Back to Journals » Journal of Asthma and Allergy » Volume 14
Spontaneous Pneumorachis – A Case-Based Review
Authors Alampoondi Venkataramanan SV , George L , Sahu KK
Received 29 June 2021
Accepted for publication 17 November 2021
Published 18 December 2021 Volume 2021:14 Pages 1539—1554
DOI https://doi.org/10.2147/JAA.S325293
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Sai Vikram Alampoondi Venkataramanan,1 Lovin George,1 Kamal Kant Sahu2
1Department of Medicine, Saint Vincent Hospital, Worcester, MA, USA; 2Department of Hematology and Medical Oncology, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA
Correspondence: Kamal Kant Sahu
Division of Hematology and Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA
Tel +1 8015874286
Email [email protected]
Abstract: Pneumorachis is characterized by the presence of free air in the spinal canal. It is referred by different names in literature such as epidural emphysema, intraspinal air, intraspinal pneumoc(o)ele, spinal epidural and subarachnoid pneumatosis, spinal and epidural emphysema, aerorachia, pneumosaccus, air myelogram, etc. Pneumorachis can be broadly classified as traumatic, iatrogenic, or spontaneous. In this case-based review, we present a case of spontaneous pneumorachis secondary to asthma exacerbation. This is followed by a systematic review of all cases of spontaneous pneumorachis identified in PubMed. The aim of this review is to understand the pathophysiology, common causes and the management of spontaneous pneumorachis.
Keywords: pneumorachis, spontaneous pneumorachis, marijuana smoking, asthma exacerbation
Introduction
Pneumorachis is a clinical condition characterized by the presence of free air in the spinal canal.1 The presence of air in the cranial cavity, termed pneumocephalus, is a well-known clinical entity associated with skull fractures; on the other hand, pneumorachis is relatively rare. As early as 1918, air was utilized as a contrast medium for neuroimaging in a technique known as air myelogram. With the advent of less toxic contrast agents, the practice slowly died out. The first case of non-iatrogenic free air in the spinal canal (cervical) was reported in 1977 in a 20-year-old male who sustained multiple skull fractures during a motor vehicle accident. The clinical entity was initially described as “traumatic air myelogram.30” The current term pneumorachis was coined by Newbold et al in 1987.31 The condition is rare, and an accurate incidence rate is not available. As per our review, 48 case reports of spontaneous pneumorachis in adults have so far been published in the literature.
 |
 |
 |
 |
 |
 |
Table 1 Recent Cases of Spontaneous Pneumorachis and Management Reported in Literature |
Based on the location of the air within the spinal cord, pneumorachis can be classified as intradural or extradural.90 Extradural pneumorachis is more common and is usually benign. Intradural pneumorachis, on the other hand, is indicative of major trauma. While neurological deficits are rarely associated with pneumorachis, it is more commonly associated with intradural pneumorachis even though there are a few case reports of extradural pneumorachis causing nerve root compression or cauda equina syndrome requiring decompression.90,91
Pneumorachis occurs most commonly due to either trauma or as a sequela of procedures involving the spinal canal.2 Pneumorachis is termed spontaneous when it occurs in the absence of trauma or iatrogenic insult.3 Spontaneous pneumorachis is extremely rare, and only a few cases have been reported in the literature.4 Many of them have been reported in conjunction with asthma exacerbation, but there are no reports of marijuana-induced asthma exacerbation leading to spontaneous pneumorachis. The use of marijuana both for recreational and medicinal purposes is on the rise and the majority of current users smoke marijuana even when using it for medicinal purposes.92 Smoking marijuana is often associated with coughing paroxysms and is a potent trigger for asthma exacerbations making its use ideal for causing pneumorachis. With the increasing popularity of smoking marijuana and the widespread use of computerised tomography as a routine imaging modality, we believe clinicians are going to encounter spontaneous pneumorachis much more frequently in the future. In this article, we present a case of spontaneous pneumorachis and do a systematic review of spontaneous pneumorachis. Our goal is to consolidate the current understanding of this clinical entity so as to aid physicians to make the best informed clinical decisions.
Case Report
A 21-year-old male with a history of mild intermittent asthma presented with productive cough, sore throat, and neck pain for one day. The patient was smoking marijuana with his friends when he developed a sore throat, a severe bout of coughing with yellowish expectoration, post-tussive emesis, shortness of breath, and wheezing. He noticed a sharp, persistent pain starting in his neck and spreading to his anterior chest following his coughing paroxysm. He felt a sensation of swelling on his neck and could feel a crunching sensation when he touched it. He was not taking any home medications. He endorsed smoking a joint of marijuana daily and drinking 200 mL of hard liquor on weekends over the last 3–4 years.
On presentation, his blood pressure was 147/74 mm Hg, heart rate was 103 beats per minute, respiratory rate was 18 per minute, temperature was 98.7 ℉, and he was saturating 94% on room air. His physical examination revealed extensive subcutaneous crepitus extending from his neck to the anterior chest wall. Auscultation of the lungs revealed diffuse expiratory wheezes with scattered rhonchi. Neurological examination did not show any motor, sensory or cranial nerve deficits.
 |
Box 1 List of Laboratory Investigations |
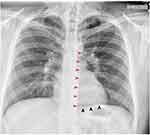
Lab investigations are shown in Box 1. EKG revealed normal sinus rhythm. A chest X-ray showed pneumomediastinum along the left cardiac border and over the cardiomediastinal silhouette anteriorly with extensive subcutaneous emphysema in the neck’s soft tissues (Figures 1 and 2). A chest CT showed pneumopericardium and pneumomediastinum with air dissecting throughout the mediastinum, into the neck, down into the spinal canal, posterior paraspinal musculature as well as adjacent chest wall musculature (Figures 3 and 4). Air in the spinal canal extended from C1 down to the T9 level. There was anterior midline pneumothorax extending from side to side, outlining the anterior and medial margins of the lungs. A transthoracic echo (TTE) showed a small amount of pneumopericardium.
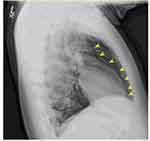 |
Figure 2 Chest X-ray (lateral view) demonstrating lucency (Yellow arrows) overlying the heart signifying pneumopericardium. |
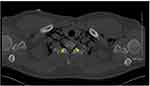 |
Figure 3 CT imaging demonstrating dissection of fascial planes in neck and invasion of trapped air into the spinal canal (yellow arrows) via intervertebral foramen. |
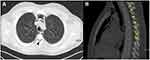 |
Figure 4 Pneumorachis demonstrated in axial (black arrow) (A) and sagittal sections (B) of thoracic CT imaging (yellow arrow). |
There was no clinical or radiological evidence of tension pneumothorax. Since the patient was not in any respiratory distress, he was initiated on the treatment of asthma exacerbation with intravenous methylprednisolone 80 mg every 8 hours, budesonide nebulizers twice daily, albuterol nebulizers as needed, and oxygen through a nasal cannula. Daily chest X-rays and continuous pulse oximetry were obtained to monitor the pneumorachis. Chest X-ray on day 2 of hospitalization showed progression of subcutaneous emphysema.
On day 3 of hospitalization, the patient reported significant improvement in his symptoms. On physical examination, his wheezing and subcutaneous crepitus had resolved. Chest X-ray also showed resolution of subcutaneous emphysema and mild improvement in pneumomediastinum. His oxygen supplementation was weaned off. He was prescribed an albuterol inhaler as needed and discharged home. He was counseled on smoking cessation and educated on avoiding activities that increase intrathoracic pressure.
Systematic Review
Search Strategy
We utilized PubMed’s search index for our systematic review. We used the search terms “pneumorachis“ OR ”spontaneous pneumorachis” under all fields to search for case reports and articles on spontaneous pneumorachis. All articles mentioned in PubMed, article bibliographies, and meeting abstracts until March 2021 were analyzed individually. Two hundred and two articles were identified, which were screened with inclusion criteria. Based on the selection criteria, 76 articles related to spontaneous pneumorachis were found, while 126 articles related to iatrogenic/traumatic pneumorachis were excluded. Of these, eight articles were written in a non-English language, two articles were inaccessible, and 18 articles on pediatric patients were excluded from the review. Forty-eight articles were included for final review and analysis, published over the past 40 years (1981-April 2021). The STrengthening, the Reporting of Observational studies in Epidemiology (STROBE) diagram, mentions in detail the steps of screening and scrutinizing the various articles during the selection process of the articles for this review (Figure 5).
 |
Figure 5 STROBE diagram depicting the selection process stepwise during the literature search for articles on nonspontaneous pneumorachis. |
Selection and Inclusion Criteria
The literature search was done by two independent and trained researchers. Abstracts of all the articles were screened to ensure appropriateness. Articles included were cases involving humans and published in the English language only. We excluded the articles that included pediatric cases (18 cases) and published in a language other than English (8 cases). Hence, 48 articles detailing 49 cases published from 1994 till March 2021 were included in the final interpretation and discussion (Figure 5).
Data Extraction
All selected articles were thoroughly studied: data were extracted and entered in a predefined table in a Word version 16.17 (Microsoft Corp, Redmond, WA) spreadsheet. Details on demographic characteristics, clinical features, comorbidities, management (antibiotics, surgery, oxygen supplementation), need for mechanical ventilation, and the outcome was extracted from each article.
Results
Patient Characteristics
There were 49 cases in 48 case reports (the case report by Martins et al had 2 cases).5 There were eight females, 40 males, and the sex of one patient was unknown. There were 12 cases between the ages of 18 to 20, 13 cases between the ages of 21 to 30, 4 cases between the ages of 31 to 40, 18 cases beyond the age of 40, and the age of 2 cases were unknown. There were no comorbidities in 25 patients. Asthma and diabetes mellitus were the most common comorbidities present in 7 patients each.6 There were no comorbidities in 24 patients, the comorbidities of 4 patients were unknown, and adenocarcinoma of the colon was present in 2 patients. Depression, anorexia nervosa, bullous lung disease, multiple sclerosis, Pancoast tumor, and diverticulitis were present in 1 patient each (Table 1).
The trigger was unknown in 7 patients, upper respiratory tract infection/bronchitis/common cold in 6 cases, asthma exacerbation in 5 cases, emphysematous pyelonephritis in 3 patients, cocaine use in 3 patients, vomiting caused by diabetic ketoacidosis in 3 patients, an acute bout of dry cough in 2 cases and vacuum intervertebral disc in 2 cases.7–9 Other causes include Valsalva maneuver, choking, ketamine sniffing, strenuous exercise, weightlifting, entero dural fistula, mucous plugging, pneumocystis pneumonia, spontaneous rupture of the bulla, bronchopleural dura subarachnoid fistula, bouts of self-induced vomiting in anorexia nervosa, vomiting induced by meningitis, transsacral cerebrospinal fluid leak, enterocutaneous fistula from the left natal cleft, and anterolisthesis of L5 over S1 in 1 patient each.10–12
Other Sites of Involvement
The spinal cord was the only site of air accumulation in 5 cases. Concomitant pneumomediastinum was reported in 30 cases, subcutaneous emphysema was reported in 33 cases, pneumothorax in 8 cases, pneumocephalus in 6 cases, kidney in 3 cases, deep spaces of the neck in 2 cases, and right iliopsoas muscle in 1 case (Figure 6).
 |
Figure 6 Various sites of air leaks in addition to pneumorachis in our study cohort. |
Management Strategies
The management was supportive in most of the cases, except for a few cases. The supportive treatment included a combination of oxygen supplementation, analgesics, and cough suppressants. Only three patients required intubation and mechanical ventilation. It was unknown whether an intubation was required in 2 patients. Patients with asthma exacerbation were treated with steroids and nebulizers. Navriya et al report that their patient with emphysematous pyelonephritis was managed with double J stenting.13 The patient in Gomez et al’s case report required vasopressor and broad-spectrum antibiotics for emphysematous pyelonephritis, as did the patient reported by Yamamoto et al14,15 Schomig et al had to do a lumbar puncture followed by repair of enterodural fistula for the treatment of pneumorachis caused by enterodural fistula.16 Mahajan et al’s patient required bronchoscopic removal of mucous plugging, while another patient of Saleem et al required bronchoscopy to diagnose pneumocystis pneumonia.17,18 Fonseca et al also performed a bronchoscopy, although the reason for the procedure is unclear.19 A total of 3 patients required chest tubes for the concomitant pneumothorax, following which pneumorachis resolved. Krishnan et al performed subcutaneous incisions to help relieve the trapped mediastinal air, subsequently leading to the resolution of pneumomediastinum.20 Lumbosacral laminectomy with duraplasty was employed by Iacoangeli et al for management of pneumorachis secondary to transacral cerebrospinal fluid leak caused by adenocarcinoma of the colon.21 C7 total laminectomy (Song et al),82 otolaryngological exploration (Oertel et al.),77 Hartman’s procedure (Amit et al), spondylolisthesis correction surgery (Kumaran et al), left partial hemilaminectomy (Lee et al), and pelvic exenteration (Tafreshi et al) were some of the other procedures employed by authors for management of the inciting factor for the pneumorachis.22–27
Outcome
The outcome was unknown in 2 cases, one patient died of sepsis, and one patient died three months after being discharged due to cancer progression.28,29 The outcome was positive in 45 out of the 49 cases, with the patients having either resolution of the pneumorachis or being discharged home.
Discussion
Pneumorachis can be classified based on etiology as traumatic, iatrogenic, or spontaneous.32 A 2010 systematic review of pneumorachis identified trauma to the respiratory tract as the causative factor in 52% of cases. Traumatic injury to the skull (especially skull base and sinus fractures) was the second most common cause.33 Iatrogenic causes include lumbar puncture, epidural analgesia, spinal surgeries, and abdominal surgeries.34,35 Epidural abscesses due to anaerobic pathogens can cause the air in the epidural space, especially in patients who have diabetes or Crohn’s disease.36 Intra-abdominal infections (especially emphysematous pyelonephritis) with anaerobic/facultative anaerobic bacteria such as Citrobacter koseri can also generate pneumoperitoneum with subsequent invasion of air into the spinal canal.15 Spontaneous pneumorachis can occur as a result of respiratory conditions and complications that incite barotrauma.37,38 Asthma is a common, chronic respiratory disease affecting 8% of adults in the U.S.A, and some of the rare but potentially life-threatening complications of an asthma exacerbation include pneumothorax and pneumomediastinum pneumopericardium, pneumorachis, tracheoesophageal fistula, and anoxic brain injury.39–41 Asthma was identified as the most common trigger for spontaneous pneumorachis in our review.42 Rarely, spontaneous extradural pneumorachis can develop as a complication of severe degenerative disc disease. Intervertebral disc vacuum phenomenon is a finding present in 1–2% of spinal radiographs and has an estimated prevalence of 20% in elderly population.93 Enlargement of this clefts in the intervertebral disc can cause a negative pressure gradient pulling gases into the space. The disk fragment containing gas could herniate into the spinal canal or the gas could get expelled into the epidural space through a weak spot in the annulus fibrosis, occasionally causing clinical presentation akin to a herniated disc.
Clinical Anatomy
Due to continuity between the cranial cavity and spinal canal, it is expected that cranial injuries communicating with the external atmosphere could cause pneumocephalus and consequent pneumorachis. However, as mentioned above, respiratory tract injuries account for most cases of pneumorachis. Defining the relations between the respiratory tract, mediastinum, and vertebral column is key to understanding the mechanism of spontaneous pneumorachis.
The mediastinum is the space within the thoracic cavity bordered superiorly by the thoracic inlet, inferiorly by the diaphragm, and excludes the lungs and pleural cavities. Within the mediastinum, the air is usually seen in the trachea, bronchi, bronchioles, alveoli, and esophagus, all of which are a potential source for pneumomediastinum and pneumorachis. Being the thinnest portion within the respiratory tree, alveolar rupture under conditions of the high trans alveolar pressure gradient is possibly the most common source of free air. If the rupture involves the visceral pleura, the patient will develop a pneumothorax. The air could also escape into the pulmonary interstitium from where it tracks along the bronchovascular bundle to reach the hilum from where it escapes into the mediastinum (Macklin effect).43 From here, air can dissect into the posterior mediastinum and separates the parietal pleura from the vertebral column gaining access into the epidural space via the intervertebral foramen.44 In the absence of any fascial barriers between the posterior mediastinum and epidural space, air can freely communicate between these compartments.45
There is no direct communication between the mediastinum and the pericardial cavity. Pneumopericardium typically occurs due to macro perforation of the pericardium and communication with the respiratory or GI tract in the setting of trauma, pneumothorax, volutrauma due to mechanical ventilation, fistulas in the setting of malignancies, etc.46 Laparoscopic surgeries could occasionally cause pneumopericardium. This is attributed to the presence of congenital patent pathways between pleura, pericardium, and peritoneal cavities. Our patient did not have a pneumothorax or pneumoperitoneum; hence, it is likely that the large volume of air was able to push into the pericardial space near the pulmonary vein Ostia where the parietal pericardium reflects onto the visceral pericardium creating the weakest spot.43
Pathophysiology
Literature review revealed one case of pneumorachis associated with cannabis use which was attributed to using a homemade bong with flow restricted intake, which forced the patient to inhale forcibly against high resistance, in turn creating a high negative intrathoracic pressure inducing barotrauma.47,48 This phenomenon is called Müller’s maneuver and can be considered the reverse of the Valsalva maneuver. A similar mechanism could explain pneumorachis and pneumomediastinum in our patient too. In this case, instead of an external resistance to airflow, bronchospasm induced by the cannabis smoke would have forced the patient to inhale against a tight airway generating high negative intrapleural pressures, while coughing against the resistance would have generated high positive intra-alveolar pressures, thereby increasing trans alveolar pressure gradient both during inspiration and expiration which resulted in the barotrauma. The chances of a pneumomediastinum causing pneumorachis increase with the amount of leaked air and acuity of onset.49
Clinical Presentation
Spontaneous pneumorachis in the absence of intraspinal causes like epidural abscesses are associated with pneumomediastinum or subcutaneous emphysema.50 Asthma exacerbation is the most common triggering factor estimated to be present in 20–30% of all cases of spontaneous pneumomediastinum in children and adolescents.51 Other predisposing conditions include respiratory tract infections, activities inducing Valsalva maneuver, activities causing Müller’s maneuver, severe vomiting, esophageal rupture, foreign body aspiration, vaping and inhalational drug use.52–63The presence of these predisposing factors along with signs and symptoms should raise the suspicion for spontaneous pneumomediastinum (SPM) and possible pneumorachis.64 SPM is more common among children and adolescents compared to older age groups. Males are more likely to be affected, and so does a tall, lean body habitus.65,66
The most common presenting complaint is retrosternal, pleuritic chest pain radiating to the neck or shoulders of acute onset after an inciting event.67,68 Even when no triggering factor is present, SPM should be considered in the differential diagnosis of any young adult presenting with acute onset chest pain.69,70 Other common symptoms include subcutaneous emphysema with crepitus, especially in the neck, neck pain, dyspnea, cough, odynophagia, and dysphagia. Esophageal perforation (Boerhaave syndrome) could also present with a similar presentation and should be considered, especially in patients who have severe vomiting; but they typically present with signs and symptoms of shock.
Subcutaneous emphysema detected over the neck or precordium is an extremely sensitive and specific sign of SPM.71,72 Hamman sign, a precordial crunching sound synchronous with systole, especially in the left lateral decubitus position, may be present in up to 18% of patients.73 Findings such as hypotension, severe respiratory distress, unilaterally diminished breath sounds, and distended neck veins should raise the suspicion for more serious etiologies such as tension pneumomediastinum, pneumothorax, or esophageal perforation.
Neurological Signs and Symptoms
Pneumorachis is asymptomatic in most cases but can cause neurologic symptoms, including spinal cord compression in about 10% of patients.74,75 As pneumorachis by itself rarely causes spinal cord compression, we should search for alternative explanations such as epidural hematomas, abscesses, herniation, etc, if neurological symptoms are present.76–81
Diagnosis
Pneumorachis is almost always found in combination with air in other cavities such as pneumothorax, pneumomediastinum, or subcutaneous emphysema.82 Ultrasound is gaining popularity as the imaging of choice in the emergency department for the fast and accurate detection of pneumomediastinum, but it is not useful for detecting pneumorachis.83,84 Pneumorachis can be diagnosed on spinal X-ray.85 A CT scan is the diagnostic test of choice.86,87 An MRI is not required unless signs of cord compression are present and other etiologies such as contusion, hematoma, herniation, or compressing bone fragments need to be ruled out. The characteristic radiological features of SPM and pneumorachis, as demonstrated in our patient, are as below:
Treatment
There are no guidelines for the management of pneumorachis.88 Serial neurological examinations are recommended, while serial imaging for pneumorachis is not required unless there is a concern for cord compression.89 The development of neurological symptoms warrants surgical decompression; otherwise, the management is conservative.4 Treatment of the inciting etiology, such as bronchodilators and oxygen supplementation for asthma exacerbation, abscess drainage, chest tube for pneumothorax, etc. are required.
Clinical Outcomes
Clinical outcomes are related to the etiology of pneumorachis. The one patient who died during the acute course of illness in our review had sepsis. Radiological resolution would occur in most cases with conservative management. Treatment of the cause will curtail ongoing air leaks allowing the intra-spinal air to get reabsorbed within hours to days. Residual neurological deficits are rare.33
Conclusion
Clinicians should be aware of the possibility of pneumorachis in the setting of acute asthma exacerbation. Although asymptomatic in most cases, it can rarely lead to serious complications such as spinal cord compression. Addressing the underlying etiology is more important than managing the pneumorachis per se. Treatment is conservative except in symptomatic cases where intervention is required.
Disclosure
The authors report no conflicts on interest in this work.
References
1. Kirkham J, Schiers KA. Pneumorrhachis. J Am Osteopath Assoc. 2016;116(2):119. doi:10.7556/jaoa.2016.027
2. Song Y, Tu L, Wu J. Pneumorrhachis with spontaneous pneumomediastinum and subcutaneous emphysema. Intern Med Tokyo Jpn. 2009;48(18):1713–1714. doi:10.2169/internalmedicine.48.2256
3. Özkan S, Yıldız OO, Ünlü İ, Karaoğlanoğlu N. Progressive subcutaneous emphysema. A rare finding: pneumorrhachis. Respir Med Case Rep. 2017;22:57–59. doi:10.1016/j.rmcr.2017.04.019
4. Oertel MF, Korinth MC, Reinges MHT, Krings T, Terbeck S, Gilsbach JM. Pathogenesis, diagnosis and management of pneumorrhachis. Eur Spine J off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc. 2006;15(Suppl 5):636–643. doi:10.1007/s00586-006-0160-6
5. Martins L, Dionísio P, Moreira S, Manique A, Correia I, Bárbara C. An unusual association in an uncommon disease: two cases of spontaneous pneumomediastinum associated with pneumorrhachis. Case Rep Pulmonol. 2016;2016:5092157. doi:10.1155/2016/5092157
6. Ripley DP, Wilson EJ, Meller MT, Cowlam S. Pneumorrhachis: a rare complication of diabetic ketoacidosis. Diabet Med J Br Diabet Assoc. 2009;26(5):566–567. doi:10.1111/j.1464-5491.2009.02703.x
7. Llewellyn K, Johnson R, Krueger EM, Seibly JM. Pneumorrhachis after an upper respiratory infection: a case report of a rare phenomenon. Cureus. 2020;12(4):e7784. doi:10.7759/cureus.7784
8. Williams J, Hsu E, Flamer-Caldera A, Ferrabolli YJ. The special k constellation, a rare presentation of ketamine use: a case report. Cureus. 2019;11(5):e4766. doi:10.7759/cureus.4766
9. Jensen VM, Støving RK, Andersen PE. Anorexia nervosa with massive pulmonary air leak and extraordinary propagation. Int J Eat Disord. 2017;50(4):451–453. doi:10.1002/eat.22674
10. Niemann MJ. [Valsalva-induced subcutaneous emphysema, pneumomediastinum and pneumorrhachis in a young man]. Ugeskr Laeger. 2019;181(9):458. Danish.
11. Germino JC, Medverd JR, Nguyen VT, Favinger JL, Marder CP. Craniocervical hyperpneumatization with concurrent pneumorrhachis, pneumomediastinum, and subcutaneous emphysema in a weightlifter. Spine J off J North Am Spine Soc. 2013;13(10):e47–53. doi:10.1016/j.spinee.2013.06.033
12. Kim C-H. Pneumorrhachis and paraspinal air with vacuum disc: case report and literature review. J Korean Neurosurg Soc. 2007;42(6):490–491. doi:10.3340/jkns.2007.42.6.490
13. Navriya SC, Kumar S, Mittal A, Bhirud DP. Pneumorrhachis in emphysematous pyelonephritis: a rare finding in an uncontrolled diabetic patient. Indian J Urol IJU J Urol Soc India. 2021;37(1):97–98.
14. Gomez CA, Vela-Duarte D, Veldkamp PJ. Infectious pneumorrhachis due to emphysematous pyelonephritis. JAMA Neurol. 2017;74(11):1374–1375. doi:10.1001/jamaneurol.2017.2340
15. Yamamoto N, Takegawa R, Seki M, et al. Pneumorachis associated with multiorgan infection due to Citrobacter koseri. Diagn Microbiol Infect Dis. 2013;77(4):370–372. doi:10.1016/j.diagmicrobio.2013.08.017
16. Schömig B, Seliger C, Schulte-Mattler W, et al. Clinical reasoning: pneumocephalus and pneumorrhachis in a 67-year-old man. Neurology. 2016;86(21):e218–221. doi:10.1212/WNL.0000000000002693
17. Mahajan PS, Al Maslamani NJ, Purayil NK. Rare case of pneumorrhachis, pneumomediastinum, pneumothorax, and surgical emphysema secondary to bronchial asthma. Int Med Case Rep J. 2014;7:35–39. doi:10.2147/IMCRJ.S60050
18. Saleem N, Parveen S, Odigwe C, Iroegbu N. Pneumomediastinum, pneumorrhachis, and subcutaneous emphysema in Pneumocystis jiroveci pneumonia in AIDS. Proc Bayl Univ Med Cent. 2016;29(2):188–190. doi:10.1080/08998280.2016.11929412
19. Fonseca AZ, Santin S, Ribeiro M. Spontaneous pneumorrhachis. Am J Emerg Med. 2016;34(12):
20. Krishnan P, Das S, Bhattacharyya C. Epidural pneumorrhachis consequent to Hamman syndrome. J Neurosci Rural Pract. 2017;8(1):118–119. doi:10.4103/0976-3147.193525
21. Iacoangeli M, Di Rienzo A, Fianchini A, et al. Acute tension pneumocephalus secondary to whole spine pneumorrhachis as an unusual presentation of a colon cancer complicated by a transsacral cerebrospinal fluid leak. J Clin Neurosci off J Neurosurg Soc Australas. 2013;20(3):469–471.
22. Tafreshi A, Liew MS, Yap LP, Azad A. Malignant enterocutaneous fistula complicated by pneumorrhachis. Spine J off J North Am Spine Soc. 2012;12(12):1161–1162. doi:10.1016/j.spinee.2012.09.038
23. Lee C-H, Cho JH, Hyun S-J, Yoon SH, Kim K-J, Kim H-J. Symptomatic gas-containing herniated disc with the vacuum phenomenon: mechanism and treatment. Case Report Neurol Med Chir. 2012;52(2):106–108. doi:10.2176/nmc.52.106
24. Kumaran SP, Gupta K, Singh SS. A rare association of spontaneous pneumorrhachis with spondylolisthesis and lumbosacral vacuum phenomenon: a case report with review of literature. Neurol India. 2011;59(1):120–121. doi:10.4103/0028-3886.76893
25. Amit A, Toll EC, Siddique S, Nelson RJ. Pneumorrhachis with pneumocranium: an unusual complication of intra-abdominal sepsis. Br J Neurosurg. 2011;25(1):111–112. doi:10.3109/02688697.2010.500420
26. Oertel MF, Korinth MC, Reinges MHT, Gilsbach JM. Pneumorrhachis of the entire spinal canal. J Neurol Neurosurg Psychiatry. 2005;76(7):1036. doi:10.1136/jnnp.2004.044636
27. Song K-J, Lee K-B. Spontaneous extradural pneumorrhachis causing cervical myelopathy. Spine J off J North Am Spine Soc. 2009;9(2):e16–18. doi:10.1016/j.spinee.2008.01.011
28. Moayedi S, Babin L. Pneumorrhachis Secondary to a Sacral Decubitus Ulcer. West J Emerg Med. 2016;17(4):466–468. doi:10.5811/westjem.2016.4.30296
29. Kazimirko DN, Parker EE, Joyner DA, et al. An unusual cause of acute headache: subarachnoid free air secondary to spontaneous bronchopleurodurosubarachnoid fistula from a Pancoast tumor. Radiol Case Rep. 2016;11(3):238–241. doi:10.1016/j.radcr.2016.06.001
30. Gordon IJ, Hardman DR. The traumatic pneumomyelogram. A previously undescribed entity. Neuroradiology. 1977;13(2):107–108. doi:10.1007/BF00339843
31. Newbold RG, Wiener MD, Vogler JB, Martinez S. Traumatic pneumorrhachis. AJR Am J Roentgenol. 1987;148(3):615–616. doi:10.2214/ajr.148.3.615
32. Drevelengas A, Kalaitzoglou I, Petridis A. Pneumorrhachis associated with spontaneous pneumomediastinum. Eur J Radiol. 1994;18(2):122–123. doi:10.1016/0720-048X(94)90277-1
33. Chaichana KL, Pradilla G, Witham TF, Gokaslan ZL, Bydon A. The clinical significance of pneumorachis: a case report and review of the literature. J Trauma. 2010;68(3):736–744. doi:10.1097/TA.0b013e3181c46dd3
34. Miguel R, Morse S, Murtagh R. Epidural air associated with multiradicular syndrome. Anesth Analg. 1991;73(1):92–94. doi:10.1213/00000539-199107000-00018
35. Dalens B, Bazin JE, Haberer JP. Epidural bubbles as a cause of incomplete analgesia during epidural anesthesia. Anesth Analg. 1987;66(7):679–683. doi:10.1213/00000539-198707000-00021
36. Drolet S, Gagné J-P, Langis P. Spontaneous pneumorrhachis associated with pneumomediastinum in a patient with diabetic ketoacidosis: an exceptional manifestation of a benign disease. Can J Surg J Can Chir. 2007;50(3):225–226.
37. Salvati F, Signora M, Pedicelli G. [Revisiting iatrogenic damage in pneumology past and current implications compared]. Clin Ter. 2012;163(4):e233–241.
38. Eesa M, Kandpal H, Sharma R, Misra A. Spontaneous pneumorrhachis in bronchial asthma. Acta Radiol Stockh Swed. 2006;47(7):672–674.
39. Sahu KK, Mishra AK, Goldman Y. A rare case of Pneumopericardium secondary to COVID-19. Heart Lung J Crit Care. 2020;49(6):679–680. doi:10.1016/j.hrtlng.2020.08.017
40. Lal A, Mishra AK, Sahu KK, Noreldin M. Spontaneous Pneumomediastinum: rare Complication of Tracheomalacia. Arch Bronconeumol. 2020;56(3):185–186. doi:10.1016/j.arbres.2019.09.017
41. Mishra AK, Sahu KK, James A. Disseminated herpes zoster following treatment with benralizumab. Clin Respir J. 2019;13(3):189–191. doi:10.1111/crj.12998
42. Papiris S, Kotanidou A, Malagari K, Roussos C. Clinical review: severe asthma. Crit Care Lond Engl. 2002;6(1):30–44. doi:10.1186/cc1451
43. Chopra V, Garg N, Mrigpuri P. Spontaneous pneumopericardium an unusual complication in a patient of HIV and pulmonary tuberculosis. Lung India off Organ Indian Chest Soc. 2013;30(2):148–150. doi:10.4103/0970-2113.110425
44. Sahu KK, Sherif AA, Mishra AK, Vyas S, George SV. Perineal ulcer: a rare cause of extensive subcutaneous emphysema. BMJ Case Rep. 2019;12(4):e229918. doi:10.1136/bcr-2019-229918
45. Defouilloy C, Galy C, Lobjoie E, Strunski V, Ossart M. Epidurual pneumatosis: a benign complication of benign pneumomediastinum. Eur Respir J. 1995;8(10):1806–1807. doi:10.1183/09031936.95.08101806
46. Lal A, Mishra AK, Sahu KK. Prevention of early ventilator-associated pneumonia. N Engl J Med. 2020;382(17):1671–1672.
47. Sahu KK, Mishra A, Naraghi L. Erythema ab igne as a complication of cannabinoid hyperemesis syndrome. BMJ Case Rep. 2019;12(1):154. doi:10.1136/bcr-2018-227836
48. Hazouard E, Koninck JC, Attucci S, Fauchier-Rolland F, Brunereau L, Diot P. Pneumorachis and pneumomediastinum caused by repeated Müller’s maneuvers: complications of marijuana smoking. Ann Emerg Med. 2001;38(6):694–697. doi:10.1067/mem.2001.118016
49. Ould-Slimane M, Ettori M-A, Lazennec J-Y, Pascal-Moussellard H, Catonne Y, Rousseau M-A. Pneumorachis: a possible source of traumatic cord compression. Orthop Traumatol Surg Res OTSR. 2010;96(7):825–828. doi:10.1016/j.otsr.2010.03.024
50. Patel V, Raval G, Gavadia K. Pneumothorax, pneumomediastinum, subcutaneous emphysema and pneumorrhachis as complications of common flu. Am J Case Rep. 2012;13:198–201. doi:10.12659/AJCR.883332
51. Stack AM, Caputo GL. Pneumomediastinum in childhood asthma. Pediatr Emerg Care. 1996;12(2):98–101. doi:10.1097/00006565-199604000-00008
52. Maeder M, Ullmer E. Pneumomediastinum and bilateral pneumothorax as a complication of cocaine smoking. Respir Int Rev Thorac Dis. 2003;70(4):407.
53. Burgwardt S, Huskic A, Schwartz G, Mason DP, Tapias L, Podgaetz E. Spontaneous pneumomediastinum secondary to electronic cigarette use. Proc Bayl Univ Med Cent. 2020;33(2):229–230. doi:10.1080/08998280.2020.1717407
54. Burton EM, Riggs W, Kaufman RA, Houston CS. Pneumomediastinum caused by foreign body aspiration in children. Pediatr Radiol. 1989;20(1–2):45–47. doi:10.1007/BF02010632
55. Engum SA, Grosfeld JL, West KW, Rescorla FJ, Scherer LR, Vaughan WG. Improved survival in children with esophageal perforation. Arch Surg Chic Ill. 1996;131(6):604–610. doi:10.1001/archsurg.1996.01430180030005
56. Forshaw MJ, Khan AZ, Strauss DC, Botha AJ, Mason RC. Vomiting-induced pneumomediastinum and subcutaneous emphysema does not always indicate Boerhaave’s syndrome: report of six cases. Surg Today. 2007;37(10):888–892. doi:10.1007/s00595-006-3493-1
57. Mumford AD, Ashkan K, Elborn S. Clinically significant pulmonary barotrauma after inflation of party balloons. BMJ. 1996;313(7072):1619. doi:10.1136/bmj.313.7072.1619
58. Yamada K, Shinmoto H, Hamamoto M, et al. Pneumonia induced by swine-origin influenza A (H1N1) infection: chest computed tomography findings in children. Jpn J Radiol. 2011;29(10):712–717. doi:10.1007/s11604-011-0620-8
59. Sahu KK, Mishra A, Patel R, Suramaethakul N. A challenging case of esophageal perforation. Monaldi Arch Chest Dis Arch Monaldi Mal Torace. 2021;91(1):548.
60. Lal A, Mishra AK, Sahu KK. Vitamin E Acetate and E-Cigarette or Vaping Product-Associated Lung Injury (EVALI): an Update. Am J Med. 2020;133(5):e204. doi:10.1016/j.amjmed.2019.11.005
61. Sahu KK, Mishra AK, Patel R, Suramaethakul N, Abraham G. Fungal empyema thoracis. QJM Mon J Assoc Physicians. 2020;113(11):823–824. doi:10.1093/qjmed/hcaa036
62. Burns J, Roby A, Jaconelli T. Pneumomediastinum, subcutaneous emphysema and pneumorrhachis following cocaine insufflation: a case report. Acute Med. 2020;19(3):154–158. doi:10.52964/AMJA.0820
63. Atmaca Temrel T, Şener A, Içme F, et al. Subcutaneous Emphysema, Pneumomediastinum, and Pneumorrhachis after Cocaine Inhalation. Case Rep Emerg Med. 2015;2015:134816. doi:10.1155/2015/134816
64. Bally K, Leikin S, Margetis K, Reynolds AS. Extensive Pneumorrhachis After Spontaneous Pneumomediastinum. World Neurosurg. 2020;142:392–395. doi:10.1016/j.wneu.2020.07.091
65. Kobashi Y, Okimoto N, Matsushima T, Soejima R. Comparative study of mediastinal emphysema as determined by etiology. Intern Med Tokyo Jpn. 2002;41(4):277–282. doi:10.2169/internalmedicine.41.277
66. Ramasamy P, Kale SB, Subramaniam S, Giridhar K. Recurrent spontaneous pneumomediastinum with concurrent pneumorrhachis: a rare clinical entity. Ann Thorac Surg. 2018;105(4):e155–7. doi:10.1016/j.athoracsur.2017.10.029
67. Macia I, Moya J, Ramos R, et al. Spontaneous pneumomediastinum: 41 cases. Eur J Cardio-Thorac Surg off J Eur Assoc Cardio Thorac Surg. 2007;31(6):1110–1114. doi:10.1016/j.ejcts.2007.03.008
68. Liao P-Y, Wang H-J. Teenager with chest pain and swollen neck: a leave-it-alone condition. Thorax. 2015;70(7):707–708. doi:10.1136/thoraxjnl-2015-206978
69. Liu H-W, Liao P-A. Young man with acute chest pain. Ann Emerg Med. 2019;73(1):e1–2. doi:10.1016/j.annemergmed.2018.06.040
70. Kanu O, Teleb M, Agrawal H, Cashin LB. Cocaine-related subcutaneous emphysema, pneumorrhachis and pneumomediastinum: a rare clinical finding. BMJ Case Rep. 2017;2017:485.
71. Radhika Nair K, Kumar UK, Shetty B, Joseph S, Jameela S. Hamman’s Syndrome - in Young Asthmatic Female. J Assoc Physicians India. 2018;66(4):70–72.
72. Pangtey GS, Das CJ, Javan N. Airlessness in airspace. Simultaneous occurrence of spontaneous pneumothorax with pneumomediastinum and pneumorrhachis: report of a case. Surg Today. 2008;38(1):49–51. doi:10.1007/s00595-007-3556-y
73. Sahni S, Verma S, Grullon J, Esquire A, Patel P, Talwar A. Spontaneous pneumomediastinum: time for consensus. North Am J Med Sci. 2013;5(8):460–464. doi:10.4103/1947-2714.117296
74. Sahu KK, Sanamandra P, Jeyaraman P, et al. Unusual cause of cord compression - a pressing issue for neurosurgeons. World Neurosurg. 2016;92:565–567. doi:10.1016/j.wneu.2015.04.061
75. Gonzales GR, Payne R, Portenoy RK, Foley KM. Epidural air from a bronchopleural-epidural-cutaneous fistula producing reversible myelopathy and radiculopathy symptoms. Neurology. 1994;44(12):2409–2410. doi:10.1212/WNL.44.12.2409
76. Mishra AK, Sahu KK, Basaula NP, Lal A. Letter to the Editor Regarding “Management of spinal emergencies in patients on direct oral anticoagulants.”. World Neurosurg. 2019;132:446. doi:10.1016/j.wneu.2019.08.107
77. Sahu KK, Rapose A, Bhandari B, et al. Letter to the Editor Regarding “Craniocervical Osteoradionecrosis Treated with Neoadjuvant and Adjuvant Hyperbaric Oxygen in Combination with Posterior Spinal Fusion.”. World Neurosurg. 2019;129:541–543. doi:10.1016/j.wneu.2019.04.124
78. Willing SJ. Epidural pneumatosis: a benign entity in trauma patients. AJNR Am J Neuroradiol. 1991;12(2):345.
79. Sahu KK, Chastain I. A rare case of holocord spinal epidural abscess. QJM Mon J Assoc Physicians. 2020;113(4):302–303. doi:10.1093/qjmed/hcz291
80. Eroglu U, Yakar F, Zaimoglu M, Ozates O, Ozgural O, Ugur HC. Pneumorrhachis. Asian J Neurosurg. 2016;11(2):172–173. doi:10.4103/1793-5482.175641
81. Amara B, Boujraf S, Benzagmout M, Labib S, Harandou M. Spontaneous pneumorrhachis and transverse myelitis complicating purulent meningitis. J Glob Infect Dis. 2013;5(4):179–182. doi:10.4103/0974-777X.122019
82. Ramses Bedolla-Pulido T, Bedolla-Barajas M, González-Mendoza T, Hollyver Sánchez-Uribe E, Delgado-Figueroa N, León-García S. Epidural pneumorrhachis, a rare complication of asthma exacerbation. J Asthma off J Assoc Care Asthma. 2019;56(12):1356–1359. doi:10.1080/02770903.2018.1539101
83. Ng L, Saul T, Lewiss RE. Sonographic evidence of spontaneous pneumomediastinum. Am J Emerg Med. 2013;31(2):
84. Eisa N, Moh’d H, Alraiyes AH, Shaheen K, Kheir F. Spontaneous epidural air entrapment. Ochsner J. 2014;14(2):240–243.
85. Goh BKP, Yeo AWY. Traumatic pneumorrhachis. J Trauma. 2005;58(4):875–879. doi:10.1097/01.TA.0000158249.77176.9A
86. Lerner EJ, Bilaniuk LT. Spontaneous bronchial-subarachnoid fistula: an unusual cause of pneumocephalus. AJNR Am J Neuroradiol. 1989;10(5 Suppl):S103.
87. Valiyakath D, Al Busaidi T, Al Shamsi S, Al Sawafi Y. Pneumorrhachis with Spontaneous Pneumomediastinum: should It Raise Special Concerns? Oman Med J. 2018;33(3):256–259. doi:10.5001/omj.2018.47
88. Al-Mufarrej F, Gharagozloo F, Tempesta B, Margolis M. Spontaneous cervicothoracolumbar pneumorrhachis, pneumomediastinum and pneumoperitoneum. Clin Respir J. 2009;3(4):239–243. doi:10.1111/j.1752-699X.2008.00116.x
89. Heckman AJ, Mohseni M, Villanueva A, Cowart JB, Graham CG. Concurrent Spontaneous Pneumomediastinum and Pneumorrhachis. J Emerg Med. 2018;54(6):e117–20. doi:10.1016/j.jemermed.2018.02.036
90. Hochhegger B, Irion KL, Hochhegger D, Santos CSP, Marchiori E. Pneumorrhachis as a complication of bronchial asthma: computed tomography findings. Radiol Bras. 2018;51(4):268. doi:10.1590/0100-3984.2016.0228
91. Shafqat A, Magableh HMF, Shafqat S, et al. Pneumorrhachis causing cauda equina syndrome: a case report and literature review. Egypt J Radiol Nucl Med. 2020;51(212). doi:10.1186/s43055-020-00330-y
92. Dai H, Richter KP. A national survey of marijuana use among US adults with medical conditions, 2016–2017. JAMA Netw Open. 2019;2(9):e1911936. doi:10.1001/jamanetworkopen.2019.11936
93. D’Anastasi M, Birkenmaier C, Schmidt GP, Wegener B, Reiser MF, Baur-Melnyk A. Correlation between vacuum phenomenon on CT and fluid on MRI in degenerative disks. AJR Am J Roentgenol. 2011;197(5):1182–1189. doi:10.2214/AJR.10.6359.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.