Back to Journals » International Journal of Nanomedicine » Volume 12
Stromelysin-2 (MMP-10) facilitates clearance and moderates inflammation and cell death following lung exposure to long multiwalled carbon nanotubes
Authors Vandivort TC, Birkland TP, Domiciano TP, Mitra S, Kavanagh TJ, Parks WC
Received 29 September 2016
Accepted for publication 17 November 2016
Published 7 February 2017 Volume 2017:12 Pages 1019—1031
DOI https://doi.org/10.2147/IJN.S123484
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Tyler C Vandivort,1,2 Timothy P Birkland,1 Talita P Domiciano,3 Somenath Mitra,4 Terrance J Kavanagh,2 William C Parks1
1Cedars-Sinai Medical Center, Women’s Guild Lung Institute, Los Angeles, CA, 2Department of Environmental and Occupational Health Sciences, University of Washington, Seattle, WA, 3Department of Pediatrics, Cedars-Sinai Medical Center, Los Angeles, CA, 4Department of Chemistry and Environmental Science, New Jersey Institute of Technology, Newark, NJ, USA
Abstract: Multiwalled carbon nanotubes (MWCNTs) are nanomaterials composed of multiple layers of graphene cylinders with unique properties that make them valuable for a number of industries. However, rising global production has led to concerns regarding potential occupational exposures to them as raw materials during handling. This is especially true for long MWCNT fibers, whose aspect ratio has been posited to initiate pathology similar to that of asbestos. Matrix metalloproteinases (MMPs) are a class of extracellular endopeptidases that control various processes related to tissue repair, inflammation, and more. Stromelysin-2 (MMP-10) has roles in modulating macrophage activation and function, and hence, we used an MMP-10 null (Mmp10-/-) mouse model to assess its role in controlling lung responses to inhaled long MWCNTs. Oropharyngeal aspiration of long MWCNTs (80 µg/mouse) by wild-type mice induced expression of Mmp10 mRNA, which was accompanied by a robust inflammatory response characterized by elevated expression of Tnfa, Il6, and Il1b. In Mmp10-/- mice, we found that absence of MMP-10 led to impaired pulmonary clearance of MWCNTs and reduced macrophage cell survival. Exposure of wild-type bone marrow-derived macrophages (BMDMs) and alveolar macrophages to MWCNTs caused a rapid, dose-dependent upregulation of Mmp10 mRNA expression, which was accompanied by expression of pro-inflammatory products (Il6 and Il1b). These products were further enhanced in Mmp10-/- macrophages, resulting in increased caspase-3-dependent cell death compared with wild-type cells. These findings indicate that MMP-10 facilitates the clearance of MWCNTs and moderates the pro-inflammatory response of exposed alveolar and infiltrated macrophages.
Keywords: MMP-10, multiwalled carbon nanotubes, lung injury, macrophages, apoptosis
Introduction
Engineered carbon nanotubes (CNTs) are a class of graphene cylinders of 1 or more layers with at least 1 external dimension between 1 and 100 nm.1 These characteristics impart unique electrical, mechanical, and thermal properties that make CNTs exceedingly valuable for a range of biomedical and commercial applications, including solar cells, adhesives, polymer composites, lithium-ion batteries, drug delivery, and medical diagnostics.2 As a result, CNT production has increased about 10-fold since 20063 to a global output of >2,000 tons in 2013.4
Concomitant with the increase in production of CNTs has been a growing urgency to understand the risks posed by occupational exposure to these nanoparticles.2 Of the 2 major subtypes of CNTs (ie, single-walled and multiwalled), multiwalled CNTs (MWCNTs) represent >99% of global production volume.5 In addition to the marked production disparity between CNT subtypes, evidence suggests that the toxicity of MWCNTs is greater than that of single-walled CNTs.6 However, other factors also augment CNT toxicity, including the presence of residual catalyst contamination from synthesis (eg, Ni, Fe, others),7 surface modifications,8–10 and length.11 Among these other factors, length is particularly important as longer MWCNTs have been suggested to contribute to inflammation and fibrosis in a manner similar to asbestos fibers.12 Lung macrophages are important targets for MWCNTs.11,13,14 Thus, understanding how these cells respond to exposure is important for understanding the mechanisms underlying MWCNT-induced lung disease.
Matrix metalloproteinases (MMPs) comprise a family of 27 distinct, but structurally related, extracellular endopeptidases. Since the first MMP was isolated from regressing tadpole tails, they have oft been assumed to function in the breakdown of extracellular matrix (ECM).15 However, while some MMPs do serve defined or limited roles in ECM turnover, this is far from being the biggest role or predominant function of these enzymes. For example, in vivo findings with genetically engineered mice have shown that MMPs are critical regulators of innate immunity by controlling leukocyte influx and activation, restoration of tissue barriers, and cleavage of latent cytokines, chemokines, and antimicrobial agents, among many other processes.16,17 Like several MMPs, MMP-10 is not expressed in unchallenged tissues, but in response to a variety of insults, including injury and infection, it is induced by macrophages and epithelial cells in many organs, including the lung.18–21 Studies with Mmp10−/− mice indicate that MMP-10 functions to control the activation status of macrophages.18,22,23
In this study, we report a protective role for macrophage MMP-10 in the acute pulmonary response to MWCNTs. We found that following aspiration of long MWCNTs into the lung, expression of MMP-10 was induced, and promoted clearance of these nanoparticles from the lung, and mononuclear cell survival, and moderated inflammation and airway fibrosis. Using bone marrow-derived macrophages (BMDMs) and alveolar macrophages, we demonstrate that macrophages respond to MWCNT exposure by initiating pro-inflammatory and apoptotic responses that are moderated by MMP-10.
Materials and methods
Animals
Mmp10−/− mice (on a C57BL/6J genetic background)19 and wild-type littermates (male and female, 8–12 weeks old) were used for these studies. All animal-handling procedures were performed according to the Guide for the Care and Use of Laboratory Animals (8th edition) of the National Research Council (National Academy of Sciences, USA) and followed the guidelines of the US Animal Welfare Act. Specific treatments and procedures were approved by the Office of Animal Welfare at the University of Washington and by the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center.
MWCNTs
MWCNTs were purchased from Cheap Tubes, Inc. (Cambridgeport, VT, USA) and provided to the University of Washington’s Nanotoxicology Center through participation in the NIEHS Centers for Nanotechnology Health Implications Research (NCNHIR) Consortium. Tube preparation for both in vivo and in vitro work consisted of suspension in dispersion medium (DM) that consisted of 0.6 mg/mL mouse serum albumin, 10 μg/mL 1,2-dipalmitoyl-sn-glycero-3-phosphocholine, and 0.1% ethanol (v/v) in PBS. Stock aliquots of MWCNTs (1.6 μg/μL) were sonicated for 19 s in a 40 kHz Branson 2510DTH bath sonicator (Branson Ultrasonics Corp., Danbury, CT, USA), and the solution was vortexed for 1 s both during preparation and immediately before treatments. Details of particle preparation and electron microscopy images were recently published.24 The manufacturer’s product data specified an outer diameter of 10–20 nm and a length of 10–30 μm. Scanning electron microscopy with energy dispersive X-ray spectroscopy analysis (SEM-EDS), conducted by the National Cancer Institute Nanotechnology Characterization Laboratory, confirmed an elemental composition of 95.8%±0.6% (SD) carbon and 3.4%±0.2% oxygen and the presence of trace amounts of Ni (0.5%±0.5%) and Fe (0.3%±0.3%). These values are consistent with their composition determined by Pulskamp et al.7
Exposures and tissue harvest
Mice (6/exposure group/genotype) were treated with a 50 μL oropharyngeal aspiration of DM or an equivalent volume containing 80 μg of MWCNTs. Mice were sacrificed 24 h later, and the left lung was occluded, flash frozen, and homogenized in either RIPA or RLT buffer (Qiagen, Valencia, CA, USA) for protein and RNA analyses. Bronchoalveolar lavage (BAL) was collected from the right lung, and total cells were identified and counted via an automated cell counter. The right lung was then inflated with 600 μL of 10% neutral buffered formalin and prepared for histology as described by Braber et al.25 Fixed lungs were paraffin-embedded, sectioned, and stained with H&E by the Cedars-Sinai Medical Center Biobank & Translational Research Core (BTRC).
MWCNT clearance
Using captured images of H&E-stained sections, the BTRC then set a threshold value with DM-alone sections to eliminate artifacts and blindly assessed both the MWCNT aggregate count and the percentage of area occupied (in pixels) in each treated lung section (n=6). These findings were supported by our own counts obtained with microscopy (10×).
Macrophage cell culture
BMDMs and alveolar macrophages were isolated and cultured under sterile techniques as described in detail by McMahan et al23 and Manicone et al.26 Briefly, for BMDMs, marrow from femurs and tibias of wild-type and Mmp10−/− mice was recovered by brief centrifugation. Red blood cells were removed by adding lysis buffer (eBioscience, San Diego CA, USA). The remaining cells were suspended in Mac medium (RPMI 1640 with 10% fetal bovine serum and 20% medium conditioned by L929 cells as a source of CSF-1) and plated at 1.5×106 cells/10 cm plate. Mac medium was replaced on days 3 and 6. Between days 7 and 10, plates were washed with PBS to remove nonadherent or dead cells. The macrophages were then removed from the plate using 5 mM ETDA in PBS, and cell counts and viability analysis were determined. Alveolar macrophages were isolated from BAL by centrifugation, pooled in pairs, and treated with 100 μg/mL of MWCNTs in low serum medium (RPMI 1640 + 2% FBS) for 2 or 24 h. Polymyxin B sulfate salt (10 μg/mL; Sigma-Aldrich, St Louis, MO, USA) was included in experimental treatments to control for potential lipopolysaccharide contamination.
Particle uptake
In vivo estimates of endocytosis were calculated from BAL cytospin slides. MWCNT-positive cells were identified by light microscopy, and percentages were determined from averages of 4 separate counts of 100 cells. The total number of MWCNT-positive cells was back-calculated from total cell estimates. In vitro endocytosis was determined in a similar manner following 3 h of MWCNT exposure. The average area occupied by MWCNT-positive vacuoles per cell was quantified from 30 measurements per animal at ×80 utilizing Digital Image Hub software version 4.0.4 (Leica Microsystems GmbH, Buffalo Grove, IL, USA).
Assays
Caspase-3 activity in lung and cell lysates was assayed using a Caspase-3 Colorimetric Assay Kit (Biovision, Milpitas, CA, USA) according to the manufacturer’s instructions. Myeloperoxidase (MPO) activity was measured in lung lysates and BAL cell pellets using the EnzChek Myeloperoxidase Activity Assay Kit (Life Technologies, Carlsbad, CA, USA). Total RNA was isolated from lung homogenates and cultured macrophages. Total RNA was isolated (Qiagen, Valencia, CA, USA), and specific transcripts were quantified by real-time PCR using TaqMan FAM-labeled probes (Applied Biosystems, Foster City, CA, USA) as described by Rohani et al.27 Total IL-1β levels were measured using the Mouse IL-1β ELISA Ready-SET-Go! Kit (eBioscience).
Statistics
Statistical analyses included either multiple t-test or two-way ANOVA where appropriate using Prism 5 (GraphPad Software, La Jolla, CA, USA). Bonferroni posttest was used to account for multiple comparisons. All data are presented as mean ± standard error of the mean (SEM), except where indicated, and a P-value of <0.05 was considered significant.
Results
MWCNT-induced expression of MMP-10
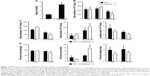
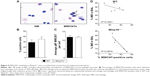
To elucidate the impact of MMP-10 on the pulmonary response to MWCNTs, wild-type (Mmp10+/+) and Mmp10−/− mice were treated with 80 μg of MWCNTs by oropharyngeal aspiration or with an equivalent volume of DM. This dose of MWCNT was based on exposures used on other studies.28–30 Lungs were collected 24 h later and processed for mRNA isolation. In lungs from unchallenged wild-type mice (ie, no MWCNTs, no DM alone), we detected a Ct range for Mmp10 mRNA between 36 and 37 indicating essentially no or very low levels of expression. Expression of Mmp10 was modestly induced by administration of DM alone, indicating that the treatment procedure caused a mild lung injury, which is common among treatment methods that involve direct instillation into the lung. However, when exposed to MWCNTs, expression of Mmp10 mRNA was stimulated >3-fold above DM-control levels (Figure 1A). As expected, Mmp10 mRNA was not detected in Mmp10−/− samples (data not shown).
Impact on inflammatory cells
Total cells in BAL did not differ between unchallenged wild-type and Mmp10−/− mice (Figure 1B) and nearly all of these (>90%) were macrophages (Figure 1C). In response to DM, total cells in BAL increased modestly, but still did not differ significantly between genotypes (Figure 1B). However, when treated with MWCNTs, the total cell count was reduced relative to the controls and was significantly lower in Mmp10−/− samples (Figure 1B). Differential cell counts revealed no significant differences in the percentage or total numbers of lymphocytes between genotypes and treatments. In contrast, we found a reduced percentage and number of macrophages, as well as a higher percentage of neutrophils in MWCNT-treated Mmp10−/− BAL compared to wild-type BAL (Figure 1C and D). However, though elevated by MWCNTs, the total numbers of neutrophils did not differ significantly between wild-type and Mmp10−/− mice (Figure 1C). Similarly, we assayed expression levels of Cxcl1 (Figure S1A), which encodes CXCL1/KC, the murine ortholog of human CXCL8/IL-8 and a critical acute-phase neutrophil chemokine.31 Consistent with our neutrophil estimates (Figure 1C and D), we found that MWCNT treatment stimulated increased Cxcl1 expression and that this increase showed only a mild trend toward being greater in Mmp10−/− mice.
We also assessed if reduced chemokine expression contributed to lowered macrophage numbers in Mmp10−/− mice. As with Cxcl1, Ccl2 levels increased significantly with MWCNT treatment, but did not differ between wild-type and Mmp10−/− mice (Figure S1B). These findings suggest that the reduced macrophage numbers in MWCNT-treated Mmp10−/− mice were due to a loss of cells rather than impaired recruitment. Overall, these findings indicate that MWCNTs mediated a pro-inflammatory response that was manifested by both an increase in neutrophil numbers and a reduction in macrophages. While both of these observations were exaggerated in Mmp10−/− mice, only the reduction of macrophages was statistically significant.
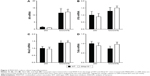
MWCNTs induce production of pro-inflammatory factors
Many groups have demonstrated that MWCNTs induce a robust pro-inflammatory response following instillation into the lung, including Il1b, Il6, Tnfa,32 and Nos2 mRNAs.33 Therefore, we assessed each of these mRNAs in the lungs of wild-type and Mmp10−/− mice (Figure 2). We found that the mRNA levels for Il6 and Nos2 were increased in MWCNT-treated mice and that their levels did not differ between wild-type and Mmp10−/− mice. Though expression of Il1b trended toward an increase in total lung RNA, the increase did not reach significance. Tnfa and mRNA levels were not affected. Consistent with these observations, we did not observe differences in pulmonary hemorrhage or total BAL protein, both markers of acute lung injury, between genotypes (Figure S2).
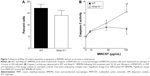
Given that the most prominent response in the cell counts was a reduction in macrophage numbers, we hypothesized that total lung transcripts might be masking an underlying pro-inflammatory phenotype in macrophages. Therefore, we used BMDMs from wild-type and Mmp10−/− mice to assess if the observed in vivo responses to MWCNTs could be attributed to macrophages. In all in vitro experiments, we added 10 μg/mL of PmB to control for possible endotoxin contamination. Mirroring what we observed in vivo, treatment of BMDMs for 2 h with 10–100 μg/mL of MWCNTs resulted in a statistically significant increase in Mmp10 mRNA vs DM control (Figure 3A). In addition, we found that MWCNT stimulated expression of mRNAs for Il1β, Il6, Il12a, and Tnfa in wild-type cells and that these values were elevated further in Mmp10−/− BMDMs (Figure 3B). Release of IL-1β protein from wild-type macrophages was also induced by MWCNTs – but not by DM – and was significantly increased from Mmp10−/− macrophages (Figure 3C).
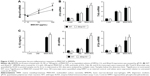
MMP-10 facilitates clearance of MWCNTs
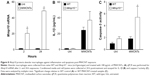
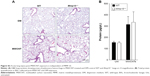
As macrophages are critical for clearing inhaled particles, we assessed if the reduced numbers of macrophages in Mmp10−/− mice impacted retention of MWCNTs. Indeed, in lung homogenates we observed more MWCNT particles in Mmp10−/− lysate pellets compared to wild-type samples, and this difference between genotypes remained evident at 28 d postexposure (Figure 4A). Other groups have found that inhaled MWCNTs persist in the lung for up to 1 year postexposure.34,35 In addition, in sections of Mmp10−/− lung we observed an accumulation of particles in alveolar macrophages (Figure 4B). By both internal counts (Figure 4A) and blinded morphometric analysis (Figure 4C and D), we measured significantly more (about 2.5-fold) retained particles (Figure 4D) and a greater percentage of total tissue occupied by MWCNT (Figure 4E) in Mmp10−/− lungs compared to wild-type tissue. No particle signal was visible (Figure 4B) or detected in the DM-control lungs (data not shown).
Despite an overall increase in retained particles in lungs, we did not find a difference in either the percentage of MWCNT-containing macrophages (Figure 5A and B) or average MWCNT-occupied area in endocytic vacuoles between wild-type and Mmp10−/− samples (Figure 5C). However, in both genotypes we found an inverse relationship between macrophage numbers and the percentage of MWCNT-positive macrophages, but this negative correlation was more robust in Mmp10−/− samples (Figure 5D). These data suggest that MMP-10 is involved in mediating both protection against macrophage cell death and particle clearance from the lung post-MWCNT exposure.
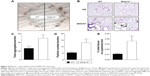
MMP-10 protects against MWCNT-mediated apoptosis in macrophages
As the number of resident macrophages did not differ between unchallenged wild-type and Mmp10−/− mice (Figure 1C and D), we assessed if the reduction of macrophages observed in Mmp10−/− mice was due to MWCNT-induced apoptosis of these cells. Caspase-3 activity, which is an established end point of MWCNT-induced cell death,13,36 in lung lysates was not affected by MWCNT exposure and did not differ between wild-type and Mmp10−/− mice (Figure 6A). However, we saw about a 2-fold increase in caspase-3 activity in BAL from MWCNT-treated Mmp10−/− mice compared to the levels measured in wild-type samples (Figure 6B). We assessed if macrophages were responsible for the robust increase in caspase-3 activity in the cellular fraction of Mmp10−/− BAL. In agreement with our in vivo findings, we found that while the number of MWCNT-positive macrophages did not differ between wild-type and Mmp10−/− mice (Figure 7A), MWCNTs mediated a significantly greater increase in caspase-3 activity in Mmp10−/− BMDM (Figure 7B). These data suggest that MMP-10 protects macrophages from MWCNT-induced apoptosis.
Because macrophages that influx into the lung – or any tissue – are distinct from the resident population,37–39 we assessed if wild-type and Mmp10−/− alveolar macrophages responded similar to MWCNT exposure as did BMDM, which model the recruited cell population. As we saw with BMDM (Figure 3A), expression of Mmp10 was significantly upregulated in wild-type alveolar macrophages exposed to MWCNT (Figure 8A). In addition, we saw a marked release of IL-1β from both wild-type and Mmp10−/− alveolar macrophages (Figure 8B), which trended (P=0.161) to be greater from Mmp10−/− cells, similar to the responses we saw with BMDM (Figure 3C). Furthermore, we found that whereas MWCNTs did not affect caspase-3 activity in wild-type alveolar macrophages, they did mediate nearly a 3-fold increase in Mmp10−/− cells (Figure 8C). These data support the conclusion that both resident alveolar macrophages and infiltrating macrophages respond similarly to MWCNT and that Mmp-10 has a protective role in both populations by moderating MWCNT-induced macrophage apoptosis.
Discussion
Despite their growing usage, much uncertainty still exists regarding the risks associated with respiratory CNT exposure. This is particularly true of occupational exposures during transfer, weighing, and blending,2 but the stability of these particles suggests that environmental CNT exposures will also become more important as the CNT market continues its transition from R&D to high-volume production.40–42 In this study, we report that MMP-10 serves a beneficial function during the acute-phase response to MWCNT by promoting clearance and moderating the pro-inflammatory, fibrotic, and apoptotic effects of MWCNT in both resident alveolar macrophages and BMDM.
Although the dosages utilized here are greater than what might be expected to occur in an occupational setting,43 they are consistent with other high-dose studies meant to uncover the mechanism(s) of MWCNT toxicity.28,44,45 While the majority of in vivo studies have focused on the long-term impact of exposure, less attention has been paid to factors that impact acute innate immune responses. This is particularly important point as macrophage apoptosis and inflammation are associated with disease in various lung models,46,47 including CNTs.48
Consistent with other studies, we found that oropharyngeal aspiration of 80 μg/mouse of MWCNTs initiated a robust damage response.30,44,49 Compared to DM controls, MWCNT-treated mice exhibited enhanced pulmonary hemorrhage, edema, neutrophilia, and macrophage cell death, as well as accumulations of MWCNTs in BAL macrophages at 24 h postexposure. In addition, mRNA analyses revealed an upregulation of multiple pro-inflammatory transcripts including Il6, Nos2, and notably, Il1β, which is important in the initiation of MWCNT-induced pathology.50 Interestingly, Mmp10 mRNA was also found to be present in this milieu.
Further analyses revealed that absence of Mmp10 in vivo was associated with enhanced mononuclear cell death and a 2- to 3-fold impairment in pulmonary clearance of MWCNTs. Given the role of endocytosis in mediating pulmonary clearance from the distal lung,51 we investigated if macrophage phagocytosis could explain the difference in clearance between wild-type and Mmp10−/− mice. However, both in vivo and cell culture analysis indicated no difference in the per-cell phagocytosis of MWCNTs between genotypes. One possible explanation for the difference in clearance between genotypes is that the increase in apoptosis of Mmp10−/− macrophages leads to fewer functional macrophages and, in turn, reduced clearance of inhaled nanoparticles. However, our data with BMDMs do not fully support this idea. As macrophage-mediated clearance of particles is thought to be secondary to mucociliary clearance,51 MMP-10 may be influencing this mucosal function. Future studies will explore the mechanistic basis for MMP-10-dependent particle clearance from lung.
Our in vivo data indicate that MMP-10 provides a protective response to macrophages by reducing their sensitivity to MWCNT-induced inflammation and cell death. In other words, while the number of positive mononuclear cells in BAL did not differ between wild-type and Mmp10−/− mice, macrophage loss was more strongly associated with the presence of MWCNT in Mmp10−/− mice than in wild types. These in vivo findings were confirmed in vitro with both BMDMs and alveolar macrophages. These experiments demonstrated that Mmp10−/− macrophages expressed higher levels of Il6 and Il1b transcripts, as well as IL-1β protein in response to MWCNTs, and that this expression was associated with an enhanced, dose-dependent death of these cells in culture. With respect to mechanism, we propose that MMP10 sheds a protein on the surface of macrophages and that this proteolytic processing mediates outside-in signaling that affects the activation state of these cells and expression of inflammatory mediators. Because we observed overlapping responses in macrophages both in vivo and in cell culture,18,23 it is likely that MMP10 controls macrophage activation via a cell autonomous mechanism. Although we do not yet know the critical substrate, identifying this macrophage protein is a major goal of our group.
Conclusion
Macrophages are critical responders to MWCNT exposure, a finding that is consistent with other in vivo30,52 and in vitro studies.8 In addition, our group and others have shown that Mmp10 is a critical determinant of macrophage responses and differentiation status in models of acute colon injury,22 skin wounds,18 and lung infection with Pseudomonas aeruginosa.23 Notably, in both the skin wound and lung infection models, adoptive transfer of wild-type macrophages into Mmp10−/− mice was sufficient to rescue the principal phenotypes (excess scar in skin wounds; morbidity in lung infection) observed in Mmp10−/− animals. While the exact mechanism(s) underlying this response is unknown (ie, the substrate MMP-10 acts on to control macrophage function), these studies all concluded that Mmp-10 is an important modifier of macrophage activation in injured tissues. In the present study, we extend these observations by reporting a role for macrophage Mmp-10 in the acute response to MWCNT exposure. In addition, our findings raise questions about the long-term impact of MMP-10 in affecting responses to MWCNT exposure.
Acknowledgments
The authors wish to thank Arkadiusz Gertych and Steven Swartwood, Cedars-Sinai Biobank and Translational Research Core, for their assistance with image analysis and tissue sectioning, Megan Cartwright, University of Washington, for assistance in particle preparation, the NIEHS Centers for Nanotechnology Health Implications Research (NCNHIR) Consortia for the MWCNTs, and the NCI Nanotechnology Characterization Laboratory for SEM-EDS elemental analysis of these nanoparticles. This work was supported by NIH grants ES019545, ES007033, ES023209, HL089455, HL098067, and HL128995. The abstract of this paper was presented at the 2016 Society of Toxicology meeting as a poster presentation containing interim findings. The poster’s abstract (no 3565) was published in “The Toxicologist: Lake Breaking Supplement” that is posted on the society’s website at: https://www.toxicology.org/events/am/AM2016/docs/2016_LB_Supplement.pdf.
Disclosure
The authors report no conflicts of interest in this work.
References
FDA. Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Silver Spring, MD: FDA; 2014. | ||
NIOSH. Current Intelligence Bulletin 65. Occupational Exposure to Carbon Nanotubes and Nanofibers. Department of Health and Human Services, editor. Atlanta, GA: NIOSH; 2013:1–184. | ||
De Volder MF, Tawfick SH, Baughman RH, Hart AJ. Carbon nanotubes: present and future commercial applications. Science. 2013;339(6119):535–539. | ||
Manke A, Luanpitpong S, Rojanasakul Y. Potential occupational risks associated with pulmonary toxicity of carbon nanotubes. Occup Med Health Aff. 2014;2. | ||
Nanotech. Nanotubes hanging in there. Nanotech Magazine. 2013:4–5. | ||
Jackson P, Jacobsen NR, Baun A, et al. Bioaccumulation and ecotoxicity of carbon nanotubes. Chem Cent J. 2013;7(1):154. | ||
Pulskamp K, Diabate S, Krug HF. Carbon nanotubes show no sign of acute toxicity but induce intracellular reactive oxygen species in dependence on contaminants. Toxicol Lett. 2007;168(1):58–74. | ||
Jiang Y, Zhang H, Wang Y, et al. Modulation of apoptotic pathways of macrophages by surface-functionalized multi-walled carbon nanotubes. PLoS One. 2013;8(6):e65756. | ||
Liu Z, Liu Y, Peng D. Hydroxylation of multi-walled carbon nanotubes reduces their cytotoxicity by limiting the activation of mitochondrial mediated apoptotic pathway. J Mater Sci Mater Med. 2014;25(4):1033–1044. | ||
Hamilton RF Jr, Wu Z, Mitra S, Shaw PK, Holian A. Effect of MWCNT size, carboxylation, and purification on in vitro and in vivo toxicity, inflammation and lung pathology. Part Fibre Toxicol. 2013;10(1):57. | ||
Chen T, Nie H, Gao X, et al. Epithelial-mesenchymal transition involved in pulmonary fibrosis induced by multi-walled carbon nanotubes via TGF-beta/Smad signaling pathway. Toxicol Lett. 2014;226(2):150–162. | ||
Donaldson K, Poland CA, Murphy FA, MacFarlane M, Chernova T, Schinwald A. Pulmonary toxicity of carbon nanotubes and asbestos – similarities and differences. Adv Drug Deliv Rev. 2013;65(15):2078–2086. | ||
van Berlo D, Wilhelmi V, Boots AW, et al. Apoptotic, inflammatory, and fibrogenic effects of two different types of multi-walled carbon nanotubes in mouse lung. Arch Toxicol. 2014;88(9):1725–1737. | ||
Hussain S, Sangtian S, Anderson SM, et al. Inflammasome activation in airway epithelial cells after multi-walled carbon nanotube exposure mediates a profibrotic response in lung fibroblasts. Part Fibre Toxicol. 2014;11:28. | ||
Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962;48:1014–1022. | ||
Chen P, Parks WC. Role of matrix metalloproteinases in epithelial migration. J Cell Biochem. 2009;108(6):1233–1243. | ||
Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4(8):617–629. | ||
Rohani MG, McMahan RS, Razumova MV, et al. MMP-10 regulates collagenolytic activity of alternatively activated resident macrophages. J Invest Dermatol. 2015;135(10):2377–2384. | ||
Kassim SY, Gharib SA, Mecham BH, Birkland TP, Parks WC, McGuire JK. Individual matrix metalloproteinases control distinct transcriptional responses in airway epithelial cells infected with Pseudomonas aeruginosa. Infect Immun. 2007;75(12):5640–5650. | ||
Konishi K, Gibson KF, Lindell KO, et al. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180(2):167–175. | ||
Murray MY, Birkland TP, Howe JD, et al. Macrophage migration and invasion is regulated by MMP10 expression. PLoS One. 2013;8(5):e63555. | ||
Koller FL, Dozier EA, Nam KT, et al. Lack of MMP10 exacerbates experimental colitis and promotes development of inflammation-associated colonic dysplasia. Lab Invest. 2012;92(12):1749–1759. | ||
McMahan RS, Birkland TP, Smigiel KS, et al. Stromelysin-2 (MMP10) moderates inflammation by controlling macrophage activation. J Immunol. 2016;197:899–909. | ||
Cartwright M, Schmuck S, Corredor C, et al. The pulmonary inflammatory response to multiwalled carbon nanotubes is influenced by gender and glutathione synthesis. Redox Biol. 2016;9:264–275. | ||
Braber S, Verheijden KA, Henricks PA, Kraneveld AD, Folkerts G. A comparison of fixation methods on lung morphology in a murine model of emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299(6):L843–L851. | ||
Manicone AM, Birkland TP, Lin M, et al. Epilysin (MMP-28) restrains early macrophage recruitment in Pseudomonas aeruginosa pneumonia. J Immunol. 2009;182(6):3866–3876. | ||
Rohani MG, Chow YH, Razumova MV, Ash S, Hung CF, Schnapp LM. uPARAP function in cutaneous wound repair. PLoS One. 2014;9(3):e92660. | ||
Mercer RR, Hubbs AF, Scabilloni JF, et al. Pulmonary fibrotic response to aspiration of multi-walled carbon nanotubes. Part Fibre Toxicol. 2011;8:21. | ||
Snyder-Talkington B, Dymacek J, Porter D, et al. System-based identification of toxicity pathways associated with multi-walled carbon nanotube-induced pathological responses. Toxicol Appl Pharmacol. 2013;272:476–489. | ||
Taylor AJ, McClure CD, Shipkowski KA, et al. Atomic layer deposition coating of carbon nanotubes with aluminum oxide alters pro-fibrogenic cytokine expression by human mononuclear phagocytes in vitro and reduces lung fibrosis in mice in vivo. PLoS One. 2014;9(9):e106870. | ||
Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17(3–4):293–307. | ||
Dong J, Porter DW, Batteli LA, Wolfarth MG, Richardson DL, Ma Q. Pathologic and molecular profiling of rapid-onset fibrosis and inflammation induced by multi-walled carbon nanotubes. Arch Toxicol. 2015;89(4):621–633. | ||
Pacurari M, Qian Y, Porter DW, et al. Multi-walled carbon nanotube-induced gene expression in the mouse lung: association with lung pathology. Toxicol Appl Pharmacol. 2011;255(1):18–31. | ||
Elgrabli D, Floriani M, Abella-Gallart S, et al. Biodistribution and clearance of instilled carbon nanotubes in rat lung. Part Fibre Toxicol. 2008;5:20. | ||
Mercer RR, Hubbs AF, Scabilloni JF, et al. Distribution and persistence of pleural penetrations by multi-walled carbon nanotubes. Part Fibre Toxicol. 2010;7:28. | ||
Alarifi S, Ali D. Mechanisms of multi-walled carbon nanotubes-induced oxidative stress and genotoxicity in mouse fibroblast cells. Int J Toxicol. 2015;34:258–265. | ||
Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70(2):163–170. | ||
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. | ||
Vermaelen K, Pauwels R. Accurate and simple discrimination of mouse pulmonary dendritic cell and macrophage populations by flow cytometry: methodology and new insights. Cytometry A. 2004;61(2):170–177. | ||
Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Crit Rev Toxicol. 2006;36(3):189–217. | ||
Invernizzi N. Nanotechnology between the lab and the shop floor: what are the effects on labor? J Nanopart Res. 2011;13(6):2249–2268. | ||
Yang ST, Luo J, Zhou Q, Wang H. Pharmacokinetics, metabolism and toxicity of carbon nanotubes for biomedical purposes. Theranostics. 2012;2(3):271–282. | ||
Erdely A, Dahm M, Chen BT, et al. Carbon nanotube dosimetry: from workplace exposure assessment to inhalation toxicology. Part Fibre Toxicol. 2013;10(1):53. | ||
Porter DW, Hubbs AF, Mercer RR, et al. Mouse pulmonary dose- and time course-responses induced by exposure to multi-walled carbon nanotubes. Toxicology. 2010;269(2–3):136–147. | ||
Chen B, Liu Y, Song WM, Hayashi Y, Ding XC, Li WH. In vitro evaluation of cytotoxicity and oxidative stress induced by multiwalled carbon nanotubes in murine RAW 264.7 macrophages and human A549 lung cells. Biomed Environ Sci. 2011;24(6):593–601. | ||
Wang L, Scabilloni JF, Antonini JM, Rojanasakul Y, Castranova V, Mercer RR. Induction of secondary apoptosis, inflammation, and lung fibrosis after intratracheal instillation of apoptotic cells in rats. Am J Physiol Lung Cell Mol Physiol. 2006;290(4):L695–L702. | ||
Yao SQ, Rojanasakul LW, Chen ZY, et al. Fas/FasL pathway-mediated alveolar macrophage apoptosis involved in human silicosis. Apoptosis. 2011;16(12):1195–1204. | ||
Andon FT, Fadeel B. Programmed cell death: molecular mechanisms and implications for safety assessment of nanomaterials. Acc Chem Res. 2013;46(3):733–742. | ||
Han SG, Andrews R, Gairola CG. Acute pulmonary response of mice to multi-wall carbon nanotubes. Inhal Toxicol. 2010;22(4):340–347. | ||
Sun B, Wang X, Ji Z, et al. NADPH oxidase-dependent NLRP3 inflammasome activation and its important role in lung fibrosis by multiwalled carbon nanotubes. Small. 2015;11(17):2087–2097. | ||
Stuart BO. Deposition and clearance of inhaled particles. Environ Health Perspect. 1984;55:369–390. | ||
Sweeney S, Grandolfo D, Ruenraroengsak P, Tetley TD. Functional consequences for primary human alveolar macrophages following treatment with long, but not short, multiwalled carbon nanotubes. Int J Nanomedicine. 2015;10:3115–3129. |
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.