty of Medicine, Universitas Padjadjaran – Dr. Hasan Sadikin General Hospital, Bandung, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Otberg N, Shapiro J. Alopecia areata. In: Kang S, Amagai M, Bruckner AL, et al., editors. Fitzpatrick’s Dermatology,
2. Amin SS, Sachdeva S. Alopecia areata: a review. J Dermatol Surg. 2013;17:37–45.
3. Beigi PKM. Alopecia Totalis/Universalis. In: Alopecia Areata. Cham: Springer; 2018:13–15.
4. Leung MC, Sutton CW, Fenton DA, Tobin DJ. Trichohyalin is a potential major autoantigen in human alopecia areata. J Proteome Res. 2010;9(10):5153–5163. doi:10.1021/pr100422u
5. Alsantali A. Alopecia areata: a new treatment plan. Clin Cosmet Investig Dermatol. 2011;4:107–115. doi:10.2147/CCID.S22767
6. Pratt CH, King LE, Messenger AG, Christiano AM, Sundberg JP. Alopecia areata. Nat Rev Dis Primers. 2017;3:1–37. doi:10.1038/nrdp.2017.11
7. Joly P. The use of methotrexate alone or in combination with low doses of oral corticosteroids in the treatment of alopecia totalis or universalis. J Am Acad Dermatol. 2006;55(4):632–636. doi:10.1016/j.jaad.2005.09.010
8. Kumaresan M. Intralesional steroids for alopecia areata. Int J Trichol. 2010;2(1):63–65. doi:10.4103/0974-7753.66920
9. Suchonwanit P, Thammarucha S, Leerunyakul K. Minoxidil and its use in hair disorders: a review. Drug Des Dev Ther. 2019;13:2777–2786. doi:10.2147/DDDT.S214907
10. Seetharam KA. Alopecia areata: an update. Indian J Dermatol Venereol Leprol. 2013;79(5):563–575. doi:10.4103/0378-6323.116725
11. Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update part I: clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62(2):177–188. doi:10.1016/j.jaad.2009.10.032
12. Olsen EA, Hordinsky MK, Price VH, et al. Alopecia areata investigational assessment guidelines-part II. J Am Acad Dermatol. 2004;51:440–447. doi:10.1016/j.jaad.2003.09.032
13. Tharumanathan S. Understanding the biological mechanism of alopecia areata. Am J Dermatol Venereol. 2015;4(1):1–4.
14. Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol. 1992;128(11):1467–1473. doi:10.1001/archderm.1992.01680210045005
15. Hammerschmidt M, Brenner FM. Efficacy and safety of methotrexate in alopecia areata. An Bras Dermatol. 2014;89(5):729–734. doi:10.1590/abd1806-4841.20142869
16. Anuset D, Perceau G, Bernard P, Reguiai Z. Efficacy and safety of methotrexate combined with low-to moderate-dose corticosteroids for severe alopecia areata. Dermatol. 2016;232(2):242–248. doi:10.1159/000441250
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.