Back to Journals » International Medical Case Reports Journal » Volume 15
Stress as Trigger Factor of HSV-1 Reactivation Causing Recurrent Intraoral Herpes Mimicking HAEM: A Case Report
Authors Hasanah NT , Hidayat W
Received 11 October 2022
Accepted for publication 9 November 2022
Published 2 December 2022 Volume 2022:15 Pages 699—706
DOI https://doi.org/10.2147/IMCRJ.S388708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Novia Tri Hasanah,1 Wahyu Hidayat2
1Oral Medicine Residency Program, Department of Oral Medicine, Universitas Padjadjaran, Bandung, Indonesia; 2Department of Oral Medicine, Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Novia Tri Hasanah, Email [email protected]
Abstract: This case highlights the role of stress as trigger factor of HSV-1 reactivation causing recurrent intraoral herpes mimicking herpes-associated erythema multiforme (HAEM). A 24-year-old female came with chief complaints of pain in the oral cavity followed by painful swallowing and fever. She admitted under stress due to family problems and also had insomnia for the last three years. Extra-oral examination revealed serosanguineous crusts on lips that were painful and easily bleed. Intra-oral examination showed the white-yellowish multiple and coalescent ulcers, irregular, and painful on the left and right buccal mucosa, upper and lower labial mucosa, dorsum of the tongue, the floor of the mouth, and oropharynx. The results of the Depression Anxiety and Stress Scale (DASS)-21 examination were moderate depression, extremely severe anxiety, and moderate stress. The results of the HSV-1 IgG examination before and after therapy were positive with titer > 200 U/mL. Pharmacological therapy included acyclovir 200 mg tablets, multivitamin, benzydamine HCl lozenges, 0.025% hyaluronic acid mouthwash, 0.9% NaCl, and 100% petroleum jelly. Non-pharmacological therapy included stress management such as self-encouragement and referral to consult with a professional. This therapy generated significant result. In conclusion, stress affects many systems in the body, including the oral cavity. Stress is one of the trigger factors for HSV-1 reactivation which can cause oral manifestation. Early detection of trigger factors is important for better treatment result.
Keywords: stress, herpes infection, immunoglobulin, reactivation, case report
Introduction
Herpes simplex virus 1 (HSV-1) is a common contagious neurotropic human pathogen that belongs to Alphaherpesvirinae subfamily of Herpesviridae family which causes oropharyngeal lesions and recurrent oral ulceration.1–3 The Greek word herpein, which means to creep, is where the word herpes originates.2,3 Large double-stranded DNA of herpesvirus are extremely common infections that can infect people for the rest of their lives.4 It is made up of 180 nm-diameter spiky enveloped particles and 100–110 nm-diameter icosahedral capsids, the latter of which contains a big DNA molecule with a 152 kbp genome. The viral envelope interacts with the host cell membrane through the fusion of receptors.5 HSV can enter keratinocytes very fast in 5 minutes at 7°C temperature.1
The pathogenesis of HSV-1 involves two life cycles, the lytic phase (primary or productive) and the latent phase (dormant).2 While the virus is actively replicating during the lytic phase, it can live in the host during the latent phase. Three methods were used to describe the latent phase: viral genome survival, restricted viral gene expression with no viral particle production, and potential reactivation to lytic replication. Alphaherpesviruses have a relatively brief (hours) replicative cycle, grow quickly in culture, and create latency in sensory neurons.6
A fundamental characteristic of herpesvirus is latency.6 Latency is defined as the silence present of a virus in the body with no ability to become a pathogen and replication. When certain triggering factors are present (internal and external triggers), it can occasionally become active.3 This trigger causes chromatin modifications in the viral episome, which encourage the de-repression and full expression of lytic viral genes.4 Latency-associated transcript (LAT) is a transcriptional gene that only exists during the latent phase and plays an important role in HSV reactivation.7 The predisposing factors are fatigue, fever, corticosteroid administration, sexual activity, trauma, stress, ultraviolet radiation, immunosuppression, menstruation, and exposure to heat, cold, or sunlight.8 When an organism fails to react adequately to unusual environmental stimuli or emotional/physical dangers, stress is the result. Unfortunately, these stress factors also continue to rise daily which weaken the immune system.9 This case report aims to report the role of stress as trigger factor of HSV-1 reactivation causing recurrent intraoral herpes that resembles herpes-associated erythema multiforme (HAEM).
Case
A 24-year-old female came to the Dental Hospital of the Faculty of Dentistry Universitas Padjadjaran with chief complaints of canker sores in the oral cavity three days ago, followed by pain in swallowing and fever two days ago. Initially, the canker sores began in the oral cavity, then appeared on the lips. The patient admitted that he had canker sores approximately six months ago caused by trauma and not periodically. She had tried gargling the salt water to relieve the symptoms, but it did not improve. Then, she went to the pharmacy to buy methylprednisolone 4 mg tablets by herself and consumed it two times a day. After taking methylprednisolone, canker sores multiply and got worse. A brief chronology of the patient showed in Figure 1.
 |
Figure 1 A brief chronology of the patient. |
There were no similar lesions on other parts of the body. She had a non-contributory medical history but she had an allergic history to shrimp. She was in a state of stress due to family problems and trouble sleeping (insomnia) for about the past three years. No history of alcohol consumption or smoking, but she had a frequent habit of licking her lips. She also had a history of chickenpox during elementary school.
Extra-oral examination showed no abnormalities in the lymph nodes. There were painful serosanguineous crusts that tend to bleed. Intra-oral examination (Figure 2) revealed irregular multiple ulcers, well-defined margins surrounded by erythema, white-yellowish concave base, varying in size, on the upper and lower labial mucosa, right and left buccal mucosa, tongue, and the mandibular posterior attached gingiva. There was a white-yellowish plaque on the entire surface of the dorsal tongue that can be scraped without leaving an erythematous area. The oral hygiene was poor, followed by halitosis.
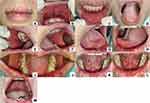 |
Figure 2 (A–M) Clinical features of the initial visit. |
The results of the DASS-21 examination were moderate depression (score 14), extremely severe anxiety (score 27), and moderate stress (score 21). The results of the Oral Health Impact Profile-14 (OHI-P 14) examination were 39 (severe). The laboratory result showed in Table 1. The diagnosis was RIH.
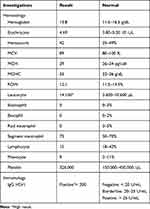 |
Table 1 Laboratory Results |
After three days, the patient complained of bleeding on her lips and could not eat normally (Figure 3). This condition was similar to HAEM. However, after one week of follow-up (Figure 4), the patient showed significant improvement. She was no longer complaining of bleeding on the lips, canker sores, and pain in swallowing, so she could open her mouth wide and eat solid food. The lips dryness caused her still lick it.
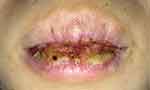 |
Figure 3 Serosanguineous crusts on lips that mimic HAEM. |
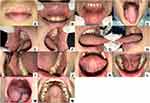 |
Figure 4 (A–N) One week of follow-up. |
After two weeks of follow-up (Figure 5), we performed an anti-HSV-1 IgG examination. The result was positive > 200 U/mL as same as the initial visit. The patient was getting better and no new lesions appeared after one month of follow-up (Figure 6). She still admitted under stress but she did not want to go to a professional. We also performed the serum cortisol examination; however, the result was within normal (12.5 ug/dl).
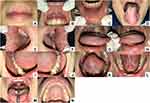 |
Figure 5 (A–N) Two weeks of follow-up. |
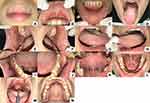 |
Figure 6 (A–N) One month of follow-up. |
Case Management
The non-pharmacological therapy included sleep hygiene, stress releases such as exercise and doing hobbies, and self-motivation or encouragement. We also referred to a professional. Oral hygiene instructions were given such as brushing teeth and tongue two times a day, sipping an amount of water or adequate hydration, and avoiding acidic, spicy, hard, and monosodium glutamate-containing foods. The pharmacological therapy included systemic and topical medications. The systemic medications included acyclovir 200 mg tablets five times a day for two weeks and a multivitamin once a day. The topical medications included three times a day of benzydamine HCl lozenges, 0.025% hyaluronic acid mouthwash, 0.9% NaCl, and 100% petroleum jelly. After one month of therapy, the patient was still instructed to consume a multivitamin once daily.
Discussion
This case report highlights the impact of stress on HSV-1 reactivation of a patient with recurrent intraoral herpes that mimics herpes-associated erythema multiforme. In this case, the patient was admitted under stress due to family problems. This long-term stress made her lack of sleep, change in sleep pattern, and fatigue. Stress is described as a force that causes a somatic reaction outside of normal body processes that can alter the mechanism in the body including the central nervous system, endocrine, and immune system.7,10 Psychological stress also increases HSV-1 titer.11
A long-term stress response is brought on by the glucocorticoid cortisol, which is released by the adrenal cortex.10 Stressors can activate the hypothalamic-pituitary-adrenal (HPA) axis, thereby increasing corticosteroid levels. Corticosteroids can cause cellular immunomodulation by indirectly suppressing the production of critical proinflammatory cytokines, chemokines, and adhesion molecules. Modulation of the cellular immune system potentially affects viral replication. The natural killer is an important defense mechanism against cancer and viral infections which is reduced by psychological stress.7 Patient also consumed methylprednisolone to relieve her symptoms, but the lesions got worse. Glucocorticoids can trigger reactivation not only by suppressing the immune system but also by providing a trigger for reactivation in neurons that harbor latent HSV, possibly by activating the cAMP adrenergic receptor or by stimulating the regulation of HSV origin.7
Epinephrine and cortisol are the main stress hormones expressed in several neurons, namely sensory neurons in the trigeminal ganglia, autonomic neurons in the superior cervical, and ciliary ganglia. Epinephrine (EPI), also known as adrenaline, is a catecholaminergic hormone secreted by the adrenal medulla and regulated by the sympathetic nervous system to induce a short-term stress response. EPI increases DNA replication and viral progeny production. Cortisol binds to glucocorticoid and mineralocorticoid receptors causing metabolic regulation and suppression of the immune system.10
HSV-1 is transmitted by direct contact through skin-to-skin or exposed mucosa, the spread of saliva or touch with active lesions, oral-to-oral, and respiratory infections.2,4,5 It can survive briefly on the skin, clothes, or plastic, making it easier for it to spread through nonsexual contact such as cheek kissing or using the same utensils.8 In contrast to primary HSV-1, lesions are most prevalent in keratinized mucosa such as attached gingiva and hard palate in HSV-1 recurrence.4,12 Since the virus is already present in the ganglia in cases of recurrent lesions, it spreads down the sensory nerves until it reaches the tissues.3
Three days after the first visit, we found the appearance of serosanguineous crusts that tend to bleed so we were suspicious of HAEM. Erythema multiforme (EM) occurrences can be brought on by recurrent HSV infections.13 The diagnosis of EM is highly supported by lip crusting, irregular and diffuse oral ulcers, and targetoid lesions on the skin.14 The patient had a habit of licking her lips. This habit allowed the saliva to wet the lips, so the virus sticked to the lips. This virus is very easily transmitted through body secretions, including saliva. Asymptomatic shedding of HSV in the saliva is considered the major form of transmission.15 This is consistent with the theory that HSV can move from ruptured vesicles to intact mucosa or skin and cause new lesions.16 If the vesicle fluid hits other areas, autoinoculation will occur.1
Investigations to confirm the diagnosis of herpes infection are Tzanck smear, serology, viral culture, and polymerase chain reaction (PCR). This patient underwent a serological IgG anti-HSV-1 examination using the ELISA technique and the result was > 200 U/mL. ELISA, a widely used serological technique, can detect antibodies from patient blood utilizing entire antigens. Compared to Western blot assays, ELISA is simpler to carry out and yields findings more quickly. These tests have a sensitivity range of 92–100% and a specificity range of 61–85% to distinguish between the two HSV types.17 A result that shows a value four times than the normal value indicates an ongoing infection or current infection.18 Antibodies appear in 4–7 days after infection and reach a peak in 2–4 weeks.19 Anti-HSV IgG antibody titers usually increase 1-2 weeks after primary infection, peak in 6-8 weeks after infection.20
Antiviral treatment decreases HSV subclinical shedding, considerably lowering transmission.21 This virus cannot be removed from the body after it has been ingested, but effective treatment can lower the virus load, one of them using analogs of viral nucleosides like acyclovir.4,5 In addition, antiviral therapy aims to reduce the duration and severity of the lesions, as well as reduce the rate of further complications.1 Acyclovir was administered five times daily as causative treatment. Benzydamine HCl and hyaluronic acid were used as symptomatic treatments. Supportive treatment included the administration of multivitamin, advice on eating a high-calorie and protein diet, and recommendation for getting adequate water and sleep. The comprehensive treatment in this patient showed significant improvement and no history of recurrence after six months of treatment. The limitation of this case report is the timing of the serum cortisol examination. Meanwhile, she satisfied with this treatment after the treatment was done. She also gave written informed consent to the publication of this case report’s data and images. The institution has approved to publish this case report.
Conclusion
Stress produces an increase in cortisol levels which suppresses the immune response and plays a role in viral reactivation of latency. It activates glucocorticoid receptors that cooperate with other stress-induced transcription factors and act as part of the transcriptional machinery for latent viral genomes in sensory ganglia. Early detection of stress could be helpful using the DASS-21 examination. Lastly, early identification of predisposing or trigger factors is crucial because it can provide adequate therapy and minimize viral reactivation in the future.
Acknowledgments
We thank the patient for the consent.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bilodeau EA, Lalla RV. Recurrent oral ulceration: etiology, classification, management, and diagnostic algorithm. Periodontol 2000. 2019;80(1):49–60. doi:10.1111/prd.12262.
2. Verzosa AL, McGeever LA, Bhark SJ, et al. Herpes simplex virus 1 infection of neuronal and non-neuronal cells elicits specific innate immune responses and immune evasion mechanisms. Front Immunol. 2021;12:1–17. doi:10.3389/fimmu.2021.644664.
3. Kumar SP, Chandy ML, Shanavas M, et al. Pathogenesis and life cycle of herpes simplex virus infection-stages of primary, latency and recurrence. J Oral Maxillofac Surg Med Pathol. 2016;28(4):350–353. doi:10.1016/j.ajoms.2016.01.006.
4. Atyeo N, Rodriguez MD, Papp B, et al. Clinical manifestations and epigenetic regulation of oral herpesvirus infections. Viruses. 2021;13(4):1–17. doi:10.3390/v13040681.
5. Moin AT, Chowdhury MAB, Riana SH, et al. An updated overview of herpes simplex virus-1 infection: insights from origin to mitigation measures. Electron J Gen Med. 2021;18:1–17 doi:10.29333/ejgm/10869.
6. Weidner-glunde M, Kruminis-kaszkiel E. Herpesviral Latency — Common Themes. Pathogens. 2020;9(2):1–26 doi:10.3390/pathogens9020125.
7. Sainz B, Loutsch JM, Marquart ME, et al. Stress-associated immunomodulation and herpes simplex virus infections. Med Hypotheses. 2001;56(3):348–356. doi:10.1054/mehy.2000.1219.
8. Fatahzadeh M, Schwartz RA. Human herpes simplex virus infections: epidemiology, pathogenesis, symptomatology, diagnosis, and management. J Am Acad Dermatol. 2007;57(5):737–763. doi:10.1016/j.jaad.2007.06.027.
9. Huang W, Xie P, Xu M, et al. The influence of stress factors on the reactivation of latent herpes simplex virus type 1 in infected mice. Cell Biochem Biophys. 2011;61(1):115–122. doi:10.1007/s12013-011-9167-7.
10. Goswami P, Ives AM, Abbott ARN, et al. Stress hormones epinephrine and corticosterone selectively reactivate HSV-1 and HSV-2 in sympathetic and sensory neurons. Viruses. 2022;14(5):21. doi:10.3390/v14051115.
11. Ives AM, Bertke AS. Stress hormones epinephrine and corticosterone selectively modulate Herpes Simplex Virus 1 (HSV-1) and HSV-2 productive infections in adult sympathetic, but not sensory, neurons. J Virol. 2017;91:e00582–17 doi:10.1128/JVI.00582-17.
12. Glick M, Greenberg MS, Lockhart PB, et al. Burket’s Oral Medicine.
13. Santosh ABR, Muddana K. Viral infections of oral cavity. J Fam Med Prim Care. 2020;9(1):36–42. doi:10.4103/jfmpc.jfmpc_807_19.
14. Farah CS, Balasubramaniam R, Mccullough MJ. Contemporary Oral Medicine. Springer International Publishing; 2019.
15. Kaufman HE, Azcuy AM, Varnell ED, et al. HSV-1 DNA in tears and saliva of normal adults. Investig Ophthalmol Vis Sci. 2005;46(1):241–247. doi:10.1167/iovs.04-0614.
16. Stock C, Guillén-Grima F, De Mendoza JH, et al. Risk factors of herpes simplex type 1 (HSV-1) infection and lifestyle factors associated with HSV-1 manifestations. Eur J Epidemiol. 2001;17(9):885–890. doi:10.1023/A:1015652713971.
17. Nath P, Kabir MA, Doust SK, et al. Diagnosis of herpes simplex virus: laboratory and point-of-care techniques. Infect Dis Rep. 2021;13(2):518–539. doi:10.3390/idr13020049.
18. Huber M. Herpes simplex type-1 virus infection. Quintessence Int. 2003;34(6):453–467.
19. Brooks GF, Jawetz E, Melnick JL, et al. Jawetz, Melnick & Adelberg’s medical microbiology; 2013.
20. Ganesha R, Sari RK, Putra NGJ. Management of herpes labialis triggered by stress. Interdental J Kedokt Gigi. 2021;17(2):56–62. doi:10.46862/interdental.v17i2.2966.
21. Singh A, Preiksaitis J, Romanowski B. The laboratory diagnosis of herpes simplex virus infections. Can J Infect Dis Med Microbiol. 2005;16:92–98 doi:10.1155/2005/318294.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
