Back to Journals » Patient Preference and Adherence » Volume 10
Spotlight on empagliflozin/metformin fixed-dose combination for the treatment of type 2 diabetes: a systematic review
Authors Kedia R, Kulkarni S, Ross M, Shivaswamy V
Received 20 April 2016
Accepted for publication 18 August 2016
Published 30 September 2016 Volume 2016:10 Pages 1999—2006
DOI https://doi.org/10.2147/PPA.S85748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Johnny Chen
Rohit Kedia,1 Supriya Kulkarni,1 Meredith Ross,1 Vijay Shivaswamy1,2
1Division of Diabetes, Endocrinology, and Metabolism, Department of Internal Medicine, University of Nebraska Medical Center, 2VA Nebraska-Western Iowa Health Care System, Omaha, NE, USA
Abstract: The dramatic rise in the prevalence of obesity and diabetes is associated with increased morbidity, mortality, and public health care costs worldwide. The need for new, effective, and long-lasting drugs is urgent. Recent research has focused on the role of the inhibitors of sodium–glucose co-transporter 2 (SGLT-2). Clinical trials have shown that SGLT-2 inhibitors have glycemic efficacy and weight-lowering potential. Dual drug therapy is a recommended therapy for patients with new-onset type 2 diabetes who need significant glycemic control. Fixed-dose combination therapy represents a particularly attractive option as it may reduce pill burden and improve adherence. The combination of metformin and empagliflozin was approved by the US Food and Drug Administration in 2014 and represents a safe and effective means to combat glycemic control and weight gain. The purpose of this systematic review is to summarize the background of the SGLT-2 inhibitors, particularly empagliflozin, and focus on the safety and efficacy of the fixed-dose combination of empagliflozin and metformin.
Keywords: diabetes mellitus, empagliflozin, metformin, hyperglycemia, systematic review
Introduction
The prevalence of diabetes mellitus type 2 (T2DM) is increasing at alarming rates and has become a national epidemic. It is estimated that 22 million Americans have diabetes and nearly 1.5 million new cases were reported in 2014.1 Current management begins with lifestyle modifications and metformin (MET) for initial management.2 A meta-analysis of the use of MET showed an average reduction in hemoglobin A1c (HgA1c) of 1.1%.3 Unfortunately, the majority of patients with type 2 diabetes will not achieve euglycemia on MET alone and will require additional diabetic medications.4 Current guidelines suggest initiating dual therapy using MET with one additional agent in patients with an HgA1c of 7.5%–9% and adding another medication to MET if the initial HgA1c is <7.5% and does not improve within 3 months.5,6 There have been new medications developed for T2DM; however, no scientific consensus has been made regarding the ideal second agent to start after MET, and there are ongoing trials to determine the ideal second-line agent.7
Sodium–glucose co-transporter 2 (SGLT-2) inhibitors are the newest class of medication that act by increasing glucose excretion in urine.8 Patients with T2DM benefit from this targeted action as they have a higher threshold for renal excretion of glucose and have upregulation of the expression of SGLT-2 compared to patients without T2DM.9 Empagliflozin (EMPA) is a potent and well-studied SGLT-2 inhibitor that was approved by the US Food and Drug Administration in 2014.9
The successful treatment of diabetes depends on medication adherence by patients, and previous studies have estimated the medication adherence to be 50%.10 Patient adherence with oral diabetic medications was estimated to be 67%–85% by prospective electronic monitoring in a review by Cramer.11 Decreased adherence to diabetic medications places patients at increased risk for hospitalizations as was found in a retrospective analysis where patients with diabetes who had <80% medication adherence were found to have increased risk of hospitalization within the next year.12 Balkrishnan et al13 demonstrated that medication nonadherence was found to be the greatest factor for increasing health care cost. In a study by Bangalore et al,14 a systematic review was done comparing adherence with fixed-dose combination versus single components of the drug given separately. The use of combination therapy demonstrated a 26% increased adherence rate compared to separately administered drug components. With respect to diabetes management, the most likely reason to cause patient dissatisfaction and alter adherence to medications is hypoglycemia.15
Methods
Data sources
Studies were identified by searching Medline and PubMed for randomized clinical trials.
Inclusion criteria
We included randomized controlled trials if they met the following criteria: 1) patients >18 years old who are diagnosed with T2DM; 2) comparison of EMPA and placebo or any other active comparator as add-on to MET, without background therapy; and 3) reporting all the following outcomes: a) HgA1c, b) body weight, c) systolic blood pressure, d) diastolic blood pressure, e) one or more adverse events, f) one or more serious adverse events, g) adverse events leading to discontinuation, h) hypoglycemic events, and i) events consistent with urinary tract and genital infections.
Exclusion criteria
Studies that reported nonhuman trials, nonrandomized trials, and EMPA monotherapy were excluded.
Mechanism of action, pharmacokinetics, and pharmacodynamics
EMPA and MET combination is a medication that contains two oral hypoglycemic agents with different mechanisms of action. EMPA lowers plasma glucose levels by inhibiting SGLT-2 in proximal tubules of nephron, thus augmenting renal excretion of glucose (Figure 1).8 Additional benefits include weight loss perhaps due to caloric loss and decrease in blood pressure due to the diuretic effect of glycosuria. The incidence of hypoglycemia is minimal due to insulin-independent mechanism of action.9 MET lowers plasma glucose levels by inhibiting gluconeogenesis in liver and reducing intestinal absorption of glucose, and it improves insulin sensitivity by increasing glucose uptake in peripheral tissues.16,17 Since MET does not cause insulin secretion, the risk of hypoglycemia is minimal.9 Efficacy results from several studies suggest that fixed-dose combinations of EMPA/MET are additive due to different mechanisms of action of the two drugs.18–20
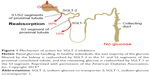  | Figure 1 Mechanism of action for SGLT-2 inhibitors. |
Empagliflozin
Pharmacokinetic studies in healthy volunteers have shown that after absorption, EMPA reaches peak plasma concentration in 1.5–2.1 hours, and mean half-life is 10–19 hours.21,22 Renal clearance ranges from 32.1 mL/min to 51.3 mL/min with half of the drug being excreted in the initial 24 hours.9 After being filtered in proximal tubule, it inhibits SGLT-2 and increases glucose excretion in a dose-dependent manner (inhibits 40% of glucose absorption at a dose of 10 mg and 60% at higher doses). SGLT-1 is mainly responsible for glucose absorption from the late proximal tubule and the gastrointestinal tract. The recommended starting dose is 10 mg/d, which can be increased to 25 mg/d.23,24 Food does not affect absorption of the drug, therefore making it convenient to be taken at any time of the day.9
Metformin
During fasting state, the bioavailability of MET is 50%–60%.24 Compared to fasting state, simultaneous food intake prolongs absorption (peak plasma concentration reached after 35 minutes).24 Apparent volume of distribution following a single dose of 850 mg MET tablet averages at 654±358 L.24 It is not extensively bound to plasma proteins, and steady-state concentrations are reached within 24–48 hours and are generally <1 mcg/mL.17,24 Intravenous administration of a single dose in normal subjects shows that 90% of the absorbed drug is excreted unchanged in urine in the first 24 hours.24 Renal clearance of MET is 3.5 times higher than creatinine clearance, indicating that tubular secretion is the main mode of excretion of the drug.25 It does not undergo hepatic metabolism. The half-life of MET is 14 hours.25
Efficacy of EMPA and MET
Multiple clinical trials have substantiated the efficacy and safety of EMPA as an add-on therapy to MET (Table 1).18–20,26–28 In a randomized, double-blind placebo-controlled trial by Haring et al,18 637 patients with uncontrolled T2DM who were already taking MET >1,500 mg/d were randomized to EMPA 10 mg/d or EMPA 25 mg/d or placebo for 24 weeks. In the placebo group, the mean reduction in HgA1c was −0.13% compared to a reduction of 0.7% and 0.77% in HgA1c in EMPA 10 mg and 25 mg groups, respectively. In this study, 37.7% of patients in EMPA 10 mg group and 38.7% of patients in EMPA 25 mg group reached target HgA1c level of <7.0% as compared to only 12.5% in the placebo group. In patients with HgA1c >10.0% at baseline, there was a reduction by 3.2% after 24 weeks of therapy with EMPA. Mean reductions in weight were 0.4 kg with placebo, 2.0 kg with EMPA 10 mg, and 2.4 kg with EMPA 25 mg. EMPA lowered systolic blood pressure in the MET plus EMPA 10 mg and 25 mg groups by 4.5 mmHg and 5.2 mmHg, respectively. There was a reduction in the placebo group of 0.4 mmHg.18
  | Table 1 Summary of efficacy in randomized clinical trials of EMPA combined with MET |
In another randomized double-blind placebo-controlled trial by Rosenstock et al,19 495 patients across 104 centers were treated with EMPA (1 mg, 5 mg, 10 mg, 25 mg, and 50 mg) in addition to MET, and comparison was made with a MET- and placebo-treated group for a 12-week period. There was reduction in HgA1c ranging from −0.1 to −0.56 among EMPA-treated groups. The MET plus placebo group had a 0.15% increase in HgA1c, compared to a reduction of 0.56% and 0.55% in the MET plus EMPA 10 mg and 25 mg groups, respectively.25 This study also included a sitagliptin 100 mg plus MET arm, which had a reduction of 0.45% in HgA1c. A total of 38.0% of patients receiving MET and EMPA 10 mg and 37.1% of patients receiving MET and EMPA 25 mg achieved HgA1c <7.0% at 12 weeks compared to 15.5% in placebo and MET group. Mean reduction in body weight for MET plus EMPA 10 mg and 25 mg was 2.7 kg and 2.6 kg, respectively. This was compared to the MET and sitagliptin group with a reduction of 0.8 kg. The reduction in systolic blood pressure was 4.39 mmHg and 8.51 mmHg in the MET plus EMPA 10 mg and 25 mg, respectively. This was compared to the MET and sitagliptin group with a reduction of 1.79 mmHg.19
In a randomized, 78-week open-label study by Ferrannini et al,26 efficacy of EMPA monotherapy was compared with MET alone, MET plus EMPA, and MET plus sitagliptin. Separate assessments were performed on the patients depending on whether they were treatment naïve or had been on a stable dose of MET immediate release of >1,500 mg/d or maximum tolerated dose for ≥10 weeks.26 The treatment-naïve patients were randomized to receive graduated dosing of EMPA (5 mg, 10 mg, or 25 mg) once daily, placebo, or open-label MET for 12 weeks. In the alternate study arm, patients who were already on a stable dose of MET were randomized to receive 1 mg, 5 mg, 10 mg, 25 mg, or 50 mg EMPA, placebo, or open-label sitagliptin 100 mg for 12 weeks. The results from patients already on background MET demonstrated a reduction of 0.34% and 0.63% in HgA1c from baseline in MET plus EMPA 10 mg and 25 mg, respectively. There was a reduction of 0.4% in the sitagliptin plus MET group. A total of 27.0% of patients on 10 mg EMPA and MET group and 44.6% of patients on 25 mg EMPA and MET group reached HgA1c of <7.0% at 78 weeks. Reduction in body weight ranged from −3.1 kg in EMPA 10 mg group to −4.0 kg in EMPA 25 mg group. There was a reduction in the systolic blood pressure of 3.3 mmHg and 3 mmHg in the EMPA 10 mg and 25 mg plus MET groups, respectively, compared to an increase of 1.8 mmHg in the MET plus sitagliptin 100 mg group.26
A head-to-head trial by Ridderstråle et al20 compared EMPA 25 mg to glimepiride (1–4 mg) as an add-on therapy to MET in patients uncontrolled on MET alone, with primary end points of the study being reduction in HgA1c at 52 weeks and 104 weeks. The study found that EMPA was noninferior to glimepiride at both 52 weeks and 104 weeks, and the adjusted mean difference in change from baseline in HbA1c was −0.11%, which determined superiority of EMPA as add-on therapy (P=0.015 for superiority) without causing significant hypoglycemia. Patients receiving EMPA and MET lost 3.1 kg at 104 weeks compared to the glimepiride group of patients who gained 1.3 kg. There was also a reduction in the mean systolic blood pressure of −3.1 mmHg in the EMPA plus MET group, as compared to a 2.5 mmHg rise in the glimepiride plus MET group.20 This study highlights that EMPA is superior to glimepiride as a second-line agent with additional benefits of weight loss and lower incidence of hypoglycemia.
In a study performed by Merker et al, patients were randomized to either 10 mg or 25 mg of EMPA or placebo as an add-on treatment to MET to evaluate changes in baseline HgA1c, weight, and blood pressure at 76 weeks. They performed an initial 24-week trial, followed by a double-blind extension trial, in which 463 of the initial 637 participants were included. At the end of the 76 weeks, the reduction in HgA1c with EMPA 10 mg and 25 mg was 0.6% and 0.7%, respectively. There was a 0% change with the placebo group. Weight reduction was seen in patients receiving EMPA 10 mg and 25 mg in addition to MET of 2.4 kg and 2.7 kg, respectively, compared to a reduction of 0.5 kg in the MET and placebo groups. Decrease in systolic blood pressure was seen in patients receiving EMPA 10 mg and 25 mg in addition to MET of 5.2 mmHg and 4.5 mmHg, respectively, compared to a reduction of 0.8 mmHg in patients receiving MET and placebo.27
A randomized trial performed by DeFronzo et al28 compared EMPA 10 mg or 25 mg with linagliptin 5 mg as an add-on therapy to MET. At the end of the 24-week trial, linagliptin plus MET had reduced the HgA1c by 0.7% compared to reductions with EMPA of 0.66% and 0.62% for 10 mg and 25 mg, respectively. The linagliptin plus MET group had a 0.3 kg weight reduction from baseline compared to EMPA, where there was a 2.9 kg and 2.8 kg weight reduction in the 10 mg and 25 mg groups, respectively. There were reductions in systolic blood pressure of 3.5 mmHg and 2.8 mmHg in the groups receiving EMPA 10 mg and 25 mg in addition to MET compared to an increase of 0.3 mmHg in the group receiving MET and linagliptin 5 mg.28
Multiple clinical trials conclude that EMPA is an effective add-on medication to MET and causes significant improvement in HgA1c, weight, and blood pressure without increasing the risk of hypoglycemia. Its efficacy is comparable to other oral antidiabetic medications such as sitagliptin and linagliptin and was found to be superior to glimepiride with respect to glycemic control.
Cardiovascular outcomes of EMPA
Type 2 diabetes is a major risk factor for cardiovascular disease, and demonstrating safety with new diabetic medications with cardiovascular outcomes is required by the US Food and Drug Administration. MET is thought to have beneficial effects on cardiovascular outcomes.29–32 The EMPRA-REG OUTCOME analyzed cardiovascular risk in patients who are receiving EMPA.33 This study was a randomized, controlled, double-blind study of 7,020 patients with established cardiovascular disease who received either placebo or 10 mg or 25 mg of EMPA with a mean monitoring of 3.1 years. Patients were randomized to add either placebo or EMPA to their own background glucose-lowering therapy. The placebo group contained 112 patients on MET, while the EMPA group had 172 patients on MET. This study defined cardiovascular events as a three-point major adverse cardiac event (MACE), which is defined as the time to first occurrence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke, and as a secondary outcome of four-point MACE, which was defined as the primary outcome plus hospitalization for unstable angina. The primary outcome occurred in greater proportion in patients who received placebo versus patients receiving EMPA (P=0.04 for superiority) and patients who received EMPA had lower rates of death from cardiovascular causes compared to the placebo group (P<0.001) and had a 38% relative risk reduction of death. Hospitalizations for heart failure and death from any cause were also lower in patients receiving EMPA. Studies are needed to elucidate the mechanisms for superior cardiovascular outcomes in patients receiving EMPA and to follow as to whether these outcomes also pertain to other SGLT-2 inhibitors.
Safety of EMPA and MET
In all the clinical trials, EMPA was overall well tolerated (Table 2). The percentage of subjects reporting at least one adverse event was equivalent among EMPA and placebo groups with the majority of events being mild to moderate in intensity. Deleterious side effects requiring discontinuation of therapy occurred in <7% of cases across all the studies.18–20,26–28 Increased occurrence of urinary tract infections was reported in EMPA-treated groups, but a vast majority of these events were mild to moderate in intensity. Importantly, the majority of the urinary tract infections occurred in females. The incidence of pyelonephritis was <1%.18–20,26–28 In EMPA groups, there were increased reports of uncomplicated genital infections ranging from 0% to 9.9%.18–20,26–28 Hypoglycemia has a significant effect on quality of life and is a major contributing factor to nonadherence, morbidity, and mortality in patients with T2DM. The incidence of hypoglycemic episodes was <5% in EMPA groups as compared to placebo across all studies.18–20,26–28 Increased hypoglycemic events were reported in the glimepiride- and MET-treated group (24%) as compared to the EMPA- and MET-treated group (2%).20 Modest increases in total cholesterol, high-density lipoprotein, and low-density lipoprotein levels were observed in EMPA groups, but their clinical relevance is minimal. EMPA caused mild increase in hematocrit levels ranging from 0.6% to 2.7% across the studies.18–20,26–28 This is probably due to the diuretic effect of enhanced glycosuria. Minor reductions in estimated glomerular filtration rate (eGFR) were observed in the EMPA-treated group as compared to baseline. However, these changes were small and comparable across all treatment groups including placebo.
  | Table 2 Summary of side effects in randomized clinical trials of EMPA combined with MET |
Available data about EMPA show that there is no significant increase in the incidence of bone fractures. When comparing EMPA to glimepiride, they were both found to have an incidence of fracture of 2%.20 In the EMPRA REG Trial, the incidence of bone fracture in the placebo group (3.9%) was comparable to the pooled EMPA group (3.8%).33
There is concern about the development of euglycemic ketosis in patients taking SGLT-2 inhibitors. In a retrospective analysis of Phase II and III trials of EMPA involving >1,300 patients, there were eight events consistent with ketoacidosis, of which three events were with EMPA and the remaining five events with placebo group.34 The EMPRA-REG OUTCOME Trial of >7,000 patients found that the incidence of euglycemic ketosis was <0.1%.33 Although the incidence is minimal, patients who experience nausea and vomiting or develop metabolic acidosis when receiving EMPA and MET combination should be assessed immediately, and immediate discontinuation of the drug is recommended along with hospitalization of the patient.35
Use of the combination of EMPA and MET in specific populations
Renal impairment
No pharmacokinetic studies have been performed using the combination pill of EMPA and MET in patients with chronic kidney disease. As eGFR declines, there is a reduction in clearance of EMPA. Dose adjustment is not required in patients with mild renal impairment (eGFR >45 mL/min), but therapy is contraindicated once eGFR falls <45 mL/min.23,24 MET has had classic contraindication in males with serum creatinine >1.5 mg/dL and in females with >1.4 mg/dL.24,36 New recommendations were updated in April 2016 that stated that there is a contraindication in the use of MET with the eGFR <30 mL/min/1.73 m2 and not to start MET if the eGFR is between 30 mL/min/1.73 m2 and 45 mL/min/1.73 m2. For patients already taking MET who have reduction of eGFR to <45 mL/min/1.73 m2, the risks and benefits of MET need to be reviewed by the provider. In addition, MET should be discontinued prior to or at the time of a procedure that involves iodinated contrast in patients with an eGFR between 30 mL/min/1.73 m2 and 60 mL/min/1.73 m2; the eGFR should then be reevaluated 48 hours after the procedure and if it remains at baseline, MET should be restarted.37
Hepatic impairment
Dose changes are not required for EMPA in patients with hepatic impairment.24,36 EMPA is mostly eliminated in feces (41.2%) or urine (54.4%), while 90% of the absorbed MET is excreted unchanged in urine in the first 24 hours.24 However, there is a black box warning for MET that it can cause lactic acidosis from MET accumulation.17,24 An increased risk of lactic acidosis is seen in conditions of: renal and hepatic impairment, acute congestive heart failure, sepsis, dehydration, and excess alcohol intake. If acidosis is suspected, then immediate discontinuation of the drug is recommended along with hospitalization of the patient.24 The combination of MET and SGLT-2 inhibitors should be avoided in patients with hepatic impairment.36
Geriatric
There is no recommendation to change the dose of EMPA and MET combination based on age alone.24 Increased age does not have an impact of pharmacokinetics of EMPA.38 Adverse effects of EMPA such as volume depletion and the risk of urinary tract infections are more frequent in patients >75 years of age.24 In addition, the clearance of MET is decreased in elderly subjects as a result of age-related decline in eGFR and warrants renal function monitoring.24
Pregnancy
The use of combination of MET and EMPA in pregnancy has a pregnancy category C risk, which indicates that there are no human studies in pregnant women with EMPA and animal studies have shown an adverse effect. EMPA may affect fetal kidney development and maturation.24 Manufacturer’s data recommend informing female patients of childbearing age that the drug has not been studied in pregnancy in humans and should only be used in pregnancy if the potential benefit outweighs the risk to the fetus.24 The use of MET has a pregnancy category B risk. There has not been evidence to suggest harm to the fetus; however, MET does cross into the placenta. The use of MET is an option for glycemic control in both T2DM and gestational DM.39
Lactation
There are no current clinical studies that have been done to our knowledge to investigate if EMPA is excreted in breast milk.24 MET is excreted in breast milk at low levels and does not appear to have adverse effects on infant growth.39
Pediatric use
There is no current clinical information regarding the use of EMPA and MET in patients who are <18 years of age.24
Drug interactions
EMPA does not interact with cytochrome P450 isoforms. Thus, EMPA does not affect concomitantly administered drugs that are substrates of the major CYP450 isoforms. In healthy volunteers, EMPA did not interact with other antidiabetic medications (MET, sitagliptin, linagliptin, glimepiride, pioglitazone), simvastatin, antihypertensive medications (hydrochlorothiazide, toresmide, verapamil, ramipril), warfarin, digoxin, and oral contraceptive pills.24 MET can potentially interact with cationic-like amiloride, triamterene, digoxin, morphine, ranitidine, and vancomycin that are excreted by tubular secretion.17 Cautious use of carbonic anhydrase inhibitors such as zonisamide, acetazolamide, and topiramate is recommended because they can induce metabolic acidosis.17,40
Conclusion
EMPA is a potent SGLT-2 inhibitor and can be combined with MET to provide dual medications in a once-daily tablet. MET and EMPA combination has been shown through clinical studies to improve long-term HgA1c when compared to MET alone. When comparing EMPA as an add-on treatment to MET, it is found to be superior to glimepiride and similar in efficacy to linagliptin and sitagliptin. The use of EMPA has shown to yield superior cardiovascular outcomes in patients with a history of cardiovascular disease. This combination provides patients a once-daily regimen that is well tolerated with minimal hypoglycemia. The use of combination therapy will likely decrease pill burden, simplify the diabetes regimen, and improve adherence rate compared to single-component medications. This may lead to improved clinical outcomes and cost savings. In addition to glycemic control, EMPA and MET may lead to weight loss and improved blood pressure control, which will improve the patients’ overall clinical condition.
Acknowledgments
This work was done through the VA Nebraska-Western Iowa Health Care System and is based upon work supported with resources and use of facilities at the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development and Biomedical Laboratory Research and Development. The contents of this review do not represent the views of the Department of Veterans Affairs or the United States Government.
Disclosure
The authors report no conflicts of interest in this work.
References
CDC [webpage on the Internet]. Diabetes Public Health Resource. Available from: http://www.cdc.gov/diabetes/statistics/prevalence_national.htm. Accessed June 17, 2016. | ||
American Diabetes Association. 7 approaches to glycemic treatment. Diabetes Care. 2016;39(suppl 1):S52–S59. | ||
Hirst JA, Farmer AJ, Ali R, Roberts NW, Stevens RJ. Quantifying the effect of metformin treatment and dose on glycemic control. Diabetes Care. 2012;35(2):446–454. | ||
Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. | ||
American Diabetes Association. 5. glycemic targets. Diabetes Care. 2016;39(suppl 1):S39–S46. | ||
Garber AJ, Abrahamson MJ, Barzilay JI, et al. Aace/Ace comprehensive diabetes management algorithm 2015. Endocr Pract. 2015;21(4):438–447. | ||
Nathan DM, Buse JB, Kahn SE, et al; GRADE Study Research Group. Rationale and design of the glycemia reduction approaches in diabetes: a comparative effectiveness study (GRADE). Diabetes Care. 2013;36(8):2254–2261. | ||
Chao ECEC. SGLT-2 inhibitors: a new mechanism for glycemic control. Clin Diabetes. 2014;32(1):4–11. | ||
Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. | ||
CDC. Medication Adherence. Available from: https://www.cdc.gov/primarycare/materials/medication/docs/medication-adherence-01ccd.pdf. Accessed June 17, 2016. | ||
Cramer J. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224. | ||
Lau D, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalization among individuals with type 2 diabetes. Diabetes Care. 2004;27(9):2149–2153. | ||
Balkrishnan R, Rajagopalan R, Camacho FT, Huston SA, Murray FT, Anderson RT. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther. 2003;25(11):2958–2971. | ||
Bangalore S, Kamalakkannan G, Parkar S, Messerli FH. Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med. 2007;120(8):713–719. | ||
Lopez JM, Annunziata K, Bailey RA, Rupnow MFT, Morisky DE. Impact of hypoglycemia on patients with type 2 diabetes mellitus and their quality of life, work productivity, and medication adherence. Patient Prefer Adherence. 2014;8:683–692. | ||
Hundal RS, Krssak M, Dufour S, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49(12):2063–2069. | ||
Powers AC, D’Alessio D. Endocrine Pancreas and Pharmacotherapy of Diabetes Mellitus and Hypoglycemia. In: Brunton LL, Chabner BA, Knollmann BC. eds. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12e. New York, NY: McGraw-Hill; 2011. | ||
Haring HU, Merker L, Seewaldt-Becker E, et al. Empagliflozin as add-on to metformin in patients with type 2 diabetes: a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Care. 2014;37(6):1650–1659. | ||
Rosenstock J, Seman LJ, Jelaska A, et al. Efficacy and safety of empagliflozin, a sodium glucose cotransporter 2 (SGLT2) inhibitor, as add-on to metformin in type 2 diabetes with mild hyperglycaemia. Diabetes Obes Metab. 2013;15(12):1154–1160. | ||
Ridderstråle M, Andersen KR, Zeller C, et al. EMPA-REG H2H-SU Trial Investigators. Comparison of empagliflozin and glimepiride as add-on to metformin in patients with type 2 diabetes: a 104-week randomised, active-controlled, double-blind, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2(9):691–700. | ||
Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–142. | ||
Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(7):613–621. | ||
Vivian EM. Sodium-glucose co-transporter 2 (SGLT2) inhibitors: a growing class of antidiabetic agents. Drugs Context. 2014;3:212264. | ||
Synjardy® (empagliflozin and metformin hydrochloride tablets) [package insert]. Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc; 2015. | ||
Kinaan M, Ding H, Triggle CR. Metformin: an old drug for the treatment of diabetes but a new drug for the protection of the endothelium. Med Princ Pract. 2015;24(5):401–415. | ||
Ferrannini E, Berk A, Hantel S, et al. Long-term safety and efficacy of empagliflozin, sitagliptin, and metformin: an active-controlled, parallel-group, randomized, 78-week open-label extension study in patients with type 2 diabetes. Diabetes Care. 2013;12:4015–4021. | ||
Merker L, Haring HU, Christiansen AV, et al. Empagliflozin as add-on to metformin in people with type 2 diabetes. Diabet Med. 2015;32(12):1555–1567. | ||
DeFronzo RA, Lewin A, Patel S, et al. Combination of empagliflozin and linagliptin as second-line therapy in subjects with type 2 diabetes inadequately controlled on metformin. Diabetes Care. 2015;38(3):384–393. | ||
Jorgensen CH, Gislason GH, Andersson C, et al. Effects of oral glucose-lowering drugs on long term outcomes in patients with diabetes mellitus following myocardial infarction not treated with emergent percutaneous coronary intervention – a retrospective nationwide cohort study. Cardiovasc Diabetol. 2010;9:54. | ||
Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK prospective diabetes study (UKPDS) group. Lancet. 1998;352(9131):854–865. | ||
Hong J, Zhang Y, Lai S, et al; SPREAD-DIMCAD Investigators. Effects of metformin versus glipizide on cardiovascular outcomes in patients with type 2 diabetes and coronary artery disease. Diabetes Care. 2013;36(5):1304–1311. | ||
Schramm TK, Gislason GH, Vaag A, et al. Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J. 2011;32(15):1900–1908. | ||
Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. | ||
Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care. 2015;38(9):1638–1642. | ||
Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care. 2015;38(9):1687–1693. | ||
Empagliflozin/metformin (synjardy) for type 2 diabetes. Med Lett Drugs Ther. 2015;57(1484):172–174. | ||
FDA [webpage on the Internet]. Available from: http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm494829.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery. Accessed April 17, 2016. | ||
Riggs MM, Staab A, Seman L, et al. Population pharmacokinetics of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with type 2 diabetes. J Clin Pharmacol. 2013;53(10):1028–1038. | ||
Blumer I, Hadar E, Hadden DR, et al. Diabetes and pregnancy: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(11):4227–4249. | ||
May M, Schindler C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther Adv Endocrinol Metab. 2016;7(2):69–83. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
