Back to Journals » Clinical Ophthalmology » Volume 15
Stratification-Based Investigation of Adjunctive Brimonidine or Timolol to a Prostaglandin Analogue in Japanese Patients with Normal-Tension Glaucoma
Authors Yoshikawa K , Mizoue S , Nitta K , Onishi H , Ikeda M , Mizuno A , Kawazoe K , Tamada Y , Takeda R , Matsumoto S
Received 1 May 2021
Accepted for publication 11 June 2021
Published 6 July 2021 Volume 2021:15 Pages 2875—2883
DOI https://doi.org/10.2147/OPTH.S318392
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Keiji Yoshikawa,1 Shiro Mizoue,2,3 Koji Nitta,4 Hiroshi Onishi,5 Masaharu Ikeda,5 Akemi Mizuno,5 Kaori Kawazoe,5 Yoshiyuki Tamada,5 Ryuji Takeda,6 Shun Matsumoto7
1Yoshikawa Eye Clinic, Tokyo, Japan; 2Ehime University Hospital, Toon, Ehime, Japan; 3Minami-Matsuyama Hospital, Matsuyama, Ehime, Japan; 4Fukui-ken Saiseikai Hospital, Fukui, Japan; 5Senju Pharmaceutical Co. Ltd., Hyogo, Japan; 6Kansai University of Welfare Sciences, Osaka, Japan; 7Ideta Eye Hospital, Kumamoto, Japan
Correspondence: Keiji Yoshikawa
Yoshikawa Eye Clinic, 1-3-1 Nakamachi, Machida, Tokyo, 194-0021, Japan
Tel +81-42-739-0781
Fax +81-42-739-0782
Email [email protected]
Purpose: We previously investigated the efficacy and safety of adding 0.1% brimonidine (Brim) or 0.5% timolol (Tim) to prostaglandin analogue (PGA) monotherapy to treat patients with normal-tension glaucoma (NTG) with intraocular pressure (IOP) of ≤ 16 mmHg. Herein, we describe an additional post-hoc stratifying analysis of the possible differences in the effect of IOP-lowering and pulse rate (PR) after adjunctive Brim or Tim to PGA.
Patients and Methods: This study included 128 subjects. Patients with NTG treated with PGA were stratified based on their baseline IOP. The changes in IOP from baseline and the effect of patient factors on IOP changes were investigated. Patients were stratified by age for investigation of their PR and blood pressure (BP). The change and the effect of patient factors on PR and BP were investigated.
Results: After stratification analysis, in 52 eyes treated with Brim and 61 eyes with Tim with baseline IOP 12 ≤ IOP ≤ 16 mmHg, both eye drops lowered IOP significantly (P < 0.0001), and the IOP-lowering efficacy of Brim was non-inferior to that of Tim. However, in 9 Brim- and 6 Tim-treated eyes with baseline IOP of < 12 mmHg, no statistically significant decrease in IOP was evident with either eye drop. In the Tim group, PR decreased significantly (P < 0.05) after stratification by age.
Conclusion: The IOP-lowering efficacy of Brim was non-inferior to that of Tim after stratification by baseline IOP (12 ≤ IOP ≤ 16 mmHg). The discrepancy in the IOP-lowering effects of Brim and Tim observed in the previous study was thought to be related to enrolled subjects with low baseline IOP. PR decreased significantly in the Tim group even after age stratification. PR should be considered when selecting β-blockers for glaucoma treatment.
Keywords: adjunctive therapy, brimonidine, intraocular pressure reduction, post-hoc analysis, pulse rate, timolol
Introduction
In Japan, normal-tension glaucoma (NTG) accounts for more than 90% of open-angle glaucoma cases.1 Prostaglandin analogues (PGA) are used in the initial treatment of NTG; however, β-blockers or brimonidine are often used for adjunctive therapy2–5 to achieve the primary target of a 20% decrease in IOP from the untreated baseline.6
In a multicenter, randomized, single-masked study, we previously investigated the IOP-lowering efficacy and safety of the adjunctive administration of 0.1% brimonidine eye drops (Brim) or 0.5% timolol eye drops (Tim) twice a day with PGA monotherapy in patients with NTG. We found that both eye drops were safe and that Brim had as efficacious an IOP-lowering effect as that of Tim; however, the statistical “non-inferiority” of Brim to Tim was not proven.
Additionally, in the previous study, a significant decrease in pulse rate (PR) was evident after adjunctive Tim administration from 4 to 12 weeks. NTG frequently occurs in patients aged over 40 years.1,8 The risk of respiratory and cardiovascular diseases, such as chronic obstructive pulmonary disease (COPD), also increases with age.1,8,9
In the present re-analysis of the previous study, we planned an additional stratification-based evaluation of the possible difference in the IOP-lowering effects of these eye drops; we also aimed to determine the factors that influence the PR and BP after administration of Brim and Tim.
Materials and Methods
Study Design
This was a post-hoc analysis of our previous study.7 The study was approved by the Ethics Committee at Minami-Matsuyama Hospital (Matsuyama, Japan) and performed in accordance with ethical principles based on the Declaration of Helsinki. Written informed consent was obtained from all patients before participation. The study was registered with the University Hospital Medical Information Network (No. UMIN000014810). Only the anonymized data of the subjects in the previous study7 were used in this post-hoc study.
Subjects
The re-analysis study subjects were patients with NTG whose IOP had been lowered to 16 mmHg or less by administration of a single PGA for at least 90 days.7 NTG was diagnosed at an independent data center (Tokyo Teishin Hospital, Tokyo, Japan) based on the visual field test that met Anderson’s criteria10 and apparent glaucomatous changes in the optic disc by fundus photography. Only the 128 subjects from the previous study7 who were eligible for its efficacy analysis were included in this re-analysis.
Inclusion criteria were: (i) age ≥ 20 years old; (ii) patients who had received PGA monotherapy in the 6 months before enrolment and had a mean IOP (based on measurements taken on three different dates) of ≤ 16 mmHg; (iii) the duration of PGA monotherapy until the screening IOP measurement was ≥ 90 days; (iv) the corrected visual acuity was ≥ 0.5; (v) the mean deviation with the Humphrey field analyzer was > −20 dB; (vi) the corneal thickness of the evaluated eyes was 450–600 μm; (vii) the IOP could be measured with a Goldmann applanation tonometer; and (viii) the investigator judged that adjunctive IOP-lowering agents were required.
Exclusion criteria were: (i) history of hypersensitivity to or major adverse effects of Brim or Tim; (ii) current or previous bronchial asthma, or bronchospasm, or severe COPD; (iii) insufficiently controlled heart failure, sinus bradycardia, second- or third-degree atrioventricular block, or cardiogenic shock; (iv) active external ocular disease, or inflammatory or infectious disease of the eye or eyelid; (v) history of corneal refractive correction surgery in the evaluated eye; (vi) fundus disease that affects the visual field test results; (vii) intraocular surgery of the evaluated eye within the previous 90 days; (viii) corticosteroid use within 7 days before IOP measurement; (ix) history of trabeculectomy and trabeculotomy; and (x) participation in this clinical study judged by the investigator to be inappropriate for any other reason.
Procedures
The previous study was conducted at eight medical institutions in Japan between September 2014 and July 2015. The study eye drops were allocated in an investigator-masked manner, based on the block randomization method, by the Drug Allocation Manager at Kindai University (Nara, Japan). PGA administration was continued by the same eye drop and dosage prior to initiation of the study. The eye drops used were Brim (0.1% brimonidine ophthalmic solution [Aiphagan®; Senju Pharmaceutical Co., Ltd., Osaka, Japan]) or Tim (0.5% timolol ophthalmic solution [Timoptol®; Santen Pharmaceutical Co., Ltd., Osaka, Japan]); these were administrated twice daily with one drop at 12-hour intervals for 12 weeks.
The ophthalmological tests, such as corrected visual acuity, refraction, anterior ocular findings (conjunctiva, eyelid, and cornea), IOP, condition of the fundus, visual field, and central corneal thickness, and BP and PR were evaluated in the previous study at baseline.7 Additionally, the anterior ocular findings, IOP, condition of the fundus, BP, and PR were evaluated after adjunctive eye drop administration for 4, 8, and 12 weeks. Corrected visual acuity, refraction, and visual field were also evaluated at the completion of the previous study.7 IOP was measured twice at each time-point by the same investigator using a Goldmann applanation tonometer, and the mean of the two measurements was calculated. When the difference between the two measurements exceeded 3 mmHg, an additional measurement was made and the median of the three measurements was used in the data analysis. PR and BP were measured twice at 1–2 minute interval after 5 minutes’ rest in the sitting position, and the second measurement data was used for analysis. The measuring equipment for PR and BP was not unified, but the same equipment was used throughout the study at each institution. At each observation time-point, the subjects were interviewed about adherence and occurrence or non-occurrence of adverse events.
Sample Size
In the current re-analysis, the setting of the non-inferiority margin was just as that in the previous study, ie the margin was determined as 0.75 mmHg by assuming baseline mean IOP on PGA monotherapy of 15.0 mmHg, change in IOP of −3.0 mmHg with add-on Brim or Tim to a PGA, and setting ratio of 50% of the change in IOP achieved with comparator Tim excluding its placebo effect (−1.5 mmHg).
Subsequently, 64 subjects were needed per group to examine that the difference in the change in IOP, relative to baseline, with Brim from that with Tim was not less than 0.75 mmHg at the end of adjunctive treatment (at a one-sided significance level (α) of 0.025, power (1-β) of 80%, and standard deviation of 1.5 mmHg). Considering a withdrawal/dropout rate of 10% of the study population, 72 subjects should have been planned to be included in each group.
Statistical Analyses
IOP
The baseline IOP was defined as IOP with PGA before adjunctive administration with Brim or Tim, and mean IOP was defined as the average IOP at 4, 8, and 12 weeks after adjunctive administration. The distributions of IOP changes with each eye drop after administration for 4, 8, and 12 weeks were investigated and analyzed using the two-sided F-test. Multiple regression analysis was performed with mean IOP decrease after adjunctive eye drop administration as the objective variable, and the adjunctive eye drop (Brim or Tim), sex, age, and baseline IOP as the explanatory variables.
The registered subjects were stratified by baseline IOP level as IOP < 12 mmHg, 12 ≤ IOP < 14 mmHg, or 14 ≤ IOP ≤ 16 mmHg. The IOP analysis after stratification with baseline IOP by eye drop group was performed using Repeated Measures Analysis of Variance using Mixed model (MMRM ANOVA). An MMRM ANOVA model included the changes from baseline IOP as the dependent variable, baseline IOP group, observation time-points, and interaction between baseline IOP group and observation time-points as fixed effect, and subjects as random effect. The P value was adjusted with the Bonferroni method. A P value of less than 0.05 was considered statistically significant.
PR and BP
Twenty-one patients were taking antihypertensive drugs, but no remedy change was observed during the study period. The eligible subjects from the previous study7 were stratified by age as follows: age < 60, 60 ≤ age < 70, 70 ≤ age < 80, and age ≥ 80 years. With Brim and Tim, the mean PR and BP after adjunctive eye drop administration was compared with two-way ANOVA using mixed model.
Multiple regression analysis was performed by applying the mean PR and BP after adjunctive eye drop administration as the objective variable, and the adjunctive eye drop (Brim or Tim), sex, age, and baseline PR and BP as the explanatory variables.
Results
In this post-hoc analysis, a total of 128 subjects with NTG were included – 61 subjects in the Brim group and 67 in the Tim group, as reported in our previous study.7
Distribution of IOP-Lowering Effects
There was no difference in the distribution of IOP changes between the Brim and Tim group at 4 and 8 weeks after adjunctive administration. After 12 weeks, the mean and SD values of IOP changes from baseline with PGA were −1.05 ± 1.81 and −1.41 ± 1.40 in the Brim and Tim group, respectively. The Brim group showed a significantly broader distribution in IOP changes than the Tim group (P = 0.0425, two-sided F-test) (Figure 1).
Effects of Patient Background Factors on IOP Decrease
Among a variety of explanatory variables, such as type of eye drops and patient background, mere baseline IOP was significantly effective on the objective variable (mean IOP decrease after adjunctive eye drop administration) (P = 0.0010) (Table 1).
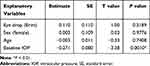 |
Table 1 Effects of Patient Background Factors on IOP Decrease |
IOP-Lowering Effects of Brim and Tim Stratified by Baseline IOP
The analysis of covariance revealed the P values of interaction effect was < 0.05 in all groups except the Brim group with baseline IOP < 12 mmHg. In both the Brim and Tim groups with baseline IOP with 12 ≤ IOP < 14 mmHg and 14 ≤ IOP ≤ 16 mmHg, the IOP at each time-point was significantly decreased relative to the baseline IOP, except that at 4 and 8 weeks in the Brim group of baseline IOP with 12 ≤ IOP < 14 mmHg. However, in either eye drop group with baseline IOP < 12 mmHg (Brim, n=9; Tim, n=6), no significant IOP decrease was found as compared to the baseline IOP at any of the observation time-points (Figure 2).
Effects of Brim and Tim on PR and BP
Although no significant difference in PR was observed in the Brim-treated subjects among any of the age groups, in the Tim-treated group, PR decreased significantly compared with the baseline in all of the age groups (Figure 3). A significant decrease of the systolic blood pressure (SBP) was observed in two groups of Brim (60 ≤ age ≤ 69 and 70 ≤ age ≤ 79 groups) and three groups of Tim (age < 60, 60 ≤ age ≤ 69, and age ≥ 80 groups). Moreover, a significant decrease of the diastolic blood pressure (DBP) was observed in two groups of Tim (age < 60 and 70 ≤ age ≤ 79 groups) (Figure 4). One patient in the Tim group was excluded because of missing PR and BP values at baseline.
 |
Figure 4 Blood pressures stratified by age. *P < 0.05, **P < 0.01. The values inside the bars indicate the mean value. Error bars represent standard error. |
Multiple regression analysis revealed that the eye drop and baseline PR were significantly associated with mean PR after adjunctive eye drop administration. Age showed the tendency of effect on PR (P=0.0587) (Table 2). Multiple regression analysis of SBP and DBP revealed that the eye drop, sex, age, and baseline BP were not significantly associated with mean BP after adjunctive eye drop administration (Table 3).
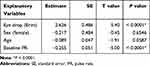 |
Table 2 Effects of Patient Background Factors on PR |
 |
Table 3 Effects of Patient Background Factors on BP (A and B) |
Discussion
In the previous study, there was no significant difference in the IOP-lowering effect of both Brim and Tim at each time point. In the present analysis, it was evident that the distribution of IOP in the Brim-treated group after 12 weeks was significantly broader than that in the Tim-treated group, suggesting that Brim and Tim differed in the mode of IOP-lowering. Among a variety of factors, baseline IOP on PGA was a mere explanatory variable that may affect IOP-lowering after adjunctive eye drop administration. Therefore, in the present study, the baseline IOP on PGA was stratified to investigate the possible difference in IOP-lowering effects between adjunctive usage of Brim and Tim. Consequently, in eyes with baseline IOP of 12 ≤ IOP < 14 mmHg and 14 ≤ IOP ≤ 16 mmHg, both Brim and Tim definitely lowered the IOP, whereas, in the eyes with baseline IOP < 12 mmHg, no significant decrease in IOP was found in either the Brim or Tim group. The number of subjects with baseline IOP < 12 mmHg on PGA monotherapy was only 9 for Brim and 6 for Tim, suggesting that the chance of showing a difference was small. The results revealed that the IOP-lowering effect was influenced by the baseline IOP levels, as reported by Lee et al.11
IOP reduction in both eye drops were −1.05±1.81 mmHg (Brim) and −1.41±1.40 mmHg (Tim), and the difference in IOP reduction between the two eye drops was 0.36 mmHg, with 95% confidence interval (CI [−0.21–0.92]) after 12 weeks. Brim was not non-inferior to Tim in our previous study7 (Table 4), and this may be attributed to that the baseline IOPs of the enrolled NTG eyes was lower than the predetermined value of 15 mmHg, ie Brim group: 13.4±1.4 mmHg, Tim group: 13.8±1.3 mmHg, and that the ratio of patients with baseline IOP < 12 mmHg was higher in the Brim group (9/61, 14.8%) than in the Tim group (6/67, 9.0%).
 |
Table 4 Non-Inferiority Analysis of Brim to Tim After Stratification by Baseline IOP on PGA Monotherapy |
In this re-analysis of the previous study, subjects were stratified to two groups by baseline IOP of < 12 mmHg and 12 ≤ IOP ≤ 16 mmHg because no significant decrease in IOP was found with either eye drop in the eyes with baseline IOP < 12 mmHg on PGA. The mean IOPs of three observation time points (4, 8, and 12 weeks) after adjunctive eye drop administration were analyzed to prevent large variations due to smaller number of patients after stratification. Hence, in those eyes with baseline IOP < 12 mmHg on PGA, the difference in IOP changes between the two eye drops was 0.82 mmHg, and its 95% CI (−0.46–2.10) exceeded the preset inferiority margin of 0.75 mmHg; therefore, Brim was not non-inferior to Tim (Table 4). The enrollment of subjects with IOP of < 12 mmHg on PGA may affect the verification of non-inferiority between the two eye drops. In the group with baseline IOP of 12 ≤ IOP ≤ 16 mmHg, both eye drops significantly decreased mean IOP relative to the baseline IOP on PGA (both P < 0.0001). The difference in IOP changes between the two eye drop groups was 0.18 mmHg with 95% CI (−0.29–0.65). Thus, 95% CI was within the 0.75-mmHg range for the non-inferiority margin; therefore, Brim was non-inferior to Tim in the eyes with baseline IOP on PGA of 12 ≤ IOP ≤ 16 mmHg (Table 4). In NTG patients with an IOP of 12 mmHg or more using PGA, therefore we believe an additional Brim use would be effective, similar to that of Tim.
In eyes on PGA monotherapy with IOP < 12 mmHg, no significant decrease in IOP was found in either the Brim or Tim group; hence, this result might question the efficacy of glaucoma eye drops application in these eyes. Glaucomatous field progression was evident in 66% of untreated NTG eyes with a mean IOP of 12.3 mmHg during the 5-year follow-up.12 In Japan, there are a certain number of NTG patients with very low IOP1 as observed in our previous study, which included up to 10% of the subjects with baseline IOP < 12 mmHg but requiring IOP-lowering treatment. As the effect of additional IOP-lowering, including glaucoma surgery,13,14 for NTG patients with IOP < 12 mmHg is controversial, treatment is aimed at neuroprotection. Brim and Tim have neuroprotective effects and long-term clinical data suggest that Brim may be a remedy of choice for NTG patients with very low IOP level.15
In our previous study,7 we found that PR decreased significantly and that this decrease lasted in patients after adjunctive administration of Tim. The incidence of NTG increases with age. In the present re-analysis of that study, P value of age in multiple regression analysis was 0.0587 and age showed the tendency of effect on PR. The Brim group showed no significant reduction in PR in any age group, whereas in the Tim group, a significant decrease in PR was observed in all groups. Brim and Tim affected PR differently. Furthermore, Tim was selected as an explanatory variable for the decrease in PR even in multivariate analysis. It has previously been reported that Tim affects respiratory and cardiovascular function even in healthy individuals aged over 65 years.16 It was reconfirmed that PR monitoring was needed for patients of all ages on Tim therapy, including those aged < 60 years. After stratification by age, SBP decreased in two and three groups of Brim and Tim, respectively, and DBP decreased in two groups of Tim. However, the decrease in SBP and DBP was less than 10 mmHg, and it was considered to be clinically acceptable.
In this re-analysis of the previous study,7 applying stratification-based investigation, Brim was non-inferior to Tim in the eyes with baseline IOP of 12 ≤ IOP ≤ 16 mmHg. On the contrary, no significant IOP decrease as compared to baseline IOP was found in the eyes with baseline IOP < 12 mmHg in either eye drop group. These results indicate that when evaluating the IOP-lowering efficacy in NTG for adjunctive glaucoma eye drops, baseline IOP level on a PGA should be a key factor, especially for NTG eyes with IOP level of < 12 mmHg. After stratification by age, PR decreased significantly compared with the baseline, as expected. This also indicates that the systemic side effect of β-blockers such as a decrease in PR should be monitored during glaucoma treatment.
This study had the following limitations. By applying stratification according to baseline IOP level on PGA, the number of subjects in each group was reduced; therefore, the accuracy of the investigation was statistically limited. It is also a limitation that we did not perform an analysis focused on each type of PGA. The original purpose of the study was to confirm the additional effects of Brim and Tim in clinical practice. We found variability among PGAs because PGA was not specified. The performance of a prospective study with an adjusted number of subjects treated with each type of PGA is needed in the future.
Conclusion
When considering adjunctive therapy to topical PGA monotherapy, we reaffirmed the importance of baseline data collection including PR and treatment strategy development in managing patients with NTG.
Data Sharing Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The authors thank Motohiro Shirakashi, Akiyoshi Nitta, Shigeki Yamabayashi, Tairo Kimura, and Toshihiko Ueda for collecting data for this study.
Funding
This study was funded by Senju Pharmaceutical Co., Ltd.
Disclosure
Keiji Yoshikawa, Shiro Mizoue, Koji Nitta, and Shun Matsumoto have received personal fees and grants from Senju Pharmaceutical Co., Ltd. Keiji Yoshikawa also reports lecture fees from Santen Pharm. Co. Ltd., Crewt Medical, RE Medical, Ellex Co. Ltd., Otsuka Pharm. Co. Ltd., Kowa, Alcon, and Pfizer, during the conduct of the study. Shiro Mizoue also reports lecture fees from Santen Pharm. Co. Ltd., Otsuka Pharm. Co. Ltd., Ellex Co. Ltd., Kowa, and Novartis Pharma. Co. Ltd., during the conduct of the study. Koji Nitta also reports lecture fees from Santen Pharm. Co. Ltd., RE Medical, Ellex Co. Ltd., Otsuka Pharm. Co. Ltd., Kowa, Alcon, NIDEK Co. Ltd., and Novartis Pharma. Co. Ltd., during the conduct of the study. Ryuji Takeda has received personal fees from Senju Pharmaceutical Co., Ltd. Hiroshi Onishi, Masaharu Ikeda, Akemi Mizuno, Kaori Kawazoe, and Yoshiyuki Tamada are employees of Senju Pharmaceutical Co., Ltd. The authors report no other conflicts of interest in this work.
References
1. Iwase A, Suzuki Y, Araie M, et al. The prevalence of primary open-angle glaucoma in Japanese: the Tajimi Study. Ophthalmology. 2004;111:1641–1648. doi:10.1016/j.ophtha.2004.03.029
2. Sakata R, Aihara M, Murata H, et al. Contributing factors for progression of visual field loss in normal-tension glaucoma patients with medical treatment. J Glaucoma. 2013;22(3):250–254. doi:10.1097/IJG.0b013e31823298fb
3. Kim M, Kim DM, Park KH, et al. Intraocular pressure reduction with topical medications and progression of normal-tension glaucoma: a 12-year mean follow-up study. Acta Ophthalmol. 2013;91(4):e270–e275. doi:10.1111/aos.12082
4. Komori S, Ishida K, Yamamoto T. Results of long-term monitoring of normal-tension glaucoma patients receiving medical therapy: results of an 18-year follow-up. Graefes Arch Clin Exp Ophthalmol. 2014;252(12):1963–1970. doi:10.1007/s00417-014-2767-3
5. Erdem E, Williams A, Kuchar SD, Waisbourd M, Spaeth GL. Long-term (>8 years) evaluation of progression in patients with low-pressure glaucoma. Eur J Ophthalmol. 2015;25(6):490–495. doi:10.5301/ejo.5000624
6. Tsumura T, Yoshikawa K, Kimura T, et al. The efficacy and safety of add-on 0.1% brimonidine tartrate preserved with sodium chlorite in on-treatment Japanese normal-tension glaucoma patients. Clin Ophthalmol. 2014;8:1681–1687. doi:10.2147/OPTH.S67366
7. Mizoue S, Nitta K, Shirakashi M, et al. Multicenter, randomized, investigator-masked study comparing brimonidine tartrate 0.1% and timolol maleate 0.5% as adjunctive therapies to prostaglandin analogues in normal-tension glaucoma. Adv Ther. 2017;34(6):1438–1448. doi:10.1007/s12325-017-0552-5
8. Yamamoto T, Iwase A, Araie M, et al. The Tajimi Study report 2: prevalence of primary angle closure and secondary glaucoma in a Japanese population. Ophthalmology. 2005;112(10):1661–1669. doi:10.1016/j.ophtha.2005.05.012
9. Fukuchi Y, Nishimura M, Ichinose M, et al. COPD in Japan: the Nippon COPD Epidemiology study. Respirology. 2004;9(4):458–465. doi:10.1111/j.1440-1843.2004.00637.x
10. Anderson DR, Patella VM. Automated Static Perimetry.
11. Lee JY, Sung KR, Lee JY. Comparison of the progression of high- and low-tension glaucoma as determined by two different criteria. Korean J Ophthalmol. 2016;30:40–47. doi:10.3341/kjo.2016.30.1.40
12. Sakata R, Yoshitomi T, Iwase A, et al. Factors associated with progression of Japanese open-angle glaucoma with lower normal intraocular pressure. Ophthalmology. 2019;126:1107–1116. doi:10.1016/j.ophtha.2018.12.029
13. Aoyama A, Ishida K, Sawada A, Yamamoto T. Target intraocular pressure for stability of visual field loss progression in normal-tension glaucoma. Jpn J Ophthalmol. 2010;54(2):117–123. doi:10.1007/s10384-009-0779-z
14. Iverson SM, Schultz SK, Shi W, Feuer WJ, Greenfield DS. Effectiveness of single-digit IOP targets on decreasing global and localized visual field progression after filtration surgery in eyes with progressive normal-tension glaucoma. J Glaucoma. 2016;25(5):408–414. doi:10.1097/IJG.0000000000000240
15. Krupin T, Liebmann JM, Greenfield DS, Ritch R, Gardiner S; Low-Pressure Glaucoma Study Group. A randomized trial of brimonidine versus timolol in preserving visual function: results from the low-pressure glaucoma treatment study. Am J Ophthalmol. 2011;151(4):671–681. doi:10.1016/j.ajo.2010.09.026
16. Araie M, Adachi M. [Effect of topical 0.1% brimonidine tartrate and 0.5% timolol maleate on pulmonary and cardiovascular functions]. Nippon Ganka Gakkai Zasshi. 2012;116(7):623–634. [in Japanese].
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



