Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Squamous Cell Carcinoma of the Nail Unit After Trauma: A Case Report
Received 11 July 2022
Accepted for publication 17 August 2022
Published 24 August 2022 Volume 2022:15 Pages 1737—1741
DOI https://doi.org/10.2147/CCID.S381877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Wen Xu, Dandan Mao, Guangdong Wen
Department of Dermatology, People’s Hospital of Peking University, Beijing, 100044, People’s Republic of China
Correspondence: Guangdong Wen, Department of Dermatology, People’s Hospital of Peking University, No. 11, Xizhimen South Street, Xicheng District, Beijing, 100044, People’s Republic of China, Tel +86 13301360712, Email [email protected]
Abstract: Squamous cell carcinoma of the nail unit (SCCNU) is a relatively uncommon tumor with a low rate of metastasis. SCCNU presents with nonspecific symptoms and signs, it is frequently misdiagnosed by dermatologists or surgeons. We report a patient with right-hand ring subungual squamous cell carcinoma who received inappropriate treatment due to a long-term misdiagnosis following trauma. This patient had been treated with acitretin combined with cefaclor, and a certain curative effect was seen, but finally, wide local excision was performed.
Keywords: squamous cell carcinoma, nail, acitretin
Case Report
A 68-year-old female presented with persistent swelling, recurrent exudation, and pain in her right ring finger after trauma three years ago. Once diagnosed with “paronychia”, she underwent nail extraction and debridement. Every time after the nail extraction treatment, the wound appeared purulent, accompanied by redness, swelling, and pain. Twenty months later, a dermatologist diagnosed the lesion as paronychia and treated it with nail avulsions repeatedly with no obvious alleviation. Eleven months ago, the patient was diagnosed with “ acrodermatitis continua of hallopeau”. Staphylococcus aureus showed positive, and acitretin combined with cefaclor was given. The affected nail plate gradually recovered and the pain was relieved. Three weeks ago, the patient felt that the growth of the nail plate stopped, the pus increased, and the nail plate thickened and turned white. The patient subsequently came to the Department of Dermatology, Peking University People’s Hospital. We considered the possibility of nail cancer and performed a nail biopsy for her. The patient’s diagnosis history flowchart can be seen in Figure 1.
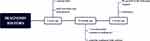 |
Figure 1 Flowchart of the patient’s diagnosis history. |
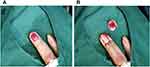
Physical examination: The right ring finger was partially swollen, and bloody exudation was seen at the edge of the nail bed. Partial separation of the deck, dark red blood scabs with purulent secretions can be seen at the separation, the deck thickens and turns white, with mild tenderness. (Figure 2) Superficial lymph nodes were not enlarged.
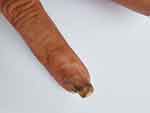 |
Figure 2 The ring finger of the right hand was partially swollen, the edge of the lesion was desquamated, and a large black scab under the nail and part of the nail plate were separated. |
Histological examinations (Figure 3): Hyperkeratosis of the superficial layer, irregular hyperplasia of the acanthus layer, the extension of the epithelium foot, partial squamous epitheliums are under-differentiated, with atypia, dyskeratotic cells can be seen, the atypical epitheliums show invasive growth, showing the appearance of squamous cell carcinoma.
 |
Figure 3 Histopathology of the lesion. (A and B) Biopsy shows atypical cells with altered nuclear cytoplasmic ratio and dyskeratotic cells. (3A HE×200/3B HE×400). |
Laboratory examinations: X-ray (Figure 4) showed degenerative changes in the right hand, and no obvious bone destruction was found in the fourth phalanx. B-ultrasound of superficial lymph nodes showed no obvious abnormality in axillary and supratrochlear lymph nodes. Positron emission tomography-computed tomography(PET-CT) scanning showed no sign of metastasis.
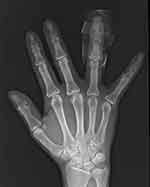 |
Figure 4 X-ray of right hand: No bone destruction. |
Final Diagnosis: Squamous cell carcinoma of the right ring nail unit.
Treatment and follow up: Wide local excision of skin malignant tumor. (Figure 5) At the 1-year follow-up, there was no sign of tumor recurrence.
Discussion
Squamous cell carcinoma of the nail unit (SCCNU) is the most common tumor of the hand and nail unit, causing 90% of all hand malignancies, but it is quite rare.1 Although SCCNU is considered a low-grade malignancy, it tends to invade the distal phalanx. Analysis of the cases surveyed revealed bone involvement in 50% of the cases.2 A delay in diagnosis and wrong treatment can result in local destruction and bone invasion. Treatment options include wide local excision, digital amputation, cryotherapy, topical modalities, and recently Mohs surgery.3
The incidence of SCCNU was approximately 0.0012% in-hospital patients and 0.028% in dermatology patients.4 It is more common in middle-aged men, and the peak age of onset is between 50 and 69 years old. The sex ratio of male to female incidence is approximately 2:1. SCCNU usually occurs only on the fingernails, especially the thumb (about 44%), and the index finger and middle finger of the dominant hand are other predisposing sites.4 SCCNU often presents with nonspecific features such as pain, destruction or discoloration of the nail plate, hyperkeratosis, erythema, ulceration, periungual or subungual mass, or paronychia. An onycholytic area with yellow discoloration of the overlying nail and frequent oozing are considered a late symptom.5 The disease takes a chronic, progressive course.5 Pain, swelling, and inflammation may suggest bone involvement.4 Clinically, it is often misdiagnosed as chronic paronychia, onychomycosis, pyogenic granuloma, parathyroid wart, glomus tumor, or enchondroma.6 Potential predisposing factors of SCCNU include chronic human papillomavirus (HPV) infection, contact to chemicals, trauma, radiation exposure, congenital conditions (eg, xeroderma pigmentosum, epidermodysplasia verruciformis), immunosuppression, and tobacco use.2 The patient in this case developed subungual squamous cell carcinoma in the ring finger after trauma, and the location was relatively rare. We believe that trauma is only an incentive, and repeated bacterial or viral infection after trauma is an important cause of nail cancer. Trauma caused the nail bed to lack the protection of the nail plate, making the nail bed more susceptible to pathogenic factors, such as virus, chemicals, and radiation.
The use of dermoscopy, a noninvasive method to better observe the nail unit, has been described as a useful instrument. Dermoscopy can help doctors better identify SCCNU. Alterations visible in SCCNU with dermoscopy are a nodular keratin mass with a white and amorphous central area, white keratin pearls, and hemorrhage associated with peripheral brown amorphous areas.7 However, none of these signs is diagnostic for the tumor.
Nail biopsy is the only way to diagnose SCCNU. Improper nail biopsy may damage the nail matrix and lead to the occurrence of nail dystrophy. It is recommended to use an open nail bed biopsy.8 Diagnosis is made by a skin biopsy deep enough to allow the pathologist to comment on depth of invasion, perineural or lymphovascular invasion, differentiation, and connection to the overlying epidermis.9 The pathological biopsy of SCCNU mainly showed atypical keratinocytes, exhibit distinct intracellular bridges, and possess hyperchromatic and pleomorphic nuclei with dyskeratosis and keratin pearls within the dermis.10 Koilocytosis, as observed in HPV-associated types, is missing.5 For patients with recurrent exudate and persistent non-healing after medication, the possibility of SCCNU should be considered, and the biopsy should be performed as soon as possible to confirm the diagnosis.
X-ray examination can evaluate for bone involvement, but the bone changes around the nail are difficult to explain because they may be due to inflammation and subungual tumors might cause compression against the periosteum.1 Since SCCNU is a low-grade tumor with a low metastatic rate, only clinical assessment of the supratrochlear and axillary lymph nodes as routine examination. Ultrasonography of lymph nodes may be performed when metastasis is suspected. A cytological needle aspirate can be performed of enlarged nodes.2 Generally, imaging is not required unless the clinical picture is suggestive of involvement of large-caliber nerves, muscle or bone, lymph node involvement, or when high-risk features are present.9
Surgical resection is the first choice for the treatment of SCCNU but requires comprehensive knowledge of the nail unit. Wide local excision can be performed when there is no bone involvement. When SCCNU does invade bone, amputation of the distal phalanx or disarticulation of the involved digit is indicated.1,11 If wide local excision is chosen, preferably with three-dimensional histological assessment or 5-mm surgical margins.5 Mohs surgery is also a great method for the treatment of SCCNU. Mohs surgery has advantages over amputation in terms of tissue sparing, digital function, appearance, and achievement of histologically clear margins. However, the recurrence rate is high and it is expensive.3 Radiation therapy is a potential treatment for patients with inoperable or multiple SCCNU, but one should be aware of its potential as a causative agent in SCCNU.2 It has been reported that acitretin alone, acitretin combined with 5-fluorouracil, and acitretin combined with clarithromycin can delay the progression of SCC and even achieve clinical remission.12 This patient had been treated with acitretin combined with cefaclor, and a certain curative effect was seen within 10 months, but the disease progressed later, and finally, wide local excision was performed. The potential possibility of acitretin in the treatment of SCCNU is still inconclusive, and further clinical studies are needed to confirm it.
Conclusion
Squamous cell carcinoma of the nail unit (SCCNU) is a relatively rare malignant tumor of the nail. One of reasons for a delay in the diagnosis is the painless and often asymptomatic presentation of this tumor, which keeps patients from seeking care. As a result, the tumor invades the lower phalanx, and eventually, only extended resection or even amputation is required. When we encounter patients with long-term repeated exudation, persistent non-healing of nail disease, and multiple treatments are ineffective, pathological biopsy should be performed as soon as possible to exclude SCCNU. After diagnosis, the treatment is decided according to the presence of bone invasion and lymph node metastasis.
Data Sharing Statement
Data is available on request due to privacy/ethical restrictions. The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Consent
The patient provided consent for publication of the manuscript and figures. The case details do not require institutional approval.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Fund program: Research and Development Foundation of Peking University People’s Hospital (RDY2020-15).
Disclosure
The authors declare no conflict of interest.
References
1. Peterson SR, Layton EG, Joseph AK. Squamous cell carcinoma of the nail unit with evidence of bony involvement: a multidisciplinary approach to resection and reconstruction. Dermatol surgery. 2004;30:218–221. doi:10.1111/j.1524-4725.2004.30068.x
2. Dijksterhuis A, Friedeman E, van der Heijden B. Squamous Cell Carcinoma of the Nail Unit: review of the Literature. J Hand Surg Am. 2018;43:374–379.e372. doi:10.1016/j.jhsa.2018.01.010
3. Dany M. Nail unit squamous cell carcinoma: updates on diagnosis, surgical approach, and the use of mohs micrographic surgery. Cutis. 2020;106:E11–e14. doi:10.12788/cutis.0122
4. Starace M, Alessandrini A, Dika E, et al. Squamous cell carcinoma of the nail unit. Dermatol Pract Concept. 2018;8:238–244. doi:10.5826/dpc.0803a017
5. Haneke E. Important malignant and new nail tumors. J Dtsch Dermatol Ges. 2017;15:367–386.
6. Meesiri S. Subungual squamous cell carcinoma masquerading as chronic common infection. J Med Assoc Thailand. 2010;93:248–251.
7. Mazzilli S, Cosio T, Diluvio L, et al. Dermoscopy and Reflectance Confocal Microscopy in the Diagnosis and Management of Nail Fold Squamous Cell Carcinoma. J Med Life. 2020;13:107–111. doi:10.25122/jml-2019-0129
8. Rtshiladze MA, Stretch JR, Stewart DA, et al. Pigmented lesions of the nail bed - Clinical assessment and biopsy. Aust Fam Physician. 2016;45:810–813.
9. Kim JYS, Kozlow JH, Mittal B, et al. Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78:560–578. doi:10.1016/j.jaad.2017.10.007
10. Lecerf P, Richert B, Theunis A, et al. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69:253–261. doi:10.1016/j.jaad.2013.02.008
11. Ruiz santiago H, Morales-Burgos A. Cryosurgery as adjuvant to Mohs micrographic surgery in the management of subungual squamous cell carcinoma. Dermatol surgery. 2011;37:256–258. doi:10.1111/j.1524-4725.2010.01860.x
12. Zhao Y, Zhu Y, Wang H, et al. Case Report: successful Treatment of Cutaneous Squamous Cell Carcinoma in Three Patients With a Combination of Acitretin and Clarithromycin. Front Oncol. 2021;11:650974. doi:10.3389/fonc.2021.650974
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.