Back to Journals » Infection and Drug Resistance » Volume 11
Statin use and the risk of Clostridium difficile infection: a systematic review with meta-analysis
Authors Tariq R, Mukhija D, Gupta A, Singh S, Pardi DS, Khanna S
Received 9 November 2017
Accepted for publication 31 January 2018
Published 13 March 2018 Volume 2018:11 Pages 405—416
DOI https://doi.org/10.2147/IDR.S156475
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Raseen Tariq,1,2 Dhruvika Mukhija,3 Arjun Gupta,4 Siddharth Singh,5 Darrell S Pardi,1 Sahil Khanna1
1Division of Gastroenterology and Hepatology, Mayo Clinic, Rochester, MN, 2Department of Internal Medicine, Rochester General Hospital, Rochester, NY, 3Department of Internal Medicine, Cleveland Clinic, Cleveland, OH, 4Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, 5Division of Gastroenterology and Hepatology, University of California San Diego, La Jolla, CA, USA
Purpose: Statins have pleiotropic effects beyond cholesterol lowering by immune modulation. The association of statins with primary Clostridium difficile infection (CDI) is unclear as studies have reported conflicting findings. We performed a systematic review and meta-analysis to evaluate the association between statin use and CDI.
Patients and methods: We searched MEDLINE, Embase, and Web of Science from January 1978 to December 2016 for studies assessing the association between statin use and CDI. The Newcastle–Ottawa Scale was used to assess the methodologic quality of included studies. Weighted summary estimates were calculated using generalized inverse variance with random-effects model.
Results: Eight studies (6 case–control and 2 cohort) were included in the meta-analysis, which comprised 156,722 patients exposed to statins and 356,185 controls, with 34,849 total cases of CDI available in 7 studies. The rate of CDI in patients with statin use was 4.3%, compared with 7.8% in patients without statin use. An overall meta-analysis of 8 studies using the random-effects model demonstrated that statins may be associated with a decreased risk of CDI (maximally adjusted odds ratio [OR], 0.80; 95% CI, 0.66–0.97; P=0.02). There was significant heterogeneity among the studies, with an I2 of 79%. No publication bias was seen. Meta-analysis of studies that adjusted for confounders revealed no protective effect of statins (adjusted OR, 0.84; 95% CI, 0.70–1.01; P=0.06, I2=75%). However, a meta-analysis of only full-text studies using the random-effects model demonstrated a decreased risk of CDI with the use of statins (OR 0.77; 95% CI, 0.61–0.99; P=0.04, I2=85%).
Conclusion: Meta-analyses of existing studies suggest that patients prescribed a statin may be at decreased risk for CDI. The results must be interpreted with caution given the significant heterogeneity and lack of benefit on analysis of studies that adjusted for confounders.
Keywords: Clostridium difficile infection, incidence, meta-analysis, statins
Introduction
Clostridium difficile infection (CDI) is the most common cause of hospital-acquired diarrhea and is increasingly being recognized in the community.1,2 Despite extensive preventive efforts, such as antibiotic stewardship, and emerging treatment strategies, an increasing incidence and worsening outcomes of CDI have been demonstrated.3 Novel risk factors for CDI, such as the use of proton pump inhibitors, have been identified that place persons previously considered to be at low risk, now at risk for CDI.4 Some risk factors for CDI such as an aging, immunocompromised population are not modifiable. In this setting, innovative methods to reduce the incidence of CDI are required.
HMG-CoA reductase inhibitors (ie, statins) are among the most common medications prescribed in the USA. From 2003 to 2012, the percentage of American adults aged 40 years and older taking a statin increased from 18% to 26%.5 The American College of Cardiology and the American Heart Association recommend statin therapy for all patients with cardiovascular disease and increased cholesterol levels and for patients aged 40–75 years who have diabetes mellitus or an estimated 10-year risk of cardiovascular disease of 7.5% or higher.6 Although the approved indications to use statins are largely cardiovascular, they have been shown to improve outcomes in infections such as pneumonia, pulmonary hypertension, new-onset inflammatory bowel disease, venous thromboembolism, autoimmune conditions such as systemic lupus erythematosus, and certain cancers such as hepatocellular carcinoma and gastric cancer.7–13 Statin use has been found to prevent infections in patients with cirrhosis and to be associated with decreased risks of severe sepsis and decompensation and all-cause mortality in compensated liver disease secondary to hepatitis C.14–16
By inhibiting the production of isoprenoid intermediates, which are required for the activation of intracellular messengers, statins have pleiotropic effects on inflammatory and immunomodulatory pathways.17 Conceivably, statins may modify the risk of CDI. This remains a pertinent question given the many number of patients taking statins and therefore are at risk for CDI. A retrospective study indicated that statin users may, in fact, be at higher risk for CDI given the ability of statins to affect the interaction of the C. difficile organism and its toxins with colonic epithelium.18 In contrast, a large case–control study demonstrated a 22% lower risk of CDI in statin users versus nonusers.19 Given the conflicting results, we performed a systematic review and meta-analysis to study the association between the use of statins and the risk of CDI.
Patients and methods
All procedures used in this meta-analysis were reported according to the Preferred Reporting Items for Systematic Reviews and meta-analyses (PRISMA) guidelines.20
Selection criteria
The studies considered in this meta-analysis were case–control studies, cohort studies, or clinical trials that included a study population of patients who did and did not receive statin therapy and that evaluated the occurrence of CDI, with no restrictions on study setting (inpatient or outpatient). We excluded studies that did not evaluate CDI as an outcome. Studies were also excluded from meta-analyses if there were insufficient data to determine an estimate of an odds ratio and 95% CI. We included published full-text articles and studies in abstract form.
Data sources and search strategy
We conducted a comprehensive search of Ovid MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE, Ovid Embase, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of Science, and Scopus from January 1978 through December 2016. The search strategy was designed and conducted by study investigators (SK and RT) and the Mayo Clinic library staff, independently. The search was limited to studies published in English. Controlled vocabulary supplemented with keywords was used to search for studies of statin use and CDI. Main keywords used in the search were the following: Clostridium difficile, C. diff, C. difficile, Clostridium difficile infection, CDI, Clostridium difficile–associated diarrhea or CDAD, or pseudomembranous colitis AND hmg coa OR hydroxymethylglutaryl OR hmg OR coa OR coenzyme OR atorvastatin OR cerivastatin OR compactin OR fluindostatin OR lovastatin OR mevinolin OR pitavastatin OR pravastatin OR rosuvastatin OR simvastatin OR statin AND outcomes, infection. The detailed search strategy is shown in Table S1.
Two authors (SK and RT) independently reviewed the titles and abstracts of the identified studies, and those that did not answer the research question of interest were excluded. The full texts of the remaining articles were reviewed to determine inclusion criteria fulfillment. The reference lists of articles with information on the topic were also reviewed for additional pertinent studies. A flow diagram of the included studies is shown in Figure 1.
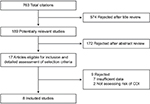  | Figure 1 Flow diagram of study selection process. Abbreviation: CDI, Clostridium difficile infection. |
The ROBINS-I risk of bias was used by 2 investigators (SK and RT) to assess the methodologic quality of case–control and cohort studies.21,22 In this scale, observational studies were scored across 3 categories using the following parameters: selection (3 questions), classification of exposure (3 questions), classification of missing data (5 questions), and bias in the selection of reported result (4 questions). For each question, 1 point was given if the study met the criterion (Table 1). Studies with a cumulative score of 10 or more were considered to be of moderate to high quality. Any discrepancies were addressed by joint re-evaluation of the original article.
Data abstraction
Data were independently abstracted to a predetermined collection form by 2 investigators (SK and RT). Data were collected for each study, including study setting and design, year of publication, location, primary outcome (CDI) reported, and number of patients in each group (exposed vs not exposed and CDI vs no CDI). Conflicts in data abstraction were resolved by consensus, referring to the original article.
Outcomes assessed
Our primary analysis focused on assessing the risk of CDI and its association with statin use in studies that adjusted for potential confounders and in full-text studies.
Statistical analyses
We used the random-effects model described by DerSimonian and Laird23 to calculate weighted summary estimates using generalized inverse variance. Adjusted odds ratios (ORs), when available, or ORs (calculated for each study) were used in the analysis. Summary estimates are presented as ORs with 95% confidence intervals (CIs). We assessed heterogeneity within groups with the I2 statistic, which estimates the proportion of total variation across studies that is due to heterogeneity in study patients, design, or interventions rather than chance; I2 values greater than 50% suggest substantial heterogeneity.21 The presence of publication bias was assessed by the visual inspection of funnel plots.21 All P-values were 2-tailed. For all tests (except for heterogeneity), a P-value <0.05 was considered statistically significant. Calculations were performed and graphs were constructed using RevMan (Review Manager, version 5.3; Cochrane Inc).
A priori-defined analyses including studies that controlled for confounders, moderate- to high-quality studies, and full-text studies only and studies that recruited inpatients only were performed.
Results
Search results
The described search strategy revealed 763 potentially relevant studies; titles were screened and relevant articles were identified (Figure 1). In all, 17 articles were reviewed, of which 9 were excluded for various reasons (Figure 1). A total of 8 studies were included in this meta-analysis, of which only 3 were abstracts; all 8 observational studies evaluated the risk of CDI with statins.18,19,24–29 Two other studies that described the risk of recurrent CDI with statins were separately analyzed.30,31
Quality of included studies
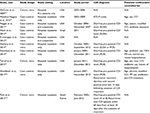
The median ROBINS-I score for case–control studies was 9.3 (range, 7–12) and for cohort studies was 8.5 (range, 7–10) out of 15 points. Four of the 8 included studies were considered to be of moderate to high quality, with a cumulative score of 10 or more. Table 1 shows the methodologic quality of all included studies.
Characteristics of included studies
The included studies comprised a total of 156,722 patients exposed to statins and 356,185 controls, with 34,849 total cases of CDI (available in 7 studies, 1 study did not report the total number of patients included). The characteristics of the 8 included studies are shown in Table 2. Seven studies were performed in USA and 1 in Israel. The earliest study recruitment period began in 2002, and the latest ended in 2015. All observational studies assessed medication exposure through review of medical records.
Statin use and CDI risk
The rate of CDI in patients taking statins was 4.3% (6,828/156,722), compared with 7.8% (28,021/356,185) in patients not taking statins. An overall meta-analysis of all 8 studies using the random-effects model demonstrated that statins were associated with a 20% decreased risk of CDI (maximally adjusted OR, 0.80; 95% CI, 0.66–0.97; P=0.02) (Figure 2A). There was a significant heterogeneity among the studies, with an I2 of 79%. No publication bias was seen (Figure 2B).
Primary analyses
Of the 8 included studies, 4 studies had been adjusted for potential confounders (Table 2). Analysis of studies that adjusted for confounders revealed no protective effect of statins (adjusted OR, 0.84; 95% CI, 0.70–1.01; P=0.06 (Figure 3). Meta-analysis of only full-text studies using the random-effects model demonstrated a decreased risk of CDI with use of statins (OR 0.77; 95% CI, 0.61–0.99; P=0.04, I2=85%) (Figure 4).
  | Figure 4 Analysis of full-text studies only. Forest plot demonstrating decreased risk of CDI with use of statins. Abbreviation: CDI, Clostridium difficile infection; SE, standard error. |
Subgroup analyses
Given the significant heterogeneity in meta-analysis of all the included studies, we performed subgroup analyses to better understand the heterogeneity. However, no single source of heterogeneity was identified; the I2 remained increased in the subgroup analyses.
Subgroup analysis of moderate- to high-quality studies
Four of the 8 included studies were considered to be of moderate to high quality based on ROBINS-I scoring. Subgroup analysis of only these studies also revealed a significantly decreased risk of CDI with the use of statins (OR 0.73; 95% CI, 0.6–0.89; P=0.04, I2=81%) (Figure 5).
  | Figure 5 Analysis of moderate- to high-quality studies. Forest plot demonstrating a decreased risk of CDI with use of statins. Abbreviation: CDI, Clostridium difficile infection; SE, standard error. |
Subgroup analysis of studies with inpatients only
Five of the 8 studies included in our meta-analysis included inpatients,19,24,25,27,28 2 included both inpatients and outpatients,26,29 and 1 study included only intensive care unit (ICU) patients.18 The subgroup analysis of studies that included only inpatients also revealed a decreased risk of CDI with use of statins (OR 0.81; 95% CI, 0.68–0.95; P=0.01, I2=76%) (Figure 6).
  | Figure 6 Analysis of studies with inpatients only. Forest plot demonstrating a decreased risk of statins on the risk of CDI. Abbreviation: CDI, Clostridium difficile infection; SE, standard error. |
Statins and CDI outcomes
During our search, we found 2 other studies that evaluated the risk of recurrent CDI with statins.30,31 Meta-analysis of these 2 studies revealed that statins had no effect on the recurrence of CDI (unadjusted OR, 0.92; 95% CI, 0.74–1.15; P=0.47) (Figure 7A). Two additional studies evaluated the association of statins with risk of 30-day CDI mortality.32,33 Meta-analysis of these 2 studies also revealed a nonsignificant result (unadjusted OR, 0.91; 95% CI, 0.34–2.39; P=0.84) (Figure 7B).
Discussion
To our knowledge, this study is the first meta-analysis to explore an association between statins and incident CDI. From our analysis of studies that were conducted over more than a decade (2002–2012), we conclude that statins may be associated with a decreased risk of CDI, with a 20% risk reduction. However, no protective effect was seen by pooling only studies that had been adjusted for potential confounders. Five of the 8 included studies showed that statins decreased the risk of incident CDI, but 1 study showed an increased risk with the use of these agents.18 However, that study was limited to ICU patients with sepsis and included very few patients with CDI.
The development of CDI is secondary to antibiotic exposure, which leads to altered intestinal microbiota.34 This is followed by infection by a toxigenic strain of C. difficile via feco-oral transmission, which leads to diarrhea secondary to inflammation from toxin exposure. Factors that facilitate infection or increase the virulence of C. difficile are being increasingly recognized. These include host-related factors such as immunosuppression, advanced age, hospitalization, severe illness, gastrointestinal surgery, obesity, and acid suppression (eg, proton pump inhibitors), 4,35–38 and organism-related factors such as expression of certain surface adhesins and flagellar genes and toxin C. difficile transferase.39,40 However, data are limited on organism- and host-specific factors that afford protection against CDI. Our study helps address a major gap in knowledge by proposing statins as having a potentially protective role in CDI. Statins are known to interfere with molecules involved in endothelial adhesion and transendothelial migration of polymorphonuclear cells to sites of inflammation.41 Statins also reduce the activation of the monocyte/macrophage system and reduce the cytotoxicity of T cells.42 It is also possible that the protective effects of statins relate to C. difficile toxins targeting Rho-GTPase proteins in the host cytosol,43 which is also a major target for the action of statins.44 Another potential mechanism for the protection from CDI offered by statins could be the alteration of microbiota by statins, although the specific effects of statins on the intestinal microbiome are unknown. Statins are also known to be bactericidal in vitro and in vivo,45 but these effects have been seen at concentrations much higher than those achieved in the blood during treatment with statins; therefore, the bactericidal effect of statins most likely does not protect against infections directly.
We performed a subgroup analysis by separating studies that included inpatients only; this analysis also demonstrated a decreased risk of CDI with statin use. The results also remained statistically significant when the analysis was limited to moderate- to high-quality studies. Analysis of studies that had been adjusted for potential confounders revealed no protective effect of statin. It is possible that the protective effect seen in the overall analysis might be a healthy effect rather than a true association between statins and decreased CDI risk.
With C. difficile being declared an emergent threat by the Centers for Disease Control and Prevention, it has become increasingly important to continue to explore novel and innovative ways to combat it, especially if the intervention can have other potential health-related benefits concurrently, as with statins. Widespread use of statins may lead to a significant decrease in health care–related costs due to their indirect effects even outside their primary indications, such as CDI inhibition. Some studies recommend the use of statins for primary prevention of cardiovascular events, even without hyperlipidemia.46
The strengths of our study include a comprehensive literature review with a large patient population and stability of our results in subgroup analysis. Our study also has several limitations. The individual studies included in our meta-analysis varied in several ways, including study design, patient population, study setting (inpatient vs outpatient vs ICU), NAP1 (North American pulsed-field gel electrophoresis type 1) status, use of International Classification of Diseases –Ninth Classification codes versus medical records, lack of information on obvious confounders such as exposure to antibiotics (class, duration, and dose) in all studies, and the duration, dose, and type of statins used. These different aspects led to substantial heterogeneity. Only 4 studies had been controlled for different confounding factors. We were unable to perform analyses in which all confounding factors could be accounted or controlled for, including continuous versus intermittent use of statin and duration and dose of statins and exposure to antibiotic use. Although no publication bias was seen on visual inspection of funnel plot, the results of this test should be interpreted with caution since fewer than 10 studies were included.
Conclusion
In conclusion, our study highlights that statins may have a role in the prevention of CDI. However, this protective effect was not seen after adjusting for potential confounders. Nevertheless, providers should continue to encourage and reinforce the use of statins when indicated in patients, not only for their direct cardiovascular benefits, but also for indirect benefits such as prevention of CDI, especially in the setting of systemic antibiotic exposure. Elective withholding of statin medications in hospitalized patients with multiple risk factors for CDI should be discouraged. However, the results of this meta-analysis must be interpreted with caution given the significant heterogeneity among the included studies and discrepancies seen on subgroup analyses. Further prospective studies would be required to evaluate the protective effect of statins on reducing the risk of CDI, including dose and duration of these medications.
Acknowledgments
The abstract of this paper was presented as a poster at the American College of Gastroenterology Conference in Orlando, FL, on October 15th, 2017. The abstract was published in the conference abstract supplement of the American Journal of Gastroenterology (Am J Gastroenterol 112:S56; doi:10.1038/ajg.2017.296).
Author contributions
RT: study design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content. DM: study design, acquisition of data, drafting of the manuscript. AG: study design, acquisition of data, drafting of the manuscript. SS: study design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content. DSP: study design, interpretation of data; critical revision of the manuscript for important intellectual content. SK: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, administrative support, study supervision. All authors have approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Gupta A, Khanna S. Community-acquired Clostridium difficile infection: an increasing public health threat. Infect Drug Resist. 2014;7:63–72. | ||
Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. | ||
Khanna S, Pardi DS. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4(4):409–416. | ||
Tariq R, Singh S, Gupta A, Pardi DS, Khanna S. Association of gastric acid suppression with recurrent Clostridium difficile infection: a systematic review and meta-analysis. JAMA Intern Med. 2017;177(6):784–791. | ||
Li SS, Zhu A, Benes V, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352(6285):586–589. | ||
Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(25 Pt B):2889–2934. | ||
Chopra V, Rogers MA, Buist M, et al. Is statin use associated with reduced mortality after pneumonia? A systematic review and meta-analysis. Am J Med. 2012;125(11):1111–1123. | ||
Singh PP, Singh S. Statins – the Holy Grail for cancer? Ann Transl Med. 2013;1(1):1. | ||
Singh S, Singh PP. Statin a day keeps cancer at bay. World J Clin Oncol. 2013;4(2):43–46. | ||
Yu HH, Chen PC, Yang YH, et al. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: a nationwide population-based cohort study. Atherosclerosis. 2015;243(1):11–18. | ||
Ungaro R, Chang HL, Cote-Daigneaut J, Mehandru S, Atreja A, Colombel JF. Statins associated with decreased risk of new onset inflammatory bowel disease. Am J Gastroenterol. 2016;111(10):1416–1423. | ||
Kunutsor SK, Seidu S, Khunti K. Statins and primary prevention of venous thromboembolism: a systematic review and meta-analysis. Lancet Haematol. 2017;4(2):e83–e93. | ||
Wright JL, Zhou S, Preobrazhenska O, et al. Statin reverses smoke-induced pulmonary hypertension and prevents emphysema but not airway remodeling. Am J Respir Crit Care Med. 2011;183(1):50–58. | ||
Almog Y, Shefer A, Novack V, et al. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation. 2004;110(7):880–885. | ||
Mohanty A, Tate JP, Garcia-Tsao G. Statins are associated with a decreased risk of decompensation and death in veterans with hepatitis C-related compensated cirrhosis. Gastroenterology. 2015;150(2):430–440.e431. | ||
Motzkus-Feagans C, Pakyz AL, Ratliff SM, Bajaj JS, Lapane KL. Statin use and infections in veterans with cirrhosis. Aliment Pharmacol Ther. 2013;38(6):611–618. | ||
Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy: molecular mechanisms and clinical results. Trends Mol Med. 2008;14(1):37–44. | ||
McGuire T, Dobesh P, Klepser D, Rupp M, Olsen K. Clinically important interaction between statin drugs and Clostridium difficile toxin? Med Hypotheses. 2009;73(6):1045–1047. | ||
Motzkus-Feagans CA, Pakyz A, Polk R, Gambassi G, Lapane KL. Statin use and the risk of Clostridium difficile in academic medical centres. Gut. 2012;61(11):1538–1542. | ||
Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. | ||
Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337(8746):867–872. | ||
Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. | ||
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. | ||
Naggie S, Miller BA, Zuzak KB, et al. A case-control study of community-associated Clostridium difficile infection: no role for proton pump inhibitors. Am J Med. 2011;124(3):276e271–276e277. | ||
Nseir W, Bishara J, Mograbi J, et al. Do statins protect against the development of Clostridium difficile-associated diarrhoea? J Antimicrob Chemother. 2013;68(8):1889–1893. | ||
Kumarappa VS, Eddi R, DeBari V, Baddoura W. Do statins protect against Clostridum difficile associated diarrhea? Paper presented at 2012 ACG Abstracts; October 19-24; 2012; Las Vegas, Nevada. | ||
Elashery A, Sohi S, Qi Y, Baig S, Chandra S. Statin use and hospital-onset Clostridium difficile infection: a case control study. Paper presented at: Infectious Diseases Week 2014; October 8-12; 2014; Philadelphia, PA. | ||
Tartof SY, Rieg GK, Wei R, Tseng HF, Jacobsen SJ, Yu KC. Comprehensive assessment across the healthcare continuum: risk of hospital-associated Clostridium difficile infection due to outpatient and inpatient antibiotic exposure. Infect Cont Hosp Ep. 2015;36(12):1409–1416. | ||
Ewelukwa O, Cheema B, Metzger S, Markert RJ, Akram S. The effect of statins on the risk of developing Clostridium difficile diarrhea: a case-control study. Gastroenterology. 2014;146(5):S252–S252. | ||
Abdelfatah M, Nayfe R, Enriquez K, Nijim A, El Zoghbi M, Watkins R. The effect of statins on the risk of recurrent Clostridium difficile infection. Paper presented at: American College of Gastroenterology Annual scientific meeting. October 17-22; 2014; Philadelphia, PA | ||
Park SW, Choi AR, Lee HJ, et al. The effects of statins on the clinical outcomes of Clostridium difficile infection in hospitalised patients. Aliment Pharmacol Therap. 2013;38(6):619–627. | ||
Saliba W, Barnett-Griness O, Elias M, Rennert G. Statins use and risk of mortality in patient with Clostridium difficile infection. Clin Microbiol Infect. 2014;20(10):1061–1066. | ||
Atamna A, Yahav D, Eliakim-Raz N, et al. The effect of statins on the outcome of Clostridium difficile infection in hospitalized patients. Eur J Clin Microbiol Infect Dis. 2016;35(5):779–784. | ||
Centers for Disease Control and Prevention (CDC). Surveillance for community-associated Clostridium difficile – Connecticut, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(13):340–343. | ||
Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006;145(10):758–764. | ||
Bishara J, Farah R, Mograbi J, et al. Obesity as a risk factor for Clostridium difficile infection. Clin Infect Dis. 2013;57(4):489–493. | ||
Kyne L, Sougioultzis S, McFarland LV, Kelly CP. Underlying disease severity as a major risk factor for nosocomial Clostridium difficile diarrhea. Infect Control Hosp Epidemiol. 2002;23(11):653–659. | ||
Loo VG, Bourgault AM, Dascal A. The authors reply. N Engl J Med. 2012;366(3):276–277. | ||
El Feghaly RE, Bangar H, Haslam DB. The molecular basis of Clostridium difficile disease and host response. Curr Opin Gastroenterol. 2014;31(1):24–29. | ||
Cowardin CA, Buonomo EL, Saleh MM, et al. The binary toxin CDT enhances Clostridium difficile virulence by suppressing protective colonic eosinophilia. Nat Microbiol. 2016;1(8):16108. | ||
Sparrow CP, Burton CA, Hernandez M, et al. Simvastatin has anti-inflammatory and antiatherosclerotic activities independent of plasma cholesterol lowering. Arterioscler, Thromb Vasc Biol. 2001;21(1):115–121. | ||
Blanco-Colio LM, Villa A, Ortego M, et al. 3-Hydroxy-3-methyl-glutaryl coenzyme A reductase inhibitors, atorvastatin and simvastatin, induce apoptosis of vascular smooth muscle cells by downregulation of Bcl-2 expression and Rho A prenylation. Atherosclerosis. 2002;161(1):17–26. | ||
Chen S, Sun C, Wang H, Wang J. The role of Rho GTPases in toxicity of Clostridium difficile toxins. Toxins. 2015;7(12):5254–5267. | ||
Cai A, Zhou Y, Li L. Rho-GTPase and atherosclerosis: pleiotropic effects of statins. J Am Heart Assoc. 2015;4(7):e002113. | ||
Bergman P, Linde C, Putsep K, et al. Studies on the antibacterial effects of statins – in vitro and in vivo. PloS one. 2011;6(8):e24394. | ||
Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195–2207. |
Supplementary material
  | Table S1 Search strategy |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.