Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Spontaneous Breathing Through Increased Airway Resistance Augments Elastase-Induced Pulmonary Emphysema
Authors Toumpanakis D, Mizi E, Vassilakopoulou V , Dettoraki M, Chatzianastasiou A, Perlikos F , Giatra G, Moscholaki M, Theocharis S, Vassilakopoulos T
Received 3 April 2020
Accepted for publication 2 July 2020
Published 12 July 2020 Volume 2020:15 Pages 1679—1688
DOI https://doi.org/10.2147/COPD.S256750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Dimitrios Toumpanakis,1,2 Eleftheria Mizi,1 Vyronia Vassilakopoulou,1 Maria Dettoraki,1 Athanasia Chatzianastasiou,1 Fotis Perlikos,1 Georgia Giatra,2 Marina Moscholaki,1 Stamatios Theocharis,3 Theodoros Vassilakopoulos1,2
1“Marianthi Simou” Applied Biomedical Research and Training Center, Medical School, University of Athens, Evangelismos Hospital, Athens, Greece; 2 3rd Department of Critical Care Medicine, Evgenideio Hospital, Medical School, University of Athens, Athens, Greece; 3Department of Pathology, Medical School, University of Athens, Athens, Greece
Correspondence: Theodoros Vassilakopoulos
3rd Department of Critical Care Medicine, Evgenideio Hospital, Medical School, University of Athens, Athens, Greece
Email [email protected]
Introduction: Resistive breathing (RB), the pathophysiologic hallmark of chronic obstructive pulmonary disease (COPD), especially during exacerbations, is associated with significant inflammation and mechanical stress on the lung. Mechanical forces are implicated in the progression of emphysema that is a major pathologic feature of COPD. We hypothesized that resistive breathing exacerbates emphysema.
Methods: C57BL/6 mice were exposed to 0.75 units of pancreatic porcine elastase intratracheally to develop emphysema. Resistive breathing was applied by suturing a nylon band around the trachea to reduce surface area to half for the last 24 or 72 hours of a 21-day time period after elastase treatment in total. Following RB (24 or 72 hours), lung mechanics were measured and bronchoalveolar lavage (BAL) was performed. Emphysema was quantified by the mean linear intercept (Lm) and the destructive index (DI) in lung tissue sections.
Results: Following 21 days of intratracheal elastase exposure, Lm and DI increased in lung tissue sections [Lm (μm), control 39.09± 0.76, elastase 62.05± 2.19, p=0.003 and DI, ctr 30.95± 2.75, elastase 73.12± 1.75, p< 0.001]. RB for 72 hours further increased Lm by 64% and DI by 19%, compared to elastase alone (p< 0.001 and p=0.02, respectively). RB induced BAL neutrophilia in elastase-treated mice. Static compliance (Cst) increased in elastase-treated mice [Cst (mL/cmH2O), control 0.067± 0.001, elastase 0.109± 0.006, p< 0.001], but superimposed RB decreased Cst, compared to elastase alone [Cst (mL/cmH2O), elastase+RB24h 0.090± 0.004, p=0.006 to elastase, elastase+RB72h 0.090± 0.005, p=0.006 to elastase].
Conclusion: Resistive breathing augments pulmonary inflammation and emphysema in an elastase-induced emphysema mouse model.
Keywords: resistive breathing, elastase, emphysema, mechanical forces
Introduction
COPD is a prevalent chronic pulmonary disease with significant worldwide mortality.1 One of the main pathologic features of COPD is emphysema, ie a permanent enlargement of airspaces distal to terminal bronchioles, without obvious fibrosis.2 Emphysema is associated with significant respiratory symptoms3 and indeed with a more rapid decline in FEV1 among COPD patients.4 Emphysema formation is mainly initiated by cigarette smoke exposure, resulting in oxidative stress, inflammation and protease-antiprotease imbalance in lung parenchyma.5 Moreover, pulmonary emphysema is a cardinal feature of patients with severe a1-antitrypsin deficiency.6
Interestingly, despite smoking cessation, pulmonary inflammation and emphysema progression persist, in severe COPD patients.7 Although, perpetuation of pulmonary inflammation in COPD patients has been attributed to several factors, such as autoimmunity, genetic susceptibility, chronic airway colonization and infection,8 several reports have implicated an independent significant role of mechanical forces for the initiation and progression of pulmonary emphysema.9 For example, in vitro, lung tissue slices from elastase-treated rats exhibited reduced threshold for mechanical failure upon stretch application.10
Undoubtedly, the most devastating mechanical insult for the emphysematous lung is the presence of a COPD exacerbation, with its attendant excessive airway narrowing, worsening of airflow limitation and dynamic hyperinflation.11 Indeed, a history of exacerbation is associated with progression of emphysema in COPD patients.12
Recently, our group has shown that resistive breathing through tracheal banding (to mimic the mechanical consequences of severe COPD exacerbations) induces pulmonary inflammation and injury in previously healthy mice.13 However, whether resistive breathing through its attendant increased mechanical stress, would exert additive inflammatory-injurious effects, when imposed onto the emphysematous lung is unknown. We hypothesized that resistive breathing would aggravate established pulmonary emphysema in mice. To test our hypothesis, we reproduced the well-described animal model of elastase-induced pulmonary emphysema and we combined it with a course of resistive breathing through tracheal banding.
Materials and Methods
Animals
Adult male C57BL/6 mice (8–10 weeks old) were used in this study. Animals were purchased by the Biomedical Sciences Research Center “A. Fleming” and were housed in a 12-hour day/night cycle at the Experimental Surgery unit of Evangelismos Hospital provided food and water ad libitum.
Elastase-Induced Emphysema Model
Pulmonary emphysema was developed in mice with the well-established model of elastase intratracheal instillation.14 Mice were anaesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (5mg/kg), an incision was performed to expose the trachea and the animals were placed in a 60° inclined surface. Then, 0.75 IU of porcine pancreatic elastase (PPE, E7885, Sigma) diluted in 60 μL of sterile normal saline was injected intratracheally, followed by a flush of 100 μL air. Control animals were injected intratracheally with only sterile normal saline. Pulmonary emphysema was evaluated at day 21 following elastase administration.
Animal Model of Resistive Breathing Through Tracheal Banding
An animal model of resistive breathing through tracheal banding was employed, as previously described by our group.13 Briefly, mice were anaesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (5mg/kg) and placed under a surgical microscope. The trachea was exposed, and a nylon band of a pre-specified length was introduced below trachea and sutured around it, to provoke a 50% reduction of its surface area. Following recovery from anesthesia, the mice were returned to their cage. Resistive breathing was applied for 24h (day 20 to day 21 from elastase or vehicle treatment) or for 72h (day 18 to day 21). Sham operated animals were used for comparisons.
Thus, in total the following groups of animals were used in the study: vehicle intratracheal administration plus sham operated (control) (n=10), vehicle plus RB for 24 hours (n=7), vehicle plus RB for 72 hours (n=6), elastase plus sham operated (n=7), elastase plus RB for 24 hours (n=6), elastase plus RB for 72 hours (n=6).
Esophageal Pressure Measurement as an Estimate of Pleural Pressure
In a separate group of animals (n=4) the esophageal pressure was measured, to estimate the mechanical stress imposed onto the lung during tracheal banding. Following anaesthesia and prior to tracheal banding, a water filled catheter (PE10 tubing), connected to a pressure transducer, was introduced to the mouth cavity and advanced forward to the esophagus until a positive value of pressure was recorded that indicated the presence of the tip of the catheter to the stomach. Then the catheter was withdrawn few millimeters, until negative values were recorded to ensure presence in the lower part of the esophagus.15,16 Pressure recordings were acquired for 8 seconds during spontaneous quietly breathing, nylon band placement under the trachea with no obstruction, 50% surface area reduction and total occlusion.
Mechanical Parameters of the Respiratory System
Following 21 days after elastase treatment, the animals were anaesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (10 mg/kg). For mice that underwent resistive breathing, the band was removed. Animals were then tracheostomized and connected to a small animal ventilator (FlexiVent, Scireq) to measure respiratory system mechanics. After 3 minutes of baseline ventilation (Tidal Volume 10 mL/kg, 150 breaths.min−1, 3 cmH2O PEEP), the volume history of the respiratory system was established with a 6-sec deep inspiration to TLC (30 cmH2O) and the mechanical parameters of the respiratory systems were estimated with three perturbations that measure the dynamic compliance and resistance of the respiratory system [single compartment linear model17] and by the forced oscillation technique, focusing on tissue elasticity parameter (H) of the constant phase model, as previously described.18 A static pressure-volume curve was performed to measured static compliance (slope of the mid linear part of the expiratory P-V curve) and hysteresis.
Bronchoalveolar Lavage Fluid Cellularity
Following measurement of the mechanical parameters of the respiratory system, the mice were sacrificed by exsanguination (vena cava dissection) under anesthesia. The thoracic cavity was opened and the left main bronchus was temporarily ligated. Three aliquots of 0.5mL normal saline were instilled into the right lung and were gently withdrawn. Bronchoalveolar lavage (BAL) fluid was then centrifuged (300xg, 5 min) and the cell pellet was reconstituted in 1 mL normal saline. Total cell count was measured with trypan blue stain and cell subpopulations were estimated in May-Grunwald stained cytospins (from 300 cells in total).
Histological Evaluation of Pulmonary Emphysema
After BAL, the right main bronchus was ligated, the temporal occlusion of the left main bronchus was removed and the left lung was inflated with 4% formaldehyde under constant pressure of 20 cmH2O. The left lung was embedded into paraffin and sagittal plane sections (4 μm thick) were cut and stained with hematoxylin-eosin. In every animal, 6 random optical fields (200x) were acquired and pulmonary emphysema was quantified by measuring the mean linear intercept (Lm) and the destruction index (DI).19 Image analysis was performed with ImageJ software.
Destruction Index (DI)
Forty-two equally distributed points were laid over the H-E tissue sections. Alveolar and duct spaces lying underneath the counting points were evaluated for the presence of destruction. Destruction was defined as one or more of the following criteria: (a) at least two alveolar wall defects, (b) at least two intraluminal parenchymal rags in alveolar ducts, (c) clearly abnormal morphology, or (d) classic emphysematous changes. The percentage of all the points falling into the several categories of destroyed air spaces was computed to reveal the destructive index, using the formula [D/(D+N)] x100%, where D = destroyed, and N = normal.
Mean Linear Intercept
Eight equally distributed horizontal lines were laid over the aforementioned tissue sections and the intercepts with alveolar walls were counted. The total length of each line of the grid was divided by the number of alveolar intercepts, to provide the mean linear intercept (Lm).
Statistical Analysis
Data are presented as mean±SEM. Statistical analysis was performed with two-way ANOVA (one factor being elastase treatment and the other resistive breathing) and the Fisher LSD test for post hoc comparisons. For esophageal pressure analysis, a repeated measures ANOVA was used (between different degrees of tracheal banding). A p value <0.05 was chosen to indicate statistical significance.
Results
Esophageal Pressure During Resistive Breathing
A 50% reduction of the tracheal surface area resulted in significantly more negative swings in esophageal pressure, compared to quiet breathing [Peso (cmH2O) quiet breathing, −4.38 ±0.75, 50% tracheal banding, −10.20±1.31, p=0.03]. Consequently, spontaneous breathing through tracheal banding resulted in a ratio of Peso/Peso,max of 22%, (Peso/Peso,max, quiet breathing, 0.09±0.01, sham-band only 0.11±0.009, p=non-significant to quiet breathing, 50% tracheal banding 0.22±0.004, p=0.006 to quiet breathing), which is well below the values observed during severe COPD exacerbations.20
Bronchoalveolar Lavage (BAL) Cellularity Following Resistive Breathing in Elastase-Treated Animals
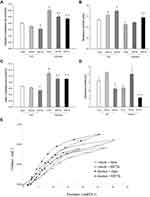
Following 21 days of elastase treatment an ~2-fold increase in total cell number was measured in BAL fluid, compared to vehicle (p=0.02), due to an increase in alveolar macrophages (p=0.02 to control) (Figure 1A). Interestingly, adding resistive breathing over elastase treatment (both 24 and 72 hours) provoked a significant increase in neutrophil count (p=0.01 and p=0.03, respectively, Figure 1B). Although sole RB for 24 hours increased macrophage count (p=0.01 to control), no additive effect was noticed, when RB was combined with elastase treatment (Figure 1C). Lymphocyte count was not affected in our experiment (ANOVA, p=0.194).
Respiratory System Mechanics Following Resistive Breathing in Elastase-Treated Animals
Elastase treatment increased respiratory system compliance (both dynamic and static, p<0.001 to control), in accordance with the presence of pulmonary emphysema (Figure 2A, C and E). In contrast, the addition of resistive breathing (both 24 and 72 hours) decreased compliance, compared to elastase alone, although compliance remained significantly elevated compared to control (Figure 2A, C and E). The same result was also obtained, when tissue elasticity (the reciprocal of compliance) was measured by the forced oscillation technique (data not shown). Following 21 days of elastase exposure, total respiratory system resistance decreased (p=0.002 to control). Resistive breathing for 72 hours increased resistance when added to elastase treatment (p<0.001, see Figure 2B). Combining resistive breathing and elastase treatment resulted in a differential effect on hysteresis, since 24h of RB increased hysteresis, compared to elastase alone (p=0.006), whereas 72h decreased hysteresis (p=0.04) (Figure 2D and E).
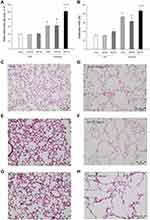
The Effect of Resistive Breathing on Pulmonary Emphysema in Elastase-Treated Animals
Elastase treatment was associated with the induction of pulmonary emphysema, as shown by the increase in both Lm and DI indices, compared to vehicle-treated mice (p=0.003 and p<0.001, to ctr, respectively). Resistive breathing for 24 hours did not affect pulmonary emphysema in elastase-treated animals. Interestingly, resistive breathing for 72 hours superimposed in elastase treatment further augmented pulmonary emphysema, as shown by the increase in Lm and DI indices (p<0.001 and p=0.02 to elastase alone, respectively, Figure 3). Note that resistive breathing alone had no effect on either Lm or DI indices in mice treated intratracheally with normal saline.
Discussion
The main finding of our study is that resistive breathing aggravates elastase-induced pulmonary emphysema in mice. COPD through increased airway resistance and hyperinflation is associated with increased mechanical stress on the lung, especially during exacerbations.11 Despite the wide recognition of the role of mechanical deformation for biological responses,21 the exact role of the mechanical forced in the pathogenesis of COPD and emphysema has not been clearly established.9 One of the main reasons for that is the absence of an animal model to mimic the mechanical consequences of severe airway obstruction, irrespective of the underlying triggering factor (eg viral or bacterial infection, air pollution e.t.c).
Our research group has provided evidence that spontaneous breathing through increased airway resistance induces pulmonary inflammation and injury in previously healthy animals.13,18 As presented in this study, tracheal banding at 50% of initial tracheal area is associated with significant negative intrathoracic pressure swings, leading to increased mechanical stress onto the lung, compared to spontaneous unloaded breathing. The time frame tracheal banding used in our study (24h to 72h) was also chosen to reflect a “usual” natural course of a COPD exacerbation.
Interestingly, 72 but not 24 hours of resistive breathing augmented elastance-induced pulmonary emphysema, as evidenced by the increased mean linear intercept and destruction indices in histological tissue sections. This suggests that the mechanical stressor has to be applied for long to implement its injurious effects. To translate this finding clinically, not only the severity (tracheal surface area) but also the duration of the exacerbation would potentially determine whether the exacerbation would result in emphysema propagation.
To our knowledge, our study provides the first evidence for the role of mechanical stress in emphysema progression in an in vivo model of severe airway obstruction, that mimics COPD patients, especially during exacerbations. Previously, Szabari et al found that one hour of mechanical ventilation with addition of deep inspirations (35 cmH2O for 3 seconds twice per minute) in elastase-treated mice produced alterations in lung structure (change in the distribution of airway diameters, increased alveolar wall thickness and decreased attachment density around airways), compared to low pressure ventilation only (without deep inspirations).22 In humans, imaging studies have shown that in COPD patients, the presence of emphysematous areas is associated with increased mechanical deformation of adjacent “normal-appearing” areas and this lung at risk is associated with lung function (FEV1) decline.23 Moreover, modelling emphysematous areas in lung CT scans showed that disease progression occurs near existing emphysematous areas.24 Our model may be especially clinically applicable to α1-antitrypsin deficiency (AATD), since the elastase emphysema model has been previously reported to replicate various features of AATD-associated pulmonary emphysema.25 Indeed, it has been shown that patients with severe AATD present frequently with exacerbations (where resistive breathing occurs)26 and exacerbations have been associated with accelerated decline in diffusion capacity of the lung for carbon monoxide26 or in FEV127 in AATD patients.
As expected, elastase treatment and emphysema formation resulted in increased compliance of the respiratory system. Addition of resistive breathing, despite augmenting the formation of pulmonary emphysema, was associated with decreased compliance compared to elastase alone, denoting the addition of some degree of lung injury, which effects predominated on the elastic properties (ie compliance). This is in accordance with findings by our group13 and others, showing that the addition of acute mechanical forces in elastase-treated animals resulted in features of injury eg increased alveolar wall thickness.22
The mechanisms that mediate resistive breathing-induced pulmonary emphysema aggravation cannot be unraveled by this study, however some conjectures are worth-attempting. A direct injurious effect of mechanical deformation due to increased transpulmonary pressure onto lung tissue may contribute to our results, especially since elastase-treated lung from rats exhibits a low threshold for mechanical failure.10 Additionally, resistive breathing was associated with increased neutrophil count in elastase-treated animals at as early as 24 hours of superimposed tracheal banding. Interestingly, although neutrophils are found in BAL fluid early after elastase administration,28 neutrophil numbers were at baseline values at day 21 in our model, suggesting that mechanical forces may re-trigger inflammatory pathogenetic mechanisms of emphysema. We have also previously shown that an acute bout of resistive breathing induces MMP-9 and MMP-12 expression in the lung of previously healthy rats,29 a proteolytic enzyme with well described role in emphysema formation,30 a mechanism which could also contribute to the emphysema propagation in our model. Interestingly, during acute exacerbations of COPD, where increased mechanical stress is imposed on the lung, significant increase in MMP-9 levels is found in the BAL fluid of patients.31
A limitation of our study is that the our model of resistive breathing through tracheal banding presents some anatomic and physiological differences with the increased airway resistance caused by exacerbations of COPD, including the anatomical location (upper versus lower airway obstruction), the homogeneity/distribution of the resistance, and the timing of this resistance within the respiratory cycle. Although these differences affect the implications of our model, overall our study provides evidence that altered respiratory physiology can contribute to alveolar remodeling and affect pulmonary inflammation.18,20,32
Conclusions
In conclusion, our data suggest that increased mechanical stress provoked by airway narrowing propagates pulmonary emphysema in elastase-treated mice. Thus, resistive breathing through tracheal banding may introduce a novel animal (preclinical) model of COPD exacerbations to investigate progression of emphysema and therapeutic interventions.
Abbreviations
AATD, α1-antitrypsin deficiency; BAL, bronchoalveolar lavage; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; PEEP, positive end-expiratory volume; TLC, total lung capacity.
Data Sharing Statement
The data used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval
All procedures, described in Methods, were approved by the ethical committee of the experimental surgery department of Evangelismos Hospital and are in accordance with the national law that follows the European Union Directive (2010/63/EU) on the protection of animals used for scientific purposes.
Acknowledgment
The authors would like to thank Dr. Vassiliki Karavana for technical help with histology sections preparation.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors have no conflict of interest to disclose.
References
1. From the global strategy for the diagnosis, management and prevention of COPD, GOLD; 2019. Available from: https://goldcopd.org.
2. Hogg JC, Timens W. The pathology of chronic obstructive pulmonary disease. Annu Rev Pathol. 2009;4:435–459. doi:10.1146/annurev.pathol.4.110807.092145
3. Grydeland TB, Dirksen A, Coxson HO, et al. Quantitative computed tomography measures of emphysema and airway wall thickness are related to respiratory symptoms. Am J Respir Crit Care Med. 2010;181(4):353–359. doi:10.1164/rccm.200907-1008OC
4. Vestbo J, Edwards LD, Scanlon PD, et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184–1192. doi:10.1056/NEJMoa1105482
5. Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med. 2000;343(4):269–280. doi:10.1056/NEJM200007273430407
6. Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185(3):246–259. doi:10.1164/rccm.201108-1428CI
7. Miller M, Cho JY, Pham A, Friedman PJ, Ramsdell J, Broide DH. Persistent airway inflammation and emphysema progression on CT scan in ex-smokers observed for 4 years. Chest. 2011;139(6):1380–1387. doi:10.1378/chest.10-0705
8. Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet. 2011;378(9795):1015–1026. doi:10.1016/S0140-6736(11)60988-4
9. Suki B, Sato S, Parameswaran H, Szabari MV, Takahashi A, Bartolak-Suki E. Emphysema and mechanical stress-induced lung remodeling. Physiology. 2013;28(6):404–413. doi:10.1152/physiol.00041.2013
10. Kononov S, Brewer K, Sakai H, et al. Roles of mechanical forces and collagen failure in the development of elastase-induced emphysema. Am J Respir Crit Care Med. 2001;164(10 Pt 1):1920–1926. doi:10.1164/ajrccm.164.10.2101083
11. O’Donnell DE, Parker CM. COPD exacerbations. 3: pathophysiology. Thorax. 2006;61(4):354–361. doi:10.1136/thx.2005.041830
12. Tanabe N, Muro S, Hirai T, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(12):1653–1659. doi:10.1164/rccm.201009-1535OC
13. Glynos C, Toumpanakis D, Loverdos K, et al. Guanylyl cyclase activation reverses resistive breathing-induced lung injury and inflammation. Am J Respir Cell Mol Biol. 2015;52(6):762–771. doi:10.1165/rcmb.2014-0092OC
14. Wright JL, Cosio M, Churg A. Animal models of chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol. 2008;295(1):L1–L15. doi:10.1152/ajplung.90200.2008
15. Hamelmann E, Schwarze J, Takeda K, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156(3 Pt 1):766–775. doi:10.1164/ajrccm.156.3.9606031
16. van Scott MR, Justice JP, Bradfield JF, Enright E, Sigounas A, Sur S. IL-10 reduces Th2 cytokine production and eosinophilia but augments airway reactivity in allergic mice. Am J Physiol Lung Cell Mol Physiol. 2000;278(4):L667–L674. doi:10.1152/ajplung.2000.278.4.L667
17. Bates JH, Irvin CG. Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol. 2003;94(4):1297–1306. doi:10.1152/japplphysiol.00706.2002
18. Toumpanakis D, Kastis GA, Zacharatos P, et al. Inspiratory resistive breathing induces acute lung injury. Am J Respir Crit Care Med. 2010;182(9):1129–1136. doi:10.1164/rccm.201001-0116OC
19. Robbesom AA, Versteeg EM, Veerkamp JH, et al. Morphological quantification of emphysema in small human lung specimens: comparison of methods and relation with clinical data. Mod Pathol. 2003;16(1):1–7. doi:10.1097/01.MP.0000043519.29370.C2
20. Vassilakopoulos T, Toumpanakis D. Can resistive breathing injure the lung? Implications for COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2016;11:2377–2384. doi:10.2147/COPD.S113877
21. Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi:10.1038/nrm2597
22. Szabari MV, Parameswaran H, Sato S, Hantos Z, Bartolak-Suki E, Suki B. Acute mechanical forces cause deterioration in lung structure and function in elastase-induced emphysema. Am J Physiol Lung Cell Mol Physiol. 2012;303(7):L567–L574. doi:10.1152/ajplung.00217.2012
23. Bhatt SP, Bodduluri S, Hoffman EA, et al. Computed tomography measure of lung at risk and lung function decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196(5):569–576. doi:10.1164/rccm.201701-0050OC
24. Mondonedo JR, Sato S, Oguma T, et al. CT imaging-based low-attenuation super clusters in three dimensions and the progression of emphysema. Chest. 2019;155(1):79–87. doi:10.1016/j.chest.2018.09.014
25. Saltini C, Krotova K. Mechanisms of lung disease. In: Strnad P, Brantly ML, Bals R, editors. Α1-Antitrypsin Deficiency (ERS Monograph). Sheffield: European Respiratory Society; 2019:52–63.
26. Needham M, Stockley RA. Exacerbations in {alpha}1-antitrypsin deficiency. Eur Respir J. 2005;25(6):992–1000. doi:10.1183/09031936.05.00074704
27. Hiller AM, Piitulainen E, Jehpsson L, Tanash H. Decline in FEV1 and hospitalized exacerbations in individuals with severe alpha-1 antitrypsin deficiency. Int J Chron Obstruct Pulmon Dis. 2019;14:1075–1083. doi:10.2147/COPD.S195847
28. Ishii Y, Itoh K, Morishima Y, et al. Transcription factor Nrf2 plays a pivotal role in protection against elastase-induced pulmonary inflammation and emphysema. J Immunol. 2005;175(10):6968–6975. doi:10.4049/jimmunol.175.10.6968
29. Toumpanakis D, Noussia O, Sigala I, et al. Inspiratory resistive breathing induces MMP-9 and MMP-12 expression in the lung. Am J Physiol Lung Cell Mol Physiol. 2015;308(7):L683–L692. doi:10.1152/ajplung.00133.2014
30. Barnes PJ, Burney PG, Silverman EK, et al. Chronic obstructive pulmonary disease. Nat Rev Dis Primers. 2015;1:15076. doi:10.1038/nrdp.2015.76
31. Papakonstantinou E, Karakiulakis G, Batzios S, et al. Acute exacerbations of COPD are associated with significant activation of matrix metalloproteinase 9 irrespectively of airway obstruction, emphysema and infection. Respir Res. 2015;16:78. doi:10.1186/s12931-015-0240-4
32. Loverdos K, Toumpanakis D, Litsiou E, et al. The differential effects of inspiratory, expiratory, and combined resistive breathing on healthy lung. Int J Chron Obstruct Pulmon Dis. 2016;11:1623–1638. doi:10.2147/COPD.S106337
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.