Back to Journals » Clinical Ophthalmology » Volume 15
Subretinal Injection of Recombinant Tissue Plasminogen Activator and Gas Tamponade to Displace Acute Submacular Haemorrhages Secondary to Age-Related Macular Degeneration
Authors Iannetta D, De Maria M , Bolletta E, Mastrofilippo V, Moramarco A, Fontana L
Received 22 June 2021
Accepted for publication 3 August 2021
Published 28 August 2021 Volume 2021:15 Pages 3649—3659
DOI https://doi.org/10.2147/OPTH.S324091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Danilo Iannetta, 1 Michele De Maria, 1 Elena Bolletta, 2 Valentina Mastrofilippo, 1 Antonio Moramarco, 1 Luigi Fontana 1
1Ophthalmology Unit, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy; 2Ocular Immunology Unit, Azienda USL-IRCCS di Reggio Emilia, Reggio Emilia, Italy
Correspondence: Danilo Iannetta
Ophthalmology Unit, AUSL-IRCCS Reggio Emilia, Viale Risorgimento 80, Reggio Emilia, Italy
Tel +39 0522296520
Fax +39 0522295839
Email [email protected]
Purpose: To analyse the efficacy of subretinal injection of recombinant tissue plasminogen activator (rtPA) and gas tamponade for the displacement of submacular haemorrhage (SMH).
Methods: This single-centre, retrospective, case series included 25 consecutive patients (25 eyes) who underwent pars plana vitrectomy (PPV) with subretinal rtPA injection and 20% sulphur hexafluoride (SF6) tamponade. The primary outcome was SMH displacement rate, defined as the absence of subretinal blood within (complete) or outside (partial) 1500 μm centred on the fovea one month after PPV. Secondary outcomes were final best-corrected visual acuity (BCVA), central macular thickness (CMT), recurrence probability, number of anti-vascular endothelial growth factor (VEGF) injections after PPV, and intra- and post-operative complications.
Results: Successful displacement was obtained in all 25 eyes (100%), with complete and partial displacement obtained in 15 (60%) and 10 (40%), respectively. BCVA significantly improved from 1.81± 0.33 to 1.37± 0.52 LogMar at 12 months from surgery (p = 0.001). The bivariate correlation analysis revealed that earlier the surgery had better visual prognosis at the end of the follow-up (p = 0.007). CMT significantly decreased from 922 ± 273.69 μm at baseline to 403.53 ± 314.64 μm at 12 months follow-up (p < 0.001). SMH recurrence was observed in two (8%) patients with a mean survival time of 11.6 ± 0.339 months and a cumulative survival probability of 88% at the end of follow-up. After PPV, the mean number of anti-VEGF injections was 3.00 ± 0.957 with no correlation with final visual acuity (p = 0.365). No intraoperative complications were recorded. Only one patient developed open funnel retinal detachment 40 days after primary PPV.
Conclusion: PPV with rtPA subretinal injection and SF6 tamponade is a safe and effective technique in displacing acute SMHs secondary to neovascular AMD. It is recommended to perform within 14 days from the onset of the symptoms to achieve BCVA improvement at 12 months and proper imaging to plan future anti-VEG treatment.
Keywords: macular degeneration, submacular haemorrhage, recombinant tissue plasminogen activator, pars plana vitrectomy, subretinal injection
A Letter to the Editor has been published for this article.
Introduction
Submacular haemorrhage (SMH) is defined as the accumulation of blood in the virtual space between the neurosensory retina and the retinal pigment epithelium (RPE), arising from the choroidal or retinal circulation within the macula and posterior pole.1 It is a potentially devastating complication of several ophthalmic or systemic diseases. Choroidal neovascularisation (CNV) is the most common cause of SMH associated primarily with age-related macular degeneration (AMD).2 Other less common ocular diseases that might complicate with CNV include polypoidal choroidal vasculopathy (PCV), myopic CNV and angioid streaks. Moreover, SMH can occur immediately after blunt or penetrating trauma from localised choroidal rupture or later from CNV that can develop at the edge of the rupture site.3,4
Independent of its primary cause, the visual prognosis of SMH is dramatically poor, mainly if left untreated,2,5,6 because of blood degradation chemical toxicity and clot mechanical damage.2,7 Blood breakdown molecules, such as hemosiderin, fibrin and iron, provoke photoreceptor necrosis.5,8 Additionally, the physical separation of the photoreceptor layer from the underlying RPE and the formation of a contractile clot trigger mechanical stress to the surrounding neuroretinal tissue, with progressive atrophy of the neurosensory cells and a consequent irreversible visual loss.9
To date, shadows remain on the best treatment options for these patients, primarily because the major clinical trial on the treatment of neovascular AMD with anti-vascular endothelial growth factor (VEGF) drugs did not include such complex cases.10
According to the current literature, the treatment rationale for large or massive SMHs relies on the displacement of blood materials away from the fovea, aiming to initiate blood reabsorption in an extramacular region and, thus, preventing macular photoreceptor damage.11–14 Various surgical techniques to displace SMH have been described, including pneumatic displacement (PD),15 intravitreal injection of recombinant tissue plasminogen activator (rtPA) with or without PD,16 pars plana vitrectomy (PPV) with subretinal injection of rtPA17 with or without the use of perfluorocarbon18 and with or without PD, and PPV with or without rtPA, in addition to surgical excision of the subretinal CNV.12 More recently, some authors have reported the use of additional subretinal air injection (subretinal PD) to enhance SMH displacement.19,20 Moreover, intravitreal anti-VEGF injections have been advocated in monotherapy or combination with rtPA, PD with or without PPV.21
Notwithstanding the vast scenario of the techniques described, evidence demonstrates that prompt treatment of SMH within seven to 14 days after its onset is crucial to prevent irreversible retinal damage.22
Moreover, treatment is suggested to ensure patients’ adequate follow-up.5,23 A large and thick clot obstructs the visualisation of the underlying retina and thus impedes proper follow-up with specific diagnostic exams. Submacular blood hampers the proper characterisation of a CNV (type, size, and location) and the definition of factors that allow making prevision on the chances of visual acuity (VA) improvement.
Despite several studies describing the effectiveness of subretinal injection of rtPA and gas injection into the vitreous chamber for SMH displacement, there are no shared guidelines or universally accepted results in terms of visual recovery, blood displacement and complications.
This study reports on the blood displacement proficiency of PPV combined with subretinal rtPA injection with SF6 gas tamponade in patients affected by SMH secondary to neovascular AMD.
Methods
Study Design
We conducted a single-centre, retrospective case series to analyse patients with a diagnosis of SMH secondary to AMD who underwent PPV with subretinal injection of rtPA and sulfur hexafluoride (SF6) gas displacement in the Ophthalmology Unit of the Azienda USL-IRCCS di Reggio Emilia (Reggio Emilia, Italy) between October 2018 and April 2020. This study obtained ethical approval from the local Institutional Review Board “Comitato Etico di Area Vasta Emilia Nord” to collect and analyze clinical data. The study was conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent, including consent to publish, was obtained from all participants.
Participants
The study included patients aged ≥50 years with a diagnosis of SMH secondary to neovascular AMD who were referred to our unit because of sudden visual impairment. Only subjects with a time from symptoms to surgery of no longer than 14 days (as determined by the patient’s history and clinical chart revision) were offered treatment.
Patients with other ocular comorbidities (diabetic retinopathy, severe myopia, advanced glaucoma and history of uveitis), history of previous vitreoretinal surgery, inadequate assessment of subretinal blood (due to severe media opacities), and SMH secondary to other forms of macular diseases other than AMD were excluded from the study.
Preoperative Assessment
Before surgery, a complete ophthalmic evaluation was conducted, including best-corrected visual acuity (BCVA), slit-lamp biomicroscopic examination, intraocular pressure measurement (IOP) by Goldman applanation tonometry, dilated fundus examination with a 90-DPT indirect lens, colour fundus photography (Optos California, Dunfermline, United Kingdom) and spectral-domain optical coherence tomography (SD-OCT) (Spectralis, Heidelberg Engineering, Heidelberg, Germany) to define the size and location of the subretinal haematoma.
Data collected included all baseline demographic information (age, gender, the affected eye, lens status, history of prior treatment with intravitreal anti-VEGF, systemic therapy with antiaggregant and/or anticoagulant drugs), duration of symptoms before surgery, BCVA before SMH, and BCVA at presentation. BCVA was converted into the logarithm of the minimal angle of resolution (LogMAR) scale for statistical analysis. “Counting fingers at 1 m” was converted into 1.8 LogMAR, “counting fingers at 30 cm” into 2.6 LogMAR, “hand movement” into 2.9 LogMAR and “light perception” into 3.2 LogMAR.24 In cases of blood accumulations that prevented a correct preoperative diagnosis, neovascular AMD was confirmed after surgery.
Surgical Technique
Surgical procedures were carried out by a single experienced vitreoretinal surgeon (DI). Patients underwent PPV in isolation or combination with cataract extraction (CE). In these cases, standard phacoemulsification with intraocular lens (IOL) implantation was completed before proceeding with vitrectomy and rtPA subretinal injection with SF6.
The technique was carried out as illustrated by Arias and Treumer in previous studies.1,25–27 A peribulbar block was obtained injecting 5–10 cc of a solution containing lidocaine and bupivacaine for local anaesthesia. All patients underwent standard three-port 23 gauge PPV with posterior hyaloid detachment induction (if not already present), followed by a core vitrectomy extended to the periphery.
Subretinal injection of 25µg/0.1 mL of rtPA (Actilyse, Boehringer Ingelheim, Germany) through a 41 gauge subretinal flexible cannula (DORC, Zuidland, The Netherlands) was performed to liquefy the haematoma and promote its displacement toward the inferior retinal quadrants. The bullous detachment of the posterior pole was obtained with a transretinal approach by entering the cannula at a single point located at one optic disc diameter below the superotemporal vascular arcade while watching for the absence of reflux toward the fovea or occurrence of an iatrogenic macular hole during the injection. After a complete fluid–air exchange, the procedure was concluded with the injection of 1.25 mg/0.05 mL bevacizumab into the vitreous cavity, followed by a complete air–SF6 20% exchange. All sclerotomies were sutured with Vicryl 8-0 at the end of the procedure.
At the end of the surgery, intracameral 1mg/mL cefuroxime sodium (Aprokam, Laboratoires THEA S.A.S., Clermont-Ferrand, France) or subconjunctival gentamicin was injected after a combined or PPV only procedure.
Alternate head-down-head-straight positioning was recommended several times per day for three days after the procedure. An anti-VEGF injection was performed one and two months after the surgery and then in the following months, depending on the optical coherence tomography (OCT) examination results.
Postoperative Assessment
Postoperatively, all patients underwent a complete ophthalmic evaluation at month 1, month 3, month 6, month 12, or, more frequently, if clinically indicated by the physician. Collected data included BCVA measurement, evaluation of the mobilisation of the macular haematoma by fundus photography and spectral-domain optical coherence tomography (SD-OCT), and number of postoperative intravitreal anti-VEGF injections and complications.
Outcome Measure
The primary outcome was the haemorrhage displacement rate one month after surgery. The efficacy of blood displacement was evaluated with the aid of fundus photographs and images from other diagnostic tools (SD-OCT) and ophthalmoscopic exam findings reported in the medical charts. Complete displacement was defined as the absence of subretinal blood within 1.500 μm centred on the fovea. Partial displacement was defined as the absence of haemorrhage under the foveola, but with persisting traces of blood within 1.500 μm centred on the fovea.
No displacement was defined as blood persisting under the foveal area on the fundus photography and OCT scan. The foveal area was defined as a zone of 1500 mm in diameter centred on the foveal avascular zone.
Secondary outcomes were:
- BCVA at the end of follow-up,
- CMT at the end of follow-up, measured from the Bruch’s membrane to the internal limiting membrane at the fovea with the calliper function of the SD-OCT,
- success rate, defined as no SMH recurrence during the follow-up period,
- number of anti-VEGF treatment after primary PPV with subretinal rtPA injection and gas tamponade, and
- intraoperative and postoperative complications.
Statistical Analysis
Demographic and clinical data were expressed using the frequency and percentage for categorical variables and the mean (± standard deviation [SD]) for continuous numerical values. To calculate each variable significance level, paired sample t-test or chi-squared test (or Fisher's exact test, where appropriate) was used. Kaplan–Meier survival curve analysis was employed to evaluate the cumulative probability of success rate throughout the follow-up period. To evaluate the statistical association between two categorical variables, the Pearson χ2 test was used. A value of p < 0.05 was considered significant for all tests. Statistical analysis was performed using v.27 of SPSS (IBM, Armonk, New York, United States).
Results
This study included 25 eyes of 25 consecutive patients referred to our unit due to SMH secondary to neovascular AMD. In 13 eyes (52%), SMH was the first clinical manifestation of age-related maculopathy (with diagnostic confirmation after primary vitrectomy), while in 12 eyes (48%), it occurred during the standard course of treatment with anti-VEGF. Patient demographics are summarised in Table 1. Only two (8%) eyes showed mild vitreous haemorrhage at presentation that did not preclude the scheduled preoperative exams for proper assessment of the SMH characteristics, and, for these reasons, all patients were offered PPV with subretinal injection of rtPA.
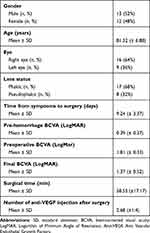 |
Table 1 Demographic Information and Clinical Preoperative and Postoperative Evaluation |
The mean time from symptoms to surgery was 9.24 ± 3.37 days, ranging from three to 14 days (95% CI = 7.85–10.63). Antiplatelet or anticoagulant therapy was suspended/replaced in time, according to the cardiologist’s indications in five (20%) and six (24%) patients, respectively, before proceeding with the scheduled surgery. Combined phacoemulsification with the PPV was performed in 17 eyes (68%) of patients. Surgery was accomplished without intraoperative complications in all cases.
Primary Outcome
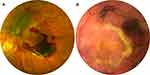
The mean ± SD preoperative SMH area at presentation was 68.34 ± 42.42 mm2 and ranged from 10.49 to 170 mm2 (95% CI = 51.24–85.43). Successful displacement of the SMH was obtained in 25 out of 25 eyes (100%) one month after surgery. A complete and partial displacement was obtained in 15 (60%) and 10 (40%) cases, respectively (Figure 1).
Secondary Outcome
Figure 2 shows the mean BCVA throughout the follow-up period. At 12 months after surgery, the mean ± SD final BCVA was 1.37 ± 0.52 LogMAR (95% CI = 1.16–1.59). The final BCVA significantly improved at the end of the follow-up compared to the baseline value (p = 0.001), although none of the patients recovered VA prior to the haemorrhagic phenomenon. We did not find any significant correlation between the final VA and the measured area of SMH.
On the contrary, we demonstrated a statistically significant positive correlation between the final VA and the time from symptoms to surgery (Figure 3), and the earlier the procedure, the better the final BCVA (p = 0.007).
 |
Figure 3 Pearson’s correlation coefficient demonstrated a positive linear relationship between time from symptoms to surgery and visual acuity at 1 year. |
SD-OCT analysis revealed an effective displacement of the subretinal component of the macular haemorrhage in all cases, with a statistically significant reduction of CMT at all the time points of the follow-up period (Table 2, Figure 4). CMT decreased from 922 ± 273.69 µm (95% CI = 790.08–1053.92) at baseline to 403.53 ± 314.64 µm (95% CI = 251.87–555.18) at 12 months follow-up (p < 0.001). The final VA at 12 months did not correlate with the final CMT (p = 0.165).
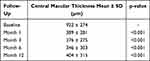 |
Table 2 Central Macular Thickness |
SMH recurrence was observed in two (8%) patients throughout the follow-up six months after the primary procedure. The Kaplan–Meier analysis (Figure 5) revealed a mean survival time of 11.6 ± 0.339 months (95% CI = 10.935–12.265). The cumulative proportion of surviving (no recurrence of SMH) at the end of the follow-up was 88%.
 |
Figure 5 Kaplan–Meier curve showing the probability of being in response vs months of follow-up. Eyes were classified as failures in case of SMH recurrence at any time points of the follow-up. |
After PPV, all patients proceeded with intravitreal injection of anti-VEGF drugs according to their treatment schedule (Figure 6). The mean number of injections at the end of the follow-up period was 3.00 ± 0.957, ranging from 2 to 5 (95% CI = 2.60–3.40). The final VA at 12 months did not correlate with the number of anti-VEGF intravitreal injections after PPV (p = 0.365).
 |
Figure 6 Colour fundus retinal photography and OCT scan before surgery (A), at month 1 (B), at month 3 (C), at month 6 (D) and at 1 year of follow-up (E). |
All procedures were uneventful. Postoperative complications included an open funnel retinal detachment in one eye 40 days after primary PPV. The patient underwent a revisional PPV and silicone oil tamponade.
Discussion
In this retrospective study, PPV with rtPA subretinal injection and SF6 tamponade was shown to be safe and effective in displacing acute SMH secondary to neovascular AMD away from the foveal region. In our cases, SMHs were partially or entirely displaced in 100% of treated patients.
Through the years, different subretinal blood displacement techniques with apparent dissimilar results have been described: intravitreal gas with or without rtPA injection,16,28–30 with or without anti-VEGF drugs,31,32 combined or not with PPV, followed by subretinal rtPA injections with gas tamponade.12–14,33 According to some authors, vitrectomy seemed to result in a higher displacement rate and better visual outcome than intravitreal rtPA and gas alone in treating SMH involving the fovea in AMD.34 Conversely, van Zeeburg et al1 reported no apparent differences in the displacement rate between the more or less invasive techniques (vitrectomy vs injection only), even if PPV showed a higher frequency of postoperative complications, such as retinal detachment, proliferative vitreoretinopathy, and vitreous haemorrhage. Despite the well-established lower complication rates with less invasive techniques, the current literature advocates a prevalent vitrectomy adoption among ophthalmologists worldwide. Our study revealed an excellent displacement rate with a low incidence of intraoperative and postoperative complications with only one patient developing retinal detachment 40 days after the primary procedure, revealing a high level of safety.
It has been demonstrated that the visual prognosis of massive SMH is dramatically poor, especially if left untreated, because of the double mechanism of damage, chemical and mechanical, of the blood under the retina.
Similar to other studies, patients in our sample achieved a mild improvement of LogMAR BCVA at 12 months follow-up, which resulted as statistically significant. Moreover, the final visual outcome showed a statistically significant positive correlation with the time from symptoms to surgery, and the earlier the vitrectomy, the better the VA. Our findings confirm the results by Hattenbach et al,6 who emphasised the importance of early surgery, suggesting that persistent haemorrhage is a predictor of final visual recovery after treatment with rtPA and gas tamponade. SMHs treated within the first two weeks demonstrated better visual results than those undergoing surgery after the third week from symptom onset. Avci et al, in a study that analysed the subretinal coapplication of rTPA and bevacizumab to treat macular haemorrhage, reported that patients with a mean SMH duration of less than 10 days significantly improved BCVA compared with patients with delayed treatment (>10 days).35 Likewise, our sample patients underwent vitrectomy within a temporal window of 14 days, and bivariate correlation analysis confirmed a significantly better LogMAR BCVA if sooner surgery was performed.6 Despite the natural history of SMH, mild improvements of VA may positively impact the quality of life of elderly patients, especially in the case of low vision in the fellow eye. Several authors reported ambulatory independence for at least three years, with a considerable reduction of care costs.36
One of the most debated topics is SMH recurrence, which is reported to range from 0% to 27% in both the least and most invasive techniques, and is dependent on the author. Notably, studies investigating intravitreal rtPA plus anti-VEGF reported no cases of SMH relapse.1,37–40 Moreover, the recurrence rate with vitrectomy seems to be lower when anti-VEGF is used. In our sample, all patients received anti-VEGF intraoperatively and after surgery (at least two injections). The probability of SMH recurrence at the end of follow-up was 2%, with only two patients reporting a bleeding relapse six months after primary PPV with subretinal rtPA and gas tamponade. Our low recurrence rate could probably be due to the adoption of anti-VEGF agents intraoperatively and a consistent anti-VEGF treatment programme during the entire follow-up period. Almost all patients in our sample received up to five injections after surgery throughout the 12 months of follow-up, with optimal control of the neovascular AMD. Although our bivariate analysis did not reveal a correlation between the cumulative injection number and final BCVA, we believe that anti-VEGF therapy contributed to the stabilisation of final VA, which significantly improved preoperatively to the final follow-up.
The half-life of intravitreally injected anti-VEGF in vitrectomized eyes was reduced by 60%, compared to non-vitrectomized eyes. Moreover, the effect of intravitreally injected anti-VEGF on the concentration of aqueous VEGF lasted for a much shorter time in the vitrectomized eyes compared with non-vitrectomized eyes.41 Despite a lack of consensus about this theory, we have not observed reduced efficacy of anti-VEGF treatments in our series, with effective response to the therapy and stabilisation of the neovascular lesion. It is noteworthy that several studies investigated the treatment of SMHs with anti-VEGF injections only, reporting improvements in or stabilisation of BCVA. Conversely, it has been established that such a conservative approach could not be applied to massive SMH. The only-injection therapy does not prevent the chemical toxicity and mechanical damage to blood breakdown molecules. It is reasonable to support the hypothesis that a thick submacular clot impedes sufficient access of anti-VEGF molecules to the choroidal neovascular lesion, drastically reducing its efficacy, and thus demanding displacement.
In our series, we treated patients with massive macular haemorrhage (up to 170 mm2). Although no statistically significant correlation was found between the extent of bleeding and final VA, prompt PPV and subretinal rtPA allowed the displacement of a bulky, oversized mass of blood. Its removal from the subretinal tissue permitted proper management of the underlying AMD, ensuring proper anti-VEGF treatment and identifying signs of disease reactivation. As stated by Maggio et al,36 haemorrhage often precludes an accurate assessment of macular features, with related difficulties in selecting the proper management strategy. The displacement of an SMH helps to perform diagnostic tests capable of revealing macular details that can guide the postoperative approach. After haemorrhage displacement and scheduled anti-VEGF therapy, only four patients (16%) were ineligible for further treatment in our population. Eyes with a complete disruption of the outer retinal layer, macular scar, no signs of active neovascularisation or complete subversion of the retinal architecture did not receive adjunctive anti-VEGF treatment. Despite the costs of primary vitrectomy, this approach made it possible to prevent significant economic losses deriving from protracted, but ineffective, injection therapies, with inconsistent benefits to patients and management difficulties for caregivers.
This study presents two main limitations: its retrospective nature and the lack of a control group. Apart from two scheduled injections after surgery, anti-VEGF treatment was not standardised and was based on physician discretion, depending on the imaging characteristics. Finally, the anti-VEGF molecules adopted were not the same for different patients receiving different anti-VEGF drugs.
In conclusion, our study demonstrated that subretinal rtPA with SF6 tamponade is safe and effective in displacing an SMH if performed within 14 days from its onset. Prompt PPV might offer significant improvement of VA at one year after surgery. Consistent treatment with anti-VEGF injections after surgery is mandatory to stabilise the neovascular lesion and maintain a visual outcome that improves patients’ quality of life. Further randomised clinical trials are necessary to establish better surgical approaches (more or less invasive), depending on SMH characteristics and the underlying disease.
Disclosure
The authors report no other potential conflicts of interest for this work.
References
1. Van Zeeburg EJT, Van Meurs JC. Literature review of recombinant tissue plasminogen activator used for recent-onset submacular hemorrhage displacement in age-related macular degeneration. Ophthalmologica. 2012;229(1):1–14. doi:10.1159/000343066
2. Bennett SR, Folk JC, Blodi CF, Klugman M. Factors prognostic of visual outcome in patients with subretinal hemorrhage. Am J Ophthalmol. 1990;109(1):33–37. doi:10.1016/S0002-9394(14)75575-8
3. Tennant MTS, Borrillo JL, Regillo CD. Management of submacular hemorrhage. Ophthalmol Clin North Am. 2002;15(4):445–452. doi:10.1016/S0896-1549(02)00049-4
4. Buschini E, Iannetta D, Lesnik Oberstein SY, Bijl HM, Mura M. Subretinal versus intravitreal injection of recombinant tissue plasminogen activator in post-traumatic submacular haemorrhages. Acta Ophthalmol. 2016;94(3):307–309. doi:10.1111/aos.12816
5. Toth CA, Morse LS, Hjelmeland LM, Landers MB. Fibrin directs early retinal damage after experimental subretinal hemorrhage. Arch Ophthalmol. 1991;109(5):723–729. doi:10.1001/archopht.1991.01080050139046
6. Hattenbach LO, Klais C, Koch FHJ, Gümbel HOC. Intravitreous injection of tissue plasminogen activator and gas in the treatment of submacular hemorrhage under various conditions. Ophthalmology. 2001;108(8):1485–1492. doi:10.1016/S0161-6420(01)00648-0
7. Avery RL, Fekrat S, Hawkins BS, Bressler NM. Natural history of subfoveal subretinal hemorrhage in age-related macular degeneration. Retina. 1996;16(3):183–189. doi:10.1097/00006982-199616030-00001
8. Gillies A, Lahav M. Absorption of retinal and subretinal hemorrhages. Ann Ophthalmol. 1983;15(11):1068–1074.
9. Glatt H, Machemer R. Experimental subretinal hemorrhage in rabbits. Am J Ophthalmol. 1982;94(6):762–773. doi:10.1016/0002-9394(82)90301-4
10. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419–1431. doi:10.1056/nejmoa054481
11. Chen CY, Hooper C, Chiu D, Chamberlain M, Karia N, Heriot WJ. Management of submacular hemorrhage with intravitreal injection of tissue plasminogen activator and expansile gas. Retina. 2007;27(3):321–328. doi:10.1097/01.iae.0000237586.48231.75
12. Haupert CL, Mccuen BW, Jaffe GJ, et al. Pars plana vitrectomy, subretinal injection of tissue plasminogen activator, and fluid-gas exchange for displacement of thick submacular hemorrhage in age-related macular degeneration. Am J Ophthalmol. 2001;131(2):208–215. doi:10.1016/S0002-9394(00)00734-0
13. Olivier S, Chow DR, Packo KH, MacCumber MW, Awh CC. Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in age-related macular degeneration. Ophthalmology. 2004;111(6):1201–1208. doi:10.1016/j.ophtha.2003.10.020
14. Chang W, Garg SJ, Maturi R, et al. Management of thick submacular hemorrhage with subretinal tissue plasminogen activator and pneumatic displacement for age-related macular degeneration. Am J Ophthalmol. 2014;157(6):1250–1257. doi:10.1016/j.ajo.2014.02.007
15. Ohji M, Saito Y, Hayashi A, Lewis JM, Tano Y. Pneumatic displacement of subretinal hemorrhage without tissue plasminogen activator. Arch Ophthalmol. 1998;116(10):1326–1332. doi:10.1001/archopht.116.10.1326
16. Tsymanava A, Uhlig CE. Intravitreal recombinant tissue plasminogen activator without and with additional gas injection in patients with submacular haemorrhage associated with age-related macular degeneration. Acta Ophthalmol. 2012;90(7):633–638. doi:10.1111/j.1755-3768.2011.02115.x
17. Peyman GA, Nelson NC, Alturki W, et al. Tissue plasminogen activating factor assisted removal of subretinal hemorrhage. Ophthalmic Surg. 1991;22(10):575–582.
18. Kamei M, Tano Y, Maeno T, Ikuno Y, Mitsuda H, Yuasa T. Surgical removal of submacular hemorrhage using tissue plasminogen activator and perfluorocarbon liquid. Am J Ophthalmol. 1996;121(3):267–275. doi:10.1016/S0002-9394(14)70274-0
19. Martel JN, Mahmoud TH. Subretinal pneumatic displacement of subretinal hemorrhage. JAMA Ophthalmol. 2013;131(12):1632–1635. doi:10.1001/jamaophthalmol.2013.5464
20. Kadonosono K, Arakawa A, Yamane S, et al. Displacement of submacular hemorrhages in age-related macular degeneration with subretinal tissue plasminogen activator and air. Ophthalmology. 2015;122(1):123–128. doi:10.1016/j.ophtha.2014.07.027
21. Fassbender JM, Sherman MP, Barr CC, Schaal S. Tissue plasminogen activator for subfoveal hemorrhage due to age-related macular degeneration. Retina. 2016;36(10):1860–1865. doi:10.1097/IAE.0000000000001030
22. Lee K, Park YG, Park YH. Visual prognosis after pneumatic displacement of submacular hemorrhage according to age-related macular degeneration subtypes. Retina. 2020;40(12):2304–2311. doi:10.1097/IAE.0000000000002762
23. Benner JD, Hay A, Landers MB, Hjelmeland LM, Morse LS. Fibrinolytic-assisted removal of experimental subretinal hemorrhage within seven days reduces outer retinal degeneration. Ophthalmology. 1994;101(4):672–681. doi:10.1016/S0161-6420(94)31279-6
24. Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287–290. doi:10.1016/j.jcrs.2004.01.014
25. Arias L, Monés J. Transconjunctival sutureless vitrectomy with tissue plasminogen activator, gas and intravitreal bevacizumab in the management of predominantly hemorrhagic age-related macular degeneration. Clin Ophthalmol. 2010;4(1):67–72. doi:10.2147/opth.s8635
26. Treumer F, Klatt C, Roider J, Hillenkamp J. Subretinal coapplication of recombinant tissue plasminogen activator and bevacizumab for neovascular age-related macular degeneration with submacular haemorrhage. Br J Ophthalmol. 2010;94(1):48–53. doi:10.1136/bjo.2009.164707
27. Treumer F, Roider J, Hillenkamp J. Long-term outcome of subretinal coapplication of rtPA and bevacizumab followed by repeated intravitreal anti-VEGF injections for neovascular AMD with submacular haemorrhage. Br J Ophthalmol. 2012;96(5):708–713. doi:10.1136/bjophthalmol-2011-300655
28. Ron Y, Ehrlich R, Axer-Siegel R, Rosenblatt I, Weinberger D. Pneumatic displacement of submacular hemorrhage due to age-related macular degeneration. Ophthalmologica. 2006;221(1):57–61. doi:10.1159/000096524
29. Gopalakrishan M, Giridhar A, Bhat S, Saikumar SJ, Elias A. Pneumatic displacement of submacular hemorrhage: safety, efficacy, and patient selection. Retina. 2007;27(3):329–334. doi:10.1097/01.iae.0000231544.43093.40
30. Fujikawa M, Sawada O, Miyake T, et al. Comparison of pneumatic displacement for submacular hemorrhages with gas alone and gas plus tissue plasminogen activator. Retina. 2013;33(9):1908–1914. doi:10.1097/IAE.0b013e318287d99d
31. Georgalas I, Papaconstantinou D, Karagiannis D, Ladas I. Pneumatic displacement of acute submacular hemorrhage with and without the use of tPA. Eur J Ophthalmol. 2011;21(2):220. doi:10.5301/ejo.2010.5685
32. Mizutani T, Yasukawa T, Ito Y, et al. Pneumatic displacement of submacular hemorrhage with or without tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1153–1157. doi:10.1007/s00417-011-1649-1
33. Hillenkamp J, Surguch V, Framme C, Gabel VP, Sachs HG. Management of submacular hemorrhage with intravitreal versus subretinal injection of recombinant tissue plasminogen activator. Graefes Arch Clin Exp Ophthalmol. 2010;248(1):5–11. doi:10.1007/s00417-009-1158-7
34. Kishikova L, Saad AAA, Vaideanu-Collins D, Isac M, Hamada D, El-Haig WM. Comparison between different techniques for treatment of submacular haemorrhage due to age-related macular degeneration. Eur J Ophthalmol. 2020;112067212095955. doi:10.1177/1120672120959551
35. Avcı R, Yıldız AM, Çınar E, et al. Subretinal coapplication of tissue plasminogen activator and bevacizumab with concurrent pneumatic displacement for submacular hemorrhages secondary to neovascular age-related macular degeneration. Turk J Ophthalmol. 2021;51(1):38–44. doi:10.4274/tjo.galenos.2020.72540
36. Maggio E, Deiro AP, Mete M, et al. Intravitreal recombinant tissue plasminogen activator and sulphur hexafluoride gas for submacular haemorrhage displacement in age-related macular degeneration: looking behind the blood. Res Artic Ophthalmol. 2020;243:224–235. doi:10.1159/000505752
37. Meyer CH, Scholl HP, Eter N, Helb HM, Holz FG. Combined treatment of acute subretinal haemorrhages with intravitreal recombined tissue plasminogen activator, expansile gas and bevacizumab: a retrospective pilot study. Acta Ophthalmol. 2008;86(5):490–494. doi:10.1111/j.1600-0420.2007.01125.x
38. Sacu S, Stifter E, Vécsei-Marlovits PV, et al. Management of extensive subfoveal haemorrhage secondary to neovascular age-related macular degeneration. Eye. 2009;23(6):1404–1410. doi:10.1038/eye.2008.267
39. Guthoff R, Guthoff T, Meigen T, Goebel W. Intravitreous injection of bevacizumab, tissue plasminogen activator, and gas in the treatment of submacular hemorrhage in age-related macular degeneration. Retina. 2011;31(1):36–40. doi:10.1097/IAE.0b013e3181e37884
40. Mayer WJ, Hakim I, Haritoglou C, et al. Efficacy and safety of recombinant tissue plasminogen activator and gas versus bevacizumab and gas for subretinal haemorrhage. Acta Ophthalmol. 2013;91(3):274–278. doi:10.1111/j.1755-3768.2011.02264.x
41. Edington M, Connolly J, Chong NV. Pharmacokinetics of intravitreal anti-VEGF drugs in vitrectomized versus non-vitrectomized eyes. Expert Opin Drug Metab Toxicol. 2017;13(12):1217–1224. doi:10.1080/17425255.2017.1404987
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.