Back to Journals » International Journal of General Medicine » Volume 14
Successful Sequential Treatment for Severe Asthma Coexisting COVID-19 via Budesonide/Glycopyrrolate/Formoterol Fumarate
Authors Liang Y, Chen M , Tan C, Tu C, Zheng X , Liu J
Received 11 November 2020
Accepted for publication 8 January 2021
Published 4 February 2021 Volume 2021:14 Pages 357—359
DOI https://doi.org/10.2147/IJGM.S291695
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yingjian Liang,* Meizhu Chen,* Cuiyan Tan, Changli Tu, Xiaobin Zheng, Jing Liu
Department of Pulmonary and Critical Care Medicine (PCCM), The Fifth Affiliated Hospital of Sun Yat-Sen University, Zhuhai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Liu; Xiaobin Zheng
The Fifth Affiliated Hospital of Sun Yat-Sen University, 52 East Meihua Road, Zhuhai City, 519000, People’s Republic of China
Tel/Fax +86(0)7562528733
Email [email protected]; [email protected]
Abstract: Awareness of the management of coronavirus disease 2019 (COVID-19) and airway diseases can effectively help clinical physician during the coronavirus pandemic. Herein, we elucidated a COVID-19 case coexisting with severe asthma. Budesonide/glycopyrrolate/formoterol fumarate (BGF) was used as sequential medicine to systemic glucocorticoids for his persisted symptoms related to bronchospasms. Our case suggests patients with long-term airway diseases like asthma probably attribute their symptoms to COVID-19 instead of primary diseases, which make it more difficult in the symptom control. BGF is able to be an effective and convenient choice as sequential medicine to systemic glucocorticoids in some refractory asthmatic patients complicated with COVID-19.
Keywords: coronavirus disease 2019, asthma, budesonide/glycopyrrolate/formoterol fumarate, sequential treatment
Background
Coronavirus disease 2019 (COVID-19) is caused by a newly notorious coronavirus (severe acute respiratory syndrome coronavirus-2, SARS-CoV-2) and is of great global public health concern as it spiraled into a pandemic.1 Recently, there has been increasing interest in recognizing the association between COVID-19 and airway diseases including asthma, especially about de-escalation therapy.2,3 However, there is no consensus regarding the sequential treatment of COVID-19 coexisting asthma. Herein, we elucidated our successful experience of using budesonide/glycopyrrolate/formoterol fumarate (BGF) in the sequential treatment of asthma coexisted with COVID-19.
Case Presentation
A 54-year-old Guinean man with 25-years smoking history was admitted to our hospital on 17th March 2020 for 3 weeks’ non-production cough and sneeze as well as 2 weeks of fever after his travel from COVID-19-ridden part of England and was quarantined as a COVID-19 suspected infection patient. Peripheral ground glass opacity involved in bilateral multi-lobes was found in chest computed tomography scan (Figure 1) and he was confirmed as COVID-19 by real time polymerase chain reaction according to Chinese Center for Disease Control recommended methods. Oxygen support was given after the COVID-19 diagnosis. However, his oxygenation index descends and PaCO2 ascends (17th March, PaCO2 30.6mmHg, pH 7.446, BE −3mmol/l; 23th March, PaCO2 102mmHg, pH 7.22, BE −5.7mmol/l) persisted even after high-flow nasal cannula (40L/min, 95%) and low-dose methylprednisolone therapy. He was transferred to ICU and received urgent intubation (23th March) for invasive ventilation (A/C, pressure control, Pi 17cmH2O, PEEP 9cmH20, f 26/min, FiO2 100%) as abrupt worsening in PaO2. However, deteriorated progression of his respiratory condition persisted. Due to the supplementary of long-term poor-controlled asthma as well as allergic rhinitis management history was provided from his family and obvious wheezing sound was caught, with a high level of total immunoglobulin E (301IU/mL) in his blood, clear diagnosis of acute asthma attack had been identified. In consideration of it, a large-dose systemic glucocorticoids therapy (intravenous methylprednisolone 4mg/kg/d) was administrated immediately. Respiratory condition improved gradually and tracheal intubation was removed. However, symptoms including chest tightness, dyspnea as well as wheezes still persisted in the course of systemic glucocorticoids converting to inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA) combination with dry powder inhaler because of his limited cooperation. Triple therapy with BGF with a novel pressure metered dose inhaler was used sequentially to prevents the adverse effect of long-term systematic glucocorticoids therapy. As a dramatic and rapid response to BGF, our patient successfully started out-of-bed activities, with recovery lung image (Figure 1).
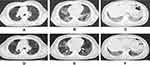 |
Figure 1 Evolution of CT scans. (A–C) represents lesions at admission. (D–F) demonstrates the recovery after out-of-bed activities. |
Antibiotic was used in the whole course. (17th to 20th March, 5th to 7th, April, moxifloxacin; 20th to 23rd March, ceftriaxone; 23rd March to 5th April, meropenem, 20th to 5th April, linezolid)
Discussion and Conclusion
To our knowledge, it is the first report about the triple therapy with BGF, a combination of ICS, long-acting muscarinic antagonists (LAMA) and LABA with co-suspension delivery technology, used in asthma complicated with COVID-19. Although the occurrence ratio of COVID-19 in asthma were low, several reports have indicated that SARS-CoV-2 commonly trigger the acute attack of asthma.2,3 Our case is able to be a potential reminder that asthma can probably increase the diagnosis and treatment difficulty of COVID-19. Two reasons maybe contribute to the diagnosis difficulty according to our clinical experience. Firstly, it is not easy to distinguish COVID-19 with airway diseases because of the similar clinical features like cough, chest tightness and dyspnea.4,5 Another is the long-standing asthma patients commonly have poor perception of own symptoms, which make it hard to describe the exact degree of their obstruction in airway and probably attribute their symptoms to COVID-19.
Triple therapy with ICS, LAMA and LABA has become increasingly popular in clinical practice as an effective and convenient treatment. Recently, triple therapy with BGF has been identified effective and convenient among airway diseases as it can result in an improvement in lung function through distinct and complementary mechanisms.6 BGF might be a good choice as sequential medicine to systemic glucocorticoids in some refractory asthma patients. In present case, BGF help our patient relieve the burden of symptoms as well as daily activities, which greatly enhance the doctors’ confidence of ICS as well as bronchodilators used in COVID-19 complicated with airway disease. We believe that it may be informative for clinicians who may encounter this type of case in their daily practice and help physicians develop a strategy for optimizing their approach to the concomitant of asthma and COVID-19 management.
Limitation
Our patient cannot provide his pre-exacerbation pulmonary function test (PFT) result as well as the past treatment. In the computed tomography scan, emphysema can be seen in the right lung, which supports the diagnosis of chronic obstructive pulmonary disease. However, our patient did not perform PFT as the precaution of COVID-19 transmission. Thus, we cannot diagnose chronic obstructive pulmonary disease without bronchodilation test result.
Abbreviations
BGF, budesonide/glycopyrrolate/formoterol fumarate; COVID-19, coronavirus disease 2019; SARS-CoV-2; ICS, severe acute respiratory syndrome coronavirus-2; LABA, long-acting β2-agonist; LAMA, long acting muscarinic antagonists.
Ethics Approval and Consent to Participate
Institutional approval is not applicable or required for publication of this manuscript.
Consent for Publication
Written and informed consent for publication was obtained from the patient. The patient was informed that de-identified data would be used in the scientific research and publications.
Funding
This work was supported by Zhuhai science and technology innovation Bureau (grant number ZH22036302200021PWC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi:10.1016/S0140-6736(20)30185-9
2. Li YK, Peng S, Li LQ, et al. Clinical and transmission characteristics of covid-19 - a retrospective study of 25 cases from a single thoracic surgery department. Curr Med Sci. 2020;40(2):295–300. doi:10.1007/s11596-020-2176-2
3. Abrams EM, Geert WJ, Yang CL. Asthma and COVID-19. CMAJ. 2020.
4. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi:10.1001/jama.2020.1585
5. Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178. doi:10.1183/09031936.00138707
6. Ishiura Y, Fujimura M, Ohkura N, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate improves inspiratory capacity in patients with asthma-chronic obstructive pulmonary disease overlap. Int J Chron Obstruct Pulmon Dis. 2020;15:269–277. doi:10.2147/COPD.S231004
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
