Back to Journals » Clinical Ophthalmology » Volume 8
Subthreshold diode-laser micropulse photocoagulation as a primary and secondary line of treatment in management of diabetic macular edema
Authors Othman I, Eissa SA, Kotb M , Sadek S
Received 24 December 2013
Accepted for publication 15 January 2014
Published 31 March 2014 Volume 2014:8 Pages 653—659
DOI https://doi.org/10.2147/OPTH.S59669
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Ihab Saad Othman,1 Sherif Ahmed Eissa,1 Mohamed S Kotb,1 Sherin Hassan Sadek2
1Cairo University, Cairo, 2Fayoum University, Al Fayoum, Egypt
Background: The purpose of this study was to evaluate subthreshold diode-laser micropulse (SDM) photocoagulation as a primary and secondary line of treatment for clinically significant diabetic macular edema (CSDME).
Methods: In this prospective nonrandomized case series, 220 cases of nonischemic CSDME were managed primarily and secondarily by SDM photocoagulation on a 15% duty cycle with a mean power of 828 mW and a spot size of 75–125 µm. SDM treatment was repeated at 3–4-month intervals if residual leakage was observed. Additional intravitreal pharmacologic therapy was used according to the response. Follow-up varied from 12 to 19 (mean 14±2.8) months. Novel software designed by the authors was used to record the subvisible threshold laser applications and their parameters on the fundus image of the eye. Evaluation of the results of treatment was done using fluorescein angiography and optical coherence tomography (OCT). Primary outcome measures included changes in visual acuity and foveal thickness at OCT. Secondary outcome measures included visual loss of one or more Snellen lines and laser scars detectable on fundus biomicroscopy or fluorescein angiography.
Results: In the primary treatment group, there was significant improvement or stabilization of visual acuity after the first 3–4 months, which was stable thereafter. Visual acuity was stable in the secondary treatment group. A corresponding reduction of macular thickness on OCT was noted during the follow-up period in both groups. Additional therapy included repeat SDM photocoagulation, intravitreal injection of triamcinolone, and pars plana vitrectomy. Laser marks seen as changes in retinal pigment epithelium on fundus biomicroscopy and fluorescein angiography were noted in 3.3% and 5.7% of cases. Our novel software could accurately record the location of all SDM-invisible applications.
Conclusion: Micropulse laser is an effective minimal intensity therapy that offers the clear advantage of minimizing or avoiding laser-induced visible retinal burn/scarring while reducing the foveal thickness in the management of selected cases of CSDME. Future prospective studies should include the use of SDM photocoagulation as a combined minimally invasive therapy to consolidate the prompt but temporary effects of anti-vascular endothelial growth factor or anti-inflammatory agents. Virtual localization of SDM-invisible applications using our proprietary software could be used to guide further retreatments.
Keywords: subthreshold diode-laser, optical coherence tomography, fluorescein angiography, clinically significant diabetic macular edema
Introduction
Laser photocoagulation has been used for the last 40 years in the management of diabetic macular edema. In 1985, the Early Treatment of Diabetic Retinopathy Study (ETDRS) showed that focal laser photocoagulation with a visible burn end point reduces the risk of moderate vision loss in patients with clinically significant diabetic macular edema (CSDME) by 50% at 3-year follow-up.1 Diabetic macular edema remains the most common cause of legal blindness in patients younger than 60 years of age.2 The long-term results of a randomized trial showed a clear advantage of focal/grid laser photocoagulation compared with triamcinolone injection in terms of stabilization or improvement of visual acuity.3 It was also shown that intravitreal ranibizumab or triamcinolone when combined with prompt or deferred laser photocoagulation is more effective than laser photocoagulation alone in improving visual acuity and macular thickness in CSDME cases at 2-year follow-up.4 Classic laser photocoagulation is a photothermal destructive therapy where the photoreceptor/retinal pigment epithelium-choriocapillaris complex is coagulated with visible burns, with elevation in tissue temperature mediating the therapeutic effect. Associated complications include expanding scarring, central field loss, subfoveal fibrosis, and choroidal neovascularization.5–7 Over the years, many investigators have shown the clear efficacy of light or invisible laser applications versus classic laser photocoagulation in the treatment of CSDME.8–10 Additionally, growing interest in subthreshold diode-laser micropulse (SDM) photocoagulation for CSDME has resulted in a number of publications.9–15
Materials and methods
This study was conducted as a single-center, prospective, nonrandomized, interventional case series at the Eye World Hospital in Giza, Egypt. Patients were enrolled from October 1, 2010 to November 30, 2012. Eligibility criteria included a diagnosis of type 2 diabetes mellitus and nonproliferative diabetic retinopathy, with CSDME documented by slit-lamp biomicroscopy, as defined by the ETDRS,3 and confirmed by optical coherence tomography (OCT), visual acuity of at least 20/80 on Snellen’s chart for virgin eyes with no prior treatment and more than 20/200 for eyes previously treated with argon laser photocoagulation and showing persistent macular edema. Increased macular thickness was established if the foveal thickness, defined as the mean thickness of the central 1 mm diameter disc of the retinal map, exceeded >210 μm. Exclusion criteria were proliferative diabetic retinopathy, foveal ischemia as evidenced by fluorescein angiography showing evidence of more than 6 clock hours of macular capillary nonperfusion, glaucoma, vitreous hemorrhage or media opacity, ocular surgery within the past year, or intravitreal injections. Informed consent was given by the patients after explaining the procedure in detail.
Baseline examination included Snellen best-corrected visual acuity, fundus photography, fluorescein angiography, and retinal thickness on OCT. OCT scans were obtained using the Stratus OCT-3 (Zeiss Humphrey Instruments, Dublin, CA, USA) with a fast macular thickness map protocol and software version 4.0. Foveal thickness was recorded for analysis from the retinal thickness maps. Whenever possible, blood pressure and glycosylated hemoglobin values were also obtained at baseline and as required along with medical consultation to assess the general condition of the patient and exclude the possibility of fluid overload. Recorded demographic details included patient age, sex, race, type and duration of diabetes, and other systemic illnesses. A comprehensive discussion with patients was undertaken preoperatively and informed consent, including for investigations done, was obtained from all patients.
Treatment technique
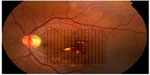
Photocoagulation was performed by the same surgeon (ISO) using an 810 nm diode-laser (OcuLight SLx; Iridex Corp, Mountain View, CA, USA) in the micro pulse emission mode. The laser power was adjusted to 1,000 mW with a duration of 300 msec and a 15% duty cycle. The spot size was set at 75–125 μm and applied confluently to cover the whole area of macular edema up to 200 μm from the center of the foveal avascular zone using an Ocular Mainster (standard) focal/grid lens (Ocular Instruments Inc, Bellevue, WA, USA). If a laser tissue reaction was noted, the power was reduced in steps of 200 mW until no reaction could be seen. The fundus picture was imported into specially designed proprietary software where an Amsler’s grid pattern was plotted on the macular area based on identifying the temporal edge of the optic nerve and the center of the foveal avascular zone (Figures 1 and 2). A color palette was used after each treatment session to mark the treated small squares corresponding to the treated zone with the performed laser parameters (Figures 2 and 3). The color palette could be changed and marked differently according to the laser parameters in each treatment session. The software could make the invisible laser spots identifiable on the Amsler’s grid square pattern. Laser parameters and applications were also recorded for each treatment session. We considered one quadrant involved if more than 50% of the area was thickened with exudates or hemorrhages. Follow-up visits were performed at one month after treatment and every 3 months thereafter, and included fundus biomicroscopy, best-corrected visual acuity, fluorescein angiography, and OCT. Supplemental treatments were performed using the same protocol as for the primary treatment and were considered at each follow-up visit in those eyes with treatable lesions on fluorescein angiography and either increased foveal thickening on fundus examination or persistent foveal thickening and/or decreased vision. The new image on the proprietary software helped to identify prior treatments, adjust and modify new laser criteria, and mark the new treatment session on the fundus picture for future reference. The study included all patients who completed the 12-month follow-up examination.
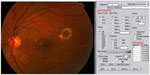  | Figure 1 Software used to import the fundus image from the patient. The laser criteria used are selected from the menu. |
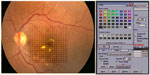  | Figure 2 Amsler’s grid pattern is projected onto the fundus picture. Each square represent a one degree projection. The color palette is chosen from the drop down menu. |
Follow-up assessment
The primary outcome measure was the proportion of patients with visual improvement or stabilization and a significant decrease in foveal thickness on OCT retina thickness maps of more than 10% of initial thickness, thus avoiding changes due to spontaneous intervisit variability. Secondary outcome measures included mean changes in visual acuity, the proportion of eyes that experienced a visual loss of one line on the Snellen’s chart, and laser marks noted on fundus photography or fluorescein angiography as window defects compared with baseline examination at 3, 6, and 12 months after study entry.
Statistical analysis
Data were analyzed using Statistical Package for Social Sciences version 10 software (SPSS Inc., Chicago, IL, USA). Qualitative data are presented as the number and percentage. Quantitative data are presented as the mean ± standard deviation. The paired t-test was used for comparison within groups. P<0.05 was considered to be statistically significant.
Results
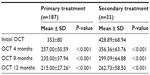
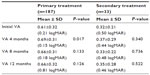
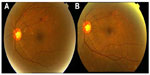
We enrolled 260 eyes from 200 patients diagnosed with CSDME. Only 220 eyes from 180 patients treated with SDM completed the 12–19-month follow-up period. Twenty patients did not attend for follow-up secondary to general illness issues (renal failure, diabetic foot, and stroke). The patients comprised 120 women (150 eyes) and 60 men (70 eyes) aged 35–69 (mean 56.07±6.75) years and with type 2 diabetes mellitus for 5–29 (mean 12.6±6.58) years. The eyes were classified in two groups, ie, group 1, which included 187 eyes with primary CSDME, and group 2, which included 33 eyes with recurrent or persistent CSDME 3 months after prior argon laser photocoagulation. Follow-up ranged from 12 to 19 months (mean 14±2.8 months). For group 1 patients, the mean baseline foveal thickness at OCT was 353±80 (270–520) μm. Mild to moderate nonproliferative retinopathy was present in 73 eyes and severe nonproliferative diabetic retinopathy was present in 114 eyes. Central foveal thickness showed significant improvement at 4 months to 257±50.59 μm (P<0.001) in 131 eyes (70.05%, Table 1). On the final follow-up, gradual improvement in macular thickness to 215±27.26 μm was noted in 159 eyes (85%, Table 1). Mean best-corrected visual acuity showed stabilization at 4 months (P<0.017, Table 2) and was maintained throughout the study in 159 eyes (85%). There was an improvement in best-corrected visual acuity in 24 (15%) of the 159 eyes over 14 months of follow-up, with corresponding improvement in foveal thickness and exudates (Figure 4). During follow-up, additional SDM treatment was necessary in 43 eyes (23%). The median number of SDM treatments was two (range one to three). Twenty-eight eyes showed persistent macular edema and worsening of visual acuity on follow-up (14.96%). These eyes were retreated with SDM at the 4-month follow-up. Further management included intravitreal injection of triamcinolone acetonide 4 mg/0.1 mL in 22 eyes (11.76%) and pars plana vitrectomy in six eyes (3.2%). For group 2 patients, the mean foveal thickness was 428.89±68.94 μm. Mild to moderate nonproliferative retinopathy was seen in ten cases and severe nonproliferative retinopathy in 22 cases. These patients were managed by SDM with a median of three sessions (range one to five) 4 months apart. At 4 months, central foveal thickness improved significantly to 356±63.76 μm (P<0.001, Table 1) and showed gradual stabilization to 262.73±58.5 μm at the 12-month follow-up in 22 cases (65%). Visual acuity was stable in 20 eyes at 4 months (P=0.441, Table 2), and one Snellen’s line of acuity improvement was documented in two eyes at the 12-month follow-up. Eleven eyes (33%) showed a poor response to SDM and were injected with triamcinolone. SDM could improve CSDME without inducing further noticeable damage to the macular area after maximum argon laser photocoagulation (Figure 5). The results were maintained at 12 months in 65% of cases. Eleven eyes needed intravitreal triamcinolone injection for diabetic macular edema that did not respond to laser treatment. Using our software, we recorded the location of microaneurysms, focal or diffuse leakage, and the power and number of laser spots delivered in the macular area in each quadrant of the projected Amsler’s grid. In our Middle Eastern population, SDM power was applied with a 15% duty cycle and the number of spots required is summarized in Table 3. In the presence of diffuse leakage affecting all four quadrants, the mean power was increased to 1,000±187 mW, with the number of spots reaching 612±215 applications. All power settings allowed laser application with no visible reaction on the retina. Laser marks seen as retinal pigment epithelium changes on fundus biomicroscopy and fluorescein angiography were noted in 3.3% and 5.7% of cases.
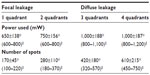  | Table 3 Mean power used and laser spots according to quadrant distribution of macular edema in the fundus |
Discussion
In 1997, Friberg and Karatza10 first reported on the clinical application of 810 nm micropulse diode laser for the treatment of macular disease. Since then, many studies have shown the efficacy of this method.9–17 Micropulse laser photocoagulation has been shown on microperimetry to be a safe method for improving vision and minimizing visual field loss with the absence of scotoma, and preservation of color vision and contrast sensitivity. Further, retinal thickness on OCT showed a reduction following SDM photocoagulation that was maintained during follow-up. In our series, there was a significant reduction in macular thickness in our primary and secondary treatment groups, mostly noticed at 4 months and ongoing during the follow-up period. Visual acuity was stable or improved in 85% of eyes in the primary treatment group and in 65% of eyes in the secondary treatment group. Our results at one-year follow-up were consistent with those of the ETDRS and National UK Diabetic Audit, where 79.6% showed stabilization and 10.3% showed improvement on Snellen’s visual acuity chart,1,18 with the advantage of no laser burns or related anatomic retinal or functional damage and collateral effects.
Despite the number of published papers in the literature, SDM photocoagulation has not been widely adopted for the management of macular retinovascular disease. A reason for this resistance might be the inability of the practitioner to see the intraoperative laser tissue reaction. This automatically creates a psychological barrier to application of an invisible spot to the retina and being unable to identify where the laser spots were positioned on follow-up. We attempted to resolve this issue by using our proprietary software that allows the surgeon to record his or her laser applications immediately after treatment by labeling the small square of the projected Amsler’s grid on the fundus picture of the patient’s macula. Further, the exact power settings are marked directly on the fundus image (Figures 1–3), allowing us to identify the treated area on follow-up and sharply define retreatment zones. In addition, we were able to determine the amount of power needed and spots applied according to the number of quadrants involved. This may serve as a reference when using SDM photocoagulation in the Middle Eastern population.
In this study of SDM photocoagulation, we aimed at painting the entire thickened and edematous retina with a pattern of confluent laser applications to maximize the beneficial effect. This method of treatment is made possible by the absence of laser scars, unlike conventional ETDRS focal/grid photocoagulation in which visible laser burns create scars and must be spaced to prevent large scotomata.1 Photothermal laser is associated with destruction of the retinal pigment epithelium/choriocapillaris/photoreceptor junction. The high intensity with a lower number of laser spots in addition to the damage induced directly at the site of application carries the risk of lateral heat spread, enlarging the laser spot over time and increasing the relative scotoma.5–7,18 It has been suggested that the benefits of thermal laser photocoagulation are derived from modification of gene expression mediated by the biological wound healing response of retinal pigment epithelium cells to thermal injury.19 This effect occurs away from the site of the impact of the photothermal laser, where retinal pigment epithelium cells are still viable and are stimulated by the laterally dispersed sublethal heat away from the site of impact. SDM photocoagulation is presumed to induce upregulation and downregulation of different vascular and permeability factors, correcting the pathologic imbalance with a lasting therapeutic effect and without any harmful photothermal effects.19 Finally, in the era of intravitreal injections of anti-inflammatory and anti-vascular endothelial growth factor agents, the Diabetic Retinopathy Clinical Research Network has confirmed that long-term treatment of diabetic macular edema may require repeat interventions as needed, where a tissue-sparing laser therapy without the propensity to cause cumulative retinal damage offers clear advantages.3
The results of this study demonstrate the benefit of SDM photocoagulation in managing CSDME in a relatively large number of patients with one-year follow-up. We introduced user-friendly software that allows the surgeon to record what he or she has done in a reproducible manner on the fundus picture, thus enabling the invisible to become visible and allowing adjustment or titration of application of SDM photocoagulation. Importantly, this technique allowed us to analyze and report the mean laser power and number of spots required for our Middle Eastern population.
Conclusion
Micropulse laser is an effective minimal intensity therapy that offers the clear advantage of minimizing or avoiding laser-induced visible retinal burn/scars while reducing foveal thickness in selected cases of CSDME. Future prospective studies might include SDM photocoagulation as a combined minimally invasive therapy to consolidate the prompt but temporary effects of anti-vascular endothelial growth factor or anti-inflammatory agents. Virtual localization of invisible SDM applications using our proprietary software could be used to guide further retreatments as needed.
Disclosure
The authors report no conflicts of interest in this work.
References
Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 1. Arch Ophthalmol. 1985;103:1796–1806. | |
Klein R, Klein BE, Moss SE, et al. The Wisconsin epidemiologic study of diabetic retinopathy. XIV. Ten-year incidence and progression of diabetic retinopathy. Arch Ophthalmol. 1994;112:1217–1228. | |
Diabetic Retinopathy Clinical Research Network (DRCR.net); Beck RW, Edwards AR, Aiello LP, et al. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–251. | |
The Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. | |
Striph GG, Hart WM Jr, Olk RJ. Modified grid laser photocoagulation for diabetic macular edema. The effect on the central visual field. Ophthalmology. 1988;95:1673–1679. | |
Schatz H, Madeira D, McDonald HR, et al. Progressive enlargement of laser scars following grid laser photocoagulation for diffuse diabetic macular edema. Arch Ophthalmol. 1991;109:1549–1551. | |
Lewis H, Schachat AP, Haimann MH, et al. Choroidal neovascularization after laser photocoagulation for diabetic macular edema. Ophthalmology. 1990;97:503–510. | |
Bandello F, Polito A, Del Borrello M, et al. “Light” versus “classic” laser treatment for clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:864–870. | |
Roider J, Brinkmann R, Wirbelauer C, et al. Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: a pilot study. Br J Ophthalmol. 2000;84:40–47. | |
Friberg TR, Karatza EC. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology. 1997;104:2030–2038. | |
Moorman CM, Hamilton AM. Clinical applications of the micropulse diode laser. Eye. 1999;13:145–150. | |
Tseng S-Y. Clinical application of micropulse diode laser in the treatment of macular edema. Am J Ophthalmol. 2005;139:S58. | |
Siviprasad S, Sandhu R, Tandon A, Sayed-Ahmed K, McHugh DA. Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: a three-year follow up. Clin Experiment Ophthalmol. 2007;35:640–644. | |
Figueira J, Khan J, Nunes S, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol. 2009;93:1341–1344. | |
Vujosevic S, Bottega E, Casciano M, et al. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina. 2010;30:908–916. | |
Laursen ML, Moeller F, Sander B, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004;88:1173–1179. | |
Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol. 2005;89:74–80. | |
Bailey CC, Sparrow JM, Grey RH, Cheng H. The National Diabetic Retinopathy Laser Treatment Audit. III. Clinical outcomes. Eye. 1999;13 Pt 2:151–159. | |
Dorin G. Subthreshold and micropulse diode laser photocoagulation. Semin Ophthalmol. 2003;18:147–153. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.