Back to Journals » Clinical Ophthalmology » Volume 14
Spectacle Independence for Pseudophakic Patients – Experience with a Trifocal Supplementary Add-on Intraocular Lens
Authors Palomino-Bautista C , Sánchez-Jean R, Carmona Gonzalez D , Romero Domínguez M, Castillo Gómez A
Received 14 November 2019
Accepted for publication 8 January 2020
Published 9 April 2020 Volume 2020:14 Pages 1043—1054
DOI https://doi.org/10.2147/OPTH.S238553
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Carlos Palomino-Bautista,1,2 Rubén Sánchez-Jean,1 David Carmona Gonzalez,1 Marta Romero Domínguez,1,2 Alfredo Castillo Gómez1,2
1Department of Ophthalmology, University Hospital Quirónsalud Madrid, Madrid, Spain; 2European University of Madrid, Madrid, Spain
Correspondence: Carlos Palomino-Bautista Calle Santa Engracia 6, Madrid 28010, Spain
Tel +34 620 858 128
Email [email protected]
Purpose: To evaluate the refractive and functional outcomes of the trifocal 1stQ AddOn® (Medicontur) supplementary intraocular lenses (IOLs) designed for implantation into the ciliary sulcus.
Patients and Methods: The study included 18 eyes of 11 pseudophakic patients with uncomplicated previous implantation of monofocal capsular bag IOLs. These patients had a desire for spectacle independence. Distance, intermediate and near visual acuities were measured, and defocus curves were plotted over a period of 6 months following implantation of the add-on IOLs. Intraocular pressure (IOP), endothelial cell density measurements and biomicroscopic evaluation were also performed.
Results: In this study, 83.3% of eyes had spherical refractions within ± 0.5 D from emetropia and 100% of eyes had spherical equivalent refractions that were within ± 1.0 D of the target refraction. Visual acuities and defocus curves clearly confirmed trifocal optical performance (UDVA=0.03 ± 0.05; UIVA=0.21 ± 0.04; UNVA=0.12 ± 0.04 logMAR; expressed as mean ±SD). Depth of focus showed identical results (DOF=0.486 D) compared to a trifocal capsular bag IOL, while the defocus curve was found to be superior in the intermediate and near ranges when compared to a trifocal capsular bag IOL. All patients achieved spectacle independence at all distances. All add-on IOLs were well positioned in the ciliary sulcus. No negative changes were noted in connection with endothelial cell counts, IOPs, the angle structure during surgery and during the follow-up period.
Conclusion: The supplementary trifocal add-on IOL seems to be a safe, efficient and stable solution for achieving spectacle independence in pseudophakic patients with monofocal primary IOLs.
Keywords: cataract surgery, supplementary IOL, trifocal, defocus curve, spectacle independence
Plain Language Summary
Cataract surgery involves the surgical removal of the opaque crystalline lens from the eye and its replacement with an artificial intraocular lens (IOL). In the majority of cases (95%) patients are implanted with a monofocal lens, which is primarily designed to correct distance vision. In this case, presbyopic patients, who also need near vision correction, require spectacles or contact lenses for near vision activities such as reading. Although multifocal IOLs have been available for cataract patients for some years, a constantly increasing demand for spectacle independence can be observed even in the pseudophakic population (those who already have an artificial IOL implanted).
Supplementary IOLs specifically designed for pseudophakic patients are also available with multifocal optics; thus, they are able to provide multifocality and real spectacle independence without explanting the primary IOL of the patient.
The aim of this study was to evaluate the safety of implanting a supplementary trifocal add-on IOL, and to assess its efficacy in terms of visual outcomes, refractive precision and spectacle independence.
Our results show that near vision improved significantly after the supplementary lens was implanted. All patients achieved spectacle independence for all distances. While the number of cases in this study was limited, implantation of the add-on lens was found to be a safe procedure.
Implanting a trifocal ciliary sulcus IOL appears to be a viable and effective option for improving vision quality and accomplishing spectacle independence for pseudophakic patients who previously had a monofocal IOL implanted in the capsular bag.
Introduction
Presbyopia is defined as the age-related accommodative deficiency of the crystalline lens, which usually develops in the fifth decade of life and leads to a progressive decline in the quality of near vision. According to a recent report, presbyopia was estimated to affect 1.8 billion people, which figure represents 25% of the world’s population. By 2020 this number is expected to reach 2.1 billion.1,2
Approximately 10 million cataract operations are performed worldwide every year. Cataract surgery involves removal of the cataractous crystalline lens, which is then followed by implantation of an artificial intraocular lens (IOL).3 Approximately 5% of cataract operations are performed with presbyopia-correcting IOLs.4–6 A monofocal lens designed to improve distance vision is implanted into the capsular bag of the eye in approximately 95% of cases.5,6 The near vision of the majority of these patients is unsatisfactory without the use of spectacles or contact lenses. A high proportion of patients would welcome the ability to be able to see clearly at all distances and to be free of spectacles.
Current surgical techniques for presbyopia correction of pseudophakic patients are based on two principal approaches: the first approach involves IOL exchange when a previously implanted monofocal IOL is explanted and a presbyopia-correcting multifocal lens is inserted into the capsular bag. This approach may result in unwanted complications.7
A less hazardous approach involves the insertion of an additional lens into the ciliary sulcus. With this method, the capsular bag is preserved and the risk of adverse complications such as vitreous loss decreases significantly compared to IOL exchange.8 Supplementary IOL implantation can be performed at any time following cataract surgery. The patient needs to have a stable posterior capsular IOL positioned within an intact capsular bag, a normal anterior chamber, a normal corneal endothelium, and no evidence of pigment dispersion syndrome.7 The presence of zonular insufficiency is a contraindication to the implantation of a secondary sulcus-fixated supplementary IOL.7,9
Unlike historical approaches, when inserting the secondary IOL into the capsular bag (piggybacking) used to be the general practice in the implantation of supplementary IOLs, the most advanced additional lenses are specifically designed for implantation into the ciliary sulcus, meaning sufficient space remains between the two lenses, and the development of interlenticular opacification is minimal.10 Furthermore, manufacturers are making great efforts to design lenses that efficiently eliminate all complications that may arise from polypseudophakia or from the ciliary sulcus being the implantation site, such as iris chafing, pigment dispersion syndrome, pupillary block, angle-closure and secondary glaucoma.7,10–13
The aim of this study was to present the initial results obtained in connection with the implantation of the 1stQ AddOn® diffractive supplementary IOL produced by Medicontur Medical Engineering Ltd (Zsámbék, Hungary) in pseudophakic patients who had previously had various monofocal capsular bag IOLs implanted. The patients in this study wished to become completely independent of spectacle correction. Our choice of this supplementary add-on IOL family was justified by that, unlike similar lenses on the market, this platform has been reported to be safe in terms of stable positioning in various eyes, leaving an adequate interlenticular space between the lens and the primary IOL, and involving hardly any complications related to either the surgery or the postoperative course.10,11,14,15 According to the manufacturer, the 1stQ AddOn diffractive supplementary IOL has an optic similar to the Medicontur Liberty® 677MY capsular bag IOL, with clinical trifocal performance and spectacle independence of patients being confirmed in related studies.16–18
Materials and Methods
Subjects
This prospective observational study included 18 eyes of eleven pseudophakic patients. These patients were pseudophakic in both eyes. Various brands of monofocal IOLs were present in the eyes of these patients. The study adhered to the principles of the Declaration of Helsinki19 and was also approved by the Institutional Research Ethics Committee of our Department at the University Hospital Quirónsalud, Madrid. Written informed consent was obtained from each patient prior to inclusion in the study. The procedure of obtaining consent included informing all patients of the advantages and possible complications of the proposed treatment.
None of the patients selected for the study reported current or previous ocular surgeries (except cataract surgery followed by capsular bag IOL implantation) or pathologies including congenital eye diseases, glaucoma, uveitis, keratitis, retinal disorders or amblyopia, and none of the patients had tear-film insufficiency (dry-eye syndrome). All of the patients were pseudophakic with monofocal capsular bag implants without any tilt or dislocation. All patients in this study expressed the explicit desire to be completely spectacle independent.
Pre- and Postoperative Examination
Prior to surgery, a detailed ocular examination was performed including corneal topography, aberrometry (iTrace; Tracey Technologies LLC; Houston, TX, USA), anterior segment examination, posterior segment examination with a macular OCT (Optovue, Optovue Inc., Fremont, CA, USA), as well as measurement of endothelial cell count (Tomey EM 3000 specular microscope, Tomey GmbH, Tennenlohe, Germany) and intraocular pressure (IOP) (measured with a Perkins Mk2 tonometer, Haag-Streit UK Ltd., Harlow, United Kingdom). Pseudophakic optical biometry measurements were performed including axial length (AXL), keratometry, central corneal thickness and anterior chamber depth (ACD) (IOL Master 700, Carl Zeiss Meditec AG, Jena, Germany). Patients with a pseudophakic ACD value of less than 2.8 mm as measured from the corneal epithelium were excluded from the study.
Patients were examined postoperatively for 6 months by the same personnel (RSJ). Endothelial cell count, IOP and anterior segment OCT were measured, autorefraction was performed (RT-900, Nidek Co., Aichi, Japan), and uncorrected and corrected distance (4 m), intermediate (67 cm) and near (33 cm) visual acuity was tested. Distance and intermediate visual acuities were tested in photopic conditions using the logMAR scale with the Early Treatment in Diabetic Retinopathy Study (ETDRS) chart in each case, and near visual acuities were recorded using the Rosenbaum card (J G. Rosenbaum, Cleveland, OH, USA).
Monocular defocus curves were plotted for each eye in photopic light conditions after correcting the patient for distance vision. Trial lenses with powers of −4.0 to +2.0 diopters were applied in 0.5 D increments, in varying order.
Objective measurement of the depth of focus (DOF; relative 90%) was performed with the iTrace aberrometer (iTrace; Tracey Technologies LLC; Houston, TX, USA) by determining the through-focus augmented visual Strehl optical transfer function (VSOTF) ratio of each eye in scotopic conditions. Each patient had depth of focus measurements performed with a pupil diameter of 3.00 mm or greater (tropicamide eye drops were used as needed). Subjective measurements of DOF were performed by recording visual acuities tested with trial lenses between −1 and +1 dioptres, with 0.12 D increments. The results were then compared to those from previous examinations performed according to the same protocol on a similar population of subjects with an implanted trifocal FineVision Pod F capsular bag IOL by PhysIOL s.a. (Liège, Belgium).
Patient Satisfaction
Patient satisfaction after the implantation of the supplementary IOL was assessed using an adaptation of the Spanish version of the NEI VFQ-25 (National Eye Institute Visual Functioning Questionnaire-25) Questionnaire.20 The questionnaire allowed the presence of dysphotopic phenomena, such as halos, glares and starbursts, to be evaluated. The questionnaire assessed independence from refractive correction (spectacles, contact lenses) and the visual satisfaction of each patient when they performed different tasks requiring good far, intermediate or near vision. The vision of each patient was given a score of from 0 (very bad) to 100 (very good) for each of the questions in the questionnaire.
The NEI VFQ-25 questionnaire consists of 25 questions in 11 topics related to vision. To simplify the analysis, we used four of the 11 topics from the original questionnaire: Dysphotopic events (Driving at night), Far vision (Watching television), Near vision (Reading tasks) and Vision in general (Overall satisfaction) were evaluated. In addition, only three possible answers were shown from the subjective assessment scale: No difficulties/Highly satisfied, Minor difficulties/Rather satisfied and Severe difficulties/Unsatisfied.
For the calculation of the satisfaction score in each topic, we used the original evaluation formula of the questionnaire:
The 1stQ Secondary Supplementary Sulcus IOL
The 1stQ add-on lens is a single-piece hydrophilic acrylic lens with 25% water content, containing an ultraviolet light absorber. The lens was specifically designed to be placed into the ciliary sulcus. The lens contains rounded optic edges with polished haptics to avoid any irritation of the sulcus.21 The anterior surface of the lens has a shallow anterior curvature. The four flexible closed loop haptics have a thickness of 0.3 mm to minimize the chances of iris chafing but still provide optimal fixation in the sulcus. The posterior surface of the lens has a concave surface to avoid contact with the primary in the capsular bag IOL, which ensures sufficient inter-lenticular space to prevent inter-lenticular opacification. The aspheric optic of the lens has a square shape to avoid pupillary capture during implantation of the IOL. The optic of the lens has a diameter of 6.0 mm with a convex–concave configuration. The overall diameter of the lens is 13.0 mm (Figure 1). This study focused on the trifocal add-on model with diffractive optics, which is designed to ensure reversible multifocality for the patient; however, monofocal and toric optic designs are also available. The diffractive model is available from −5.0 to +5.0 diopters in 0.5 D increments (including 0.00 D, a refractive neutral model), with added powers equivalent to 1.5 and 3.0 diopters at the IOL plane. The optical surface is similar to the Liberty 677MY trifocal capsular bag IOL by Medicontur: it has a 3.00 mm central apodized diffractive structure with 6 diffractive rings on the anterior surface, which includes the patented Elevated Phase Shift (EPS) technology to create the additional focal points for sharp intermediate and near vision. The outer 75% of the optic surface is left refractive or neutral, depending on the model.
The appropriate power for the sulcus lens was determined using the online calculator available on the manufacturer’s website.22 The target refraction (always as close as possible to plano in this study), manifest subjective refraction, axial length, current keratometry and pseudophakic anterior chamber depth were entered into the calculator to determine the appropriate lens power. The refractive goal for all eyes was emetropia. In 27.8% of cases (5 eyes), a plano powered add-on lens was used (A45RD2 A4DW0M). In the remaining 72.2% of cases (13 eyes), lenses with refractive correction other than plano (A45RD2 A4EW0M) were used (Figure 2).
Surgery
All surgery was performed between February 2017 and December 2018 with topical anesthesia by the same experienced surgeon (CPB) using the same surgical protocol. The supplementary IOL implantations were performed after an average interval of 11.4 ±5.91 years (range: 0.6 to 18.8 years) following the implantation of the primary monofocal capsular bag IOL. Bilateral IOL implantation was performed with seven patients. Four patients received unilateral implantation of the diffractive supplementary IOL because the contralateral eye in these patients had previously been implanted with a diffractive lens in the capsular bag (3 eyes with the TECNIS Symfony® ZXR00 IOL by Johnson & Johnson, Santa Ana, CA, USA; and 1 eye with the FineVision PodF IOL by PhysIOL s.a., Liège, Belgium). The diffractive supplementary lens was always injected into the dominant eye through a 2.75-mm clear corneal incision with the Medjet B1B 2.2 one-piece, single-use injector (Medicontur Medical Engineering Ltd, Zsámbék, Hungary). Healon PRO viscoelastic material (Abbott Medical Optics Inc., Santa Ana, CA, USA) was used, and the wound was left sutureless in all cases. Topical corticosteroids were administered during each implantation of the sulcus IOL to minimize the risk of inflammation.
Statistical Analysis
The data were entered into a Microsoft Excel spreadsheet (Redmond, WA, USA) and was further analyzed using the GraphPad Prism 8.2.0 statistical software (San Diego, CA, USA). All of the data collected in this study were de-identified and later entered into the Mendeley Data depository database from doi:10.17632/msbfkx4gv8.1.23 The dataset will be available immediately following publication, without any end date previously defined.
Pre- and post-operative data of 11 patients (18 eyes) were included in the analyses. Descriptive statistics was used in the analysis (mean, standard deviation, median, minimum, maximum, 95% confidence intervals). All variables were tested for normal distribution using the D’Agostino & Pearson test. Depending on the results, comparisons between matching pre- and postoperative variables were performed using either the paired two-tailed t-test (in case of normal distribution) or the Mann–Whitney test (when a non-parametric test was required). Multiple t-tests using the two-stage linear step-up procedure of Benjamini, Krieger and Yekutieli, with Q = 1%, were used for the comparison of the defocus and DOF-curves of the lens being studied and the FineVision PodF IOL. Each row was analyzed individually, without assuming a consistent SD.
Results are presented as mean ± standard deviation (SD) along with the range defined by the minimum and maximum values in brackets in the case of each variable. P values of 0.05 or less were considered to be statistically significant in all cases.
Results
Pre-and postoperative data of 18 eyes (11 patients) were collected and analyzed. The preoperative demographics of the study population is presented in Table 1.
 |
Table 1 Preoperative Demographics of the Patients |
Safety
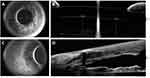
No intraocular complications or adverse events, including anterior chamber bleeding, corneal or iris damage occurred during any of the operations. Similarly, no adverse events were reported during the 6-month follow-up period. Thorough examinations were performed to assess the possible presence of inflammation, corneal edema, angle closure, pupillary block, iris capture or pigment dispersion syndrome. The anterior and posterior segments were found to be normal in all cases. The position and appearance of both the capsular bag and supplementary IOLs were examined and recorded during the postoperative visit at 6 months. All lenses were found to be well positioned without any tilt, decentration, dislocation, discoloration or opacity. A distance of at least 500 µM could be measured between the two IOLs in each eye included in the investigation (Figure 3A and B). Furthermore, cornea angle was found to be in the physiological range in each eye (Figure 3C and D). There were no reports of narrow-angle or secondary glaucoma in any case. Endothelial cell density (cells/mm2) did not differ significantly compared to the preoperative values (Figure 4). An average loss of only 2.00 ±1.58% (range: 0.23% to 5.14%) cells was observed. Intraocular pressures were similar pre- and postoperatively (p=0.8163; preoperative: 15.6 ±1.50 mmHg; range: 13 to 18 mmHg; postoperative: 15.7 ±1.26 mmHg; range: 14 to 17 mmHg). None of the patients had tear-film insufficiency during the postoperative period.
Visual Outcomes
Autorefractometry measurements and visual acuity assessment for distance, intermediate and near were performed in all cases. The pre- and postoperative results of autorefractometry measurements of spherical refraction (SPH, sphere; expressed in diopters), along with the subjective spherical refraction for distance and near are presented in Figure 5. A significant improvement in near vision, and subsequently a remarkable reduction in the required refractive correction was achieved (p<0.0001). Prior to the implantation of the supplementary lens, 16.7% of the eyes had spherical refraction (SPH) within ±0.5 D from emetropia, and 72.2% of the eyes were within the ±1.0 D range. Postoperative measurements showed significant improvement towards the target refraction, emetropia. The spherical refraction in 83.3% of the eyes was within ±0.5 D from emetropia, while all (100%) eyes were within ±1.0 D (Figure 6) from the intended target.
Figure 7 displays the pre- and postoperative visual acuities expressed with the logMAR scale. Table 2 shows the required spherical and cylindrical corrections in diopters along with the average magnitude of improvement in each case. A significant improvement in distance and near visual acuities was observed. Preoperative intermediate visual acuities were not measured. Intermediate visual acuity was measured in the postoperative period and the values displayed functional intermediate visual acuity.
 |
Table 2 Visual Acuities and Required Visual Correction Before and After Supplementary IOL Implantation |
Defocus Evaluation
The monofocal defocus curves did not differ between patients that received diffractive supplementary lenses with plano distance power and patients that received lenses with distance powers other than plano (data not shown). Therefore, the monocular defocus curves obtained in this study are presented as a pooled data set of all eyes (n=18). The defocus curves obtained confirmed the trifocal performance of the diffractive sulcus lenses with appropriate visual acuities in all ranges of vision (Figure 8A). The defocus curves obtained in this study with the diffractive add-on lenses were compared to the binocular defocus curves of patients previously implanted with the FineVision PodF capsular bag IOL by the same surgeon following the same measurement protocol under the same conditions. Multiple t-tests were used to compare matching defocus measurement points. Visual acuities with the addition of −1.5 and −2.0 diopters (intermediate range) were superior in the add-on trifocal group (p=0.0005 and p=0.0019, respectively). The supplementary add-on IOL also provided better visual acuity in the near segment of the curve (p=0.0240 for −3.5 D, and p=0.0003 for −4.0 D addition). All other sections of the defocus curves were found to be identical between the trifocal add-on and the trifocal capsular bag IOL groups.
When comparing the depth of focus (DOF) curve of the patients implanted with the secondary trifocal IOL to the curve derived from the eyes implanted with the trifocal capsular bag IOL, multiple t-tests showed that the two curves are identical (Figure 8B; n=12 eyes for the add-on, and n=20 eyes for the capsular bag lens; p>0.05 at each defocus measurement point). Subjective depth of focus assessments resulted in a DOF 90% of 0.486 ±0.09 (range 0.57 to 0.30) for the add-on, and 0.490 ±0.09 (range 0.59 to 0.30) for the tested capsular bag lens (p=0.8097). Objective measurements with the iTrace aberrometer confirmed the subjective results: DOF 90% was 0.300 ±0.07 (range 0.45 to 0.21) for the supplementary lens, and 0.284 ±0.06 (range 0.45 to 0.20) for the capsular bag IOL (p=0.4460).
Patient Satisfaction
Spectacle independence was achieved at all distances in all eyes implanted with the examined supplementary lens. The majority of patients reported being highly satisfied with the surgical outcome in all vision-related issues examined (Figure 9).
Discussion
The object of our investigation was to evaluate the safety of implanting a trifocal supplementary intraocular lens. An assessment of the efficacy of achieving spectacle independence following implantation of the investigated secondary IOL into the ciliary sulcus in pseudophakic patients previously implanted with monofocal capsular bag IOLs was also performed.
Eighteen eyes of eleven patients were implanted either monocularly or binocularly with the IOL under investigation. No complications were observed during surgery or in the postoperative period, which is a finding similar to previous studies involving the same ciliary sulcus lens family.10,11,14,15,24 The design of the tested lenses supports the integrity and physiologic function of the anterior segment of the eye. Intraocular pressure remained practically unchanged in these studies.11,15,24 Not even a transient increase in IOP was observed in this study; however, such changes have been reported in connection with the Sulcoflex Trifocal 703F supplementary IOL (Rayner Intraocular Lenses Ltd; Worthing, UK).25 The convex–concave geometry, along with the four flexible loop haptics ensure enough space in the sulcus. No angle-closure or secondary glaucoma was observed in this study. The pupils and irises were normal. No pigment dispersion syndrome was observed during the entire 6 months of follow-up.
All of the 18 IOLs implanted remained stable in terms of position in the eye over the entire 6 months of follow-up. No tilt, decentration, or any displacement was observed. Contrary to what McLintock et al reported in their cohort of 51 eyes followed for 3 months postoperatively, the mean lens rotation was 8.23° (maximum 17.63°), and after multiple repositioning of the Sulcoflex 653T IOL, a mean final rotation of 6.17° could be achieved.26 Based on our own experience, we believe that the special square design with the four flexible haptics ensures superior stability for the tested supplementary add-on IOL in the ciliary sulcus; hence, it can also provide a good foundation for an optic efficiently correcting astigmatism – nevertheless, in our current investigation, we did not use the toric model in any of our cases.
Generally, cataract surgery with phacoemulsification leads to a 4.01–16.0% loss of endothelial cells.27 Contrary to this, the decrease in endothelial cell count in this study was negligible (2.00 ±1.58%). The endothelial cell count in this study decreased from an average of 2146.8 ±86.5 to 2104.1 ±98.0 cells/mm2, which is far from the minimal numerical density of 400–500 cells/mm2 required to sustain the pumping activity of the corneal endothelium.28 Based on these results, we concluded that the implantation of the investigated supplementary add-on lens did not threaten corneal integrity or function. Similar values have been reported with the HumanOptics Add-On IOL (HumanOptics AG, Erlangen, Germany) by Basarir et al29; however, this 3-piece silicone lens is no longer available. To date, no comparable values relating to the Sulcoflex Trifocal 703F ciliary sulcus lens have been published.
It was observed in this study that the type and power of the primary IOL in the capsular bag does not have a significant impact on the inter-lenticular distance between the IOL in the capsular bag and the supplementary sulcus-fixated IOL of the same eye. Further studies involving a larger number of eyes are needed to further qualify this observation.
Refractive and visual outcomes all met our expectations: residual refractive error, which resulted from the surgery involving the primary IOL in the bag, was corrected with a high level of predictability: 83.3% of the eyes had a residual refraction (SPH) of no more than 0.5 D, and all eyes (100%) were within ±1.0 D of the target refraction, emetropia. Autorefraction and subjective refraction confirmed that the ciliary sulcus lens under investigation is a useful and effective tool to correct residual refractive errors and to provide complete spectacle independence in pseudophakic patients. Using the clearly trifocal performance optics near vision was improved from an average of 0.51 ±0.15 logMAR to 0.12 ±0.04, and subsequently none of the patients required further near vision correction.
Monocular defocus curves confirmed the trifocal performance of the investigated supplementary IOL. The defocus curves of the add-on lens were found to be similar to those of the Liberty trifocal capsular bag IOL made by the same manufacturer.16–18 In this study, we also compared the defocus curves of the supplementary IOL to a subset of patients previously implanted with a primary capsular bag trifocal IOL, which is considered the “gold standard” for trifocal lenses. We found that the add-on lens provided better visual acuities in the intermediate (−1.5 to −2.0 D) and near (−3.5 D and −4.0 D addition) ranges of vision. One limitation of this comparison is that only binocular defocus curves were available for the primary in-the-bag trifocal IOL group retrospectively with the mean age of this group being younger (mean 59.5 ±3.19 years) than that of the patients implanted with the add-on lens (mean 73.1 ±7.22 years). This difference in age between the two groups might be acceptable, as the add-on patients received implantation of the sulcus lens as a secondary procedure (they had already had cataract surgery performed with primary IOL implantation several years earlier). We do believe that taking these factors (age and mono- vs binocular results) into consideration only strengthens the potential lying in the examined supplementary IOL, and trifocality with good visual acuities was shown to be achievable in this study for pseudophakic patients previously implanted with monofocal capsular bag intraocular lenses. The defocus tolerances (DOF 90%) were shown to be identical for the two groups, both with objective and subjective measurement techniques.
This study revealed high patient satisfaction following the implantation of the supplementary add-on IOL. Six months postoperatively all patients reported a high level of satisfaction with their vision in general, with all patients experiencing complete spectacle independence. The majority of our patients (90.6%) did not mention any difficulties in connection with dysphotopic events and the remaining 9.4% experienced only minor disturbances. In contrast, 81% of the patients implanted with the refractive Sulcoflex 653F supplementary IOL experienced photic phenomena, and the disturbances were assessed as mild to moderate in most cases (93%/100%).30
Based on our current investigation we aim to extend the follow-up of the enrolled patients in order to evaluate the stability of the visual and refractive outcomes over a longer period. This longer follow-up will enable us to examine patients for any development of inter-lenticular opacification between the surfaces of the sulcus IOL and the primary in-the-bag IOL. However, inter-lenticular opacification is not expected to be observed due to the convex-concave design of the sulcus IOL and the clearance between the supplementary and primary in-the-bag IOL, which was also observed in our present study.
Conclusion
Based on our experiences we conclude that the investigated supplementary add-on IOL designed for ciliary sulcus implantation is a safe and effective option for providing spectacle independence in pseudophakic patients. When the add-on IOL is implanted into the eye of carefully selected patients it represents no risk to ocular integrity and functionality and preoperatively estimated refractive outcomes can be achieved in most cases. The diffractive optic surface ensures good visual quality in all ranges of vision, without any further refractive correction required.
Abbreviations
ACD, Anterior chamber depth; AUC, Area under the curve; AXL, Axial length; CYL, Cylindrical power; DOF, Depth of focus; EPS, Elevated Phase Shift; ETDRS, Early Treatment in Diabetic Retinopathy Study; ILO, Inter-lenticular opacification; IOL, Intraocular lens; IOP, Intraocular pressure; logMAR, Tenth-based logarithm of the Minimal Angle Resolution; OCT, Optical coherence tomography; SD, Standard deviation; SEQ, Spherical equivalent refraction; SPH, Spherical power; Sphere; UDVA, Uncorrected Distance Visual Acuity; UIVA, Uncorrected Intermediate Visual Acuity; UNVA, Uncorrected Near Visual Acuity; VSOTF, Visual Strehl optical transfer function.
Disclosure
The authors report no conflicts of interest in this work. The authors also declare that none of them has any financial interest in the subject of the investigation or receives payment from any mentioned company.
References
1. Fricke TR, Tahhan N, Resnikoff S, et al. Global prevalence of presbyopia and vision impairment from uncorrected presbyopia: systematic review, meta-analysis, and modelling. Ophthalmology. 2018;125(10):1492–1499. doi:10.1016/j.ophtha.2018.04.013
2. Arlt E, Krall E, Moussa S, Grabner G, Dexl A. Implantable inlay devices for presbyopia: the evidence to date. Clin Ophthalmol. 2015;9:129–137. doi:10.2147/OPTH.S57056
3. World Health Organization. [homepage on the internet] Priority eye diseases: cataract. Prevention of Blindness and Visual Impairment. Available from: https://www.who.int/blindness/causes/priority/en/index1.html.
4. Marcos S. Ocular ageing: improving the quality of sight for cataract and presbyopia sufferers. Lychnos R&D Aging Dev New Technol. 2010;2:60–65.
5. Mertens EL, Is a robust growth of premium IOL use in the cards? - The answer is likely yes, according to manufacturers of premium IOLs and market scope research. Cataract Refract Surg Eur. 2015;10:8.
6. Slade SG, Corley JA, Doane JF, et al. The premium IOL channel: pitfalls & prospects. Cataract Refract Surg Eur. 2018. Issue ‘Is Lens surgery the new LASIK?’. Available from: https://crstoday.com/articles/2018-may/the-premium-iol-channel-pitfalls-prospects/. Accessed January 10, 2020.
7. Manzouri B, Dari M, Claoué C. Supplementary IOLs: monofocal and multifocal, their applications and limitations. Asia Pac J Ophthalmol (Phila). 2017;6:358–363. doi:10.22608/APO.2017110
8. Hill WE. Refractive enhancement with piggybacking intraocular lenses. In: Chang D, editor. Mastering Refractive IOLs - the Art and Science. Chapter 217. Thorofare: Slack Inc.; 2008:
9. Rubenstein JB. Piggyback IOLs for residual refractive error after cataract surgery. Cataract Refract Surg Today. 2012;12:28–30.
10. Reiter N, Werner L, Guan J, et al. Assessment of a new hydrophilic acrylic supplementary IOL for sulcus fixation in pseudophakic cadaver eyes. Nat Eye (London). 2017;31(5):802–809. doi:10.1038/eye.2016.310
11. Gundersen KG, Potvin R. A review of results after implantation of a secondary intraocular lens to correct residual refractive error after cataract surgery. Clin Ophthalmol. 2017;3(11):1791–1796. doi:10.2147/OPTH.S144675
12. Gerten G, Kermani O, Schmiedt K, Farvili E, Foerster A, Oberheide U. Dual intraocular lens implantation: monofocal lens in the bag and additional diffractive multifocal lens in the sulcus. J Cataract Refract Surg. 2009;35(12):2136–2143. doi:10.1016/j.jcrs.2009.07.014
13. Kahraman G, Amon M. New supplementary intraocular lens for refractive enhancement in pseudophakic patients. J Cataract Refract Surg. 2010;36(7):1090–1094. doi:10.1016/j.jcrs.2009.12.045
14. Scharioth GB. New add-on intraocular lens for patients with age-related macular degeneration. J Cataract Refract Surg. 2015;41(8):1559–1563. doi:10.1016/j.jcrs.2015.07.018
15. Nekolova J, Rozsival P, Sin M, Jiraskova N. Scharioth macula lens: a new intraocular implant for low-vision patients with stabilized maculopathy- first experience. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161(2):206–209. doi:10.5507/bp.2017.014
16. Fernández J, Rodríguez-Vallejo M, Martínez J, Tauste A, Piñero DP. Biometric factors associated with the visual performance of a high addition multifocal intraocular lens. Curr Eye Res. 2018;43(8):998–1005. doi:10.1080/02713683.2018.1478981
17. García-Bella J, Ventura-Abreu N, Morales-Fernández L, et al. Visual outcomes after progressive apodized diffractive intraocular lens implantation. Eur J Ophthalmol. 2018;28(3):282–286. doi:10.5301/ejo.5001030
18. Gyory JF, Madár E, Srinivasan S. Implantation of a diffractive-refractive trifocal intraocular lens with centralized diffractive rings: two-year results. J Cataract Refract Surg. 2019;45(5):639–646. doi:10.1016/j.jcrs.2019.01.024
19. World Medical Association. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
20. Mangione CM, Lee PP, Gutierrez PR, Spritzer K, Berry S, Hays RD. Development of the 25-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119(7):1050–1058. doi:10.1001/archopht.119.7.1050
21. LeBoyer RM, Werner L, Snyder ME, Mamalis N, Riemann CD, Augsberger JJ. Acute haptic-induced ciliary sulcus irritation associated with single-piece AcrySof intraocular lenses. J Cataract Refract Surg. 2005;31(7):1421–1427. doi:10.1016/j.jcrs.2004.12.056
22. 1stQ AddOn® Calculator. [homepage on the Internet] Available from: https://www.1stq.de/en/34-addoncalculator.
23. Palomino-Bautista C. Experiences with the 1stQ AddOn® trifocal supplementary intraocular lens. Mendeley Data. 2019. doi:10.17632/msbfkx4gv8.1
24. Srinivasan S, Scharioth G, Riehl A, et al. Implantation of Scharioth macula lens in patients with age-related macular degeneration: results of a prospective European multicentre clinical trial. BMJ Open Ophthalmol. 2019;4:e000322. doi:10.1136/bmjophth-2019-000322
25. Amon M, Mularoni A, Spedale F, et al. Prospective multicentre evaluation assessing visual acuity and patient satisfaction in pseudophakic patients with bilaterally implanted supplementary trifocal intraocular lens. Available from: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=2ahUKEwiS2OTO9obkAhXjwosKHX5HCtQQFjABegQIARAC&url=https%3A%2F%2Frayner.com%2Fwp-content%2Fuploads%2F2019%2F05%2F337.Prospective-multicentre-evaluation-assessing-visual-acuity-and-patient-satisfaction-in-pseudophakic-patientsSulcoflexTrifocal.pdf&usg=AOvVaw3lGX7LvbBB9i13fCEySKR7.
26. McLintock CA, McKelvie J, Gatzioufas Z, Wilson JJ, Stephensen DC, Apel AJG. Outcomes of toric supplementary intraocular lenses for residual astigmatic refractive error in pseudophakic eyes. Int Ophthalmol. 2019;39(9):1965–1972. doi:10.1007/s10792-018-1027-7
27. Hwang HB, Lyu B, Yim HB, Lee NY. Endothelial cell loss after phacoemulsification according to different anterior chamber depths. J Ophthalmol. Epub 2015 Aug 31.
28. Sobottka-Ventura AC, Wälti R, Böhnke M. Corneal thickness and endothelial density before and after cataract surgery. Br J Ophthalmol. 2001;85(1):18–20. doi:10.1136/bjo.85.1.18
29. Basarir B, Kaya V, Altan C, Karakus S, Pinarci EY, Demirok A. The use of a supplemental sulcus fixated IOL (HumanOptics Add-On IOL) to correct pseudophakic refractive errors. Eur J Ophthalmol. 2012;22(6):898–903. doi:10.5301/ejo.5000156
30. Schrecker J, Blass S, Langenbucher A. Silicone-diffractive versus acrylic-refractive supplementary IOLs: visual performance and manual handling. J Refract Surg. 2014;30(1):41–48. doi:10.3928/1081597X-20131217-05
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.