Back to Journals » Cancer Management and Research » Volume 12
Sparing Organs at Risk with Simultaneous Integrated Boost Volumetric Modulated Arc Therapy for Locally Advanced Non-Small Cell Lung Cancer: An Automatic Treatment Planning Study
Authors Wang D, Chen J , Zhang X, Zhang T , Wang L, Feng Q, Zhou Z, Dai J, Bi N
Received 21 July 2020
Accepted for publication 28 August 2020
Published 6 October 2020 Volume 2020:12 Pages 9643—9653
DOI https://doi.org/10.2147/CMAR.S273197
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Daquan Wang,1,* Jiayun Chen,1,* Xiaodong Zhang,2 Tao Zhang,1 Luhua Wang,1 Qinfu Feng,1 Zongmei Zhou,1 Jianrong Dai,1 Nan Bi1
1Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2Department of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX, USA
*These authors contributed equally to this work
Correspondence: Nan Bi
Department of Radiation Oncology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No. 17 Panjiayuannanli, Chaoyang District, Beijing 100021, People’s Republic of China
Tel +86 10 87788995
Email [email protected]
Background: The technique of simultaneous integrated boost volumetric modulated arc therapy (SIB-VMAT) has been widely used in locally advanced non-small cell lung cancer; however, its dosimetric advantages are seldom reported. This study aimed to quantify dosimetric advantages of SIB-VMAT.
Methods: Forty patients with stage III non-small cell lung cancer in our hospital were retrospectively included. SIB-VMAT and conventional VMAT (C-VMAT) plans were generated for every patient using the automatic treatment planning system. A reduced dose was delivered to PTV in SIB-VAMT plans compared to C-VMAT plans (50.4Gy vs 60Gy). The prescribed dose was 50.4 Gy in 28 fractions to PTV and 59.92 Gy in 28 fractions to PGTV in SIB-VMAT plans, while 60 Gy in 30 fractions to PTV in C-VMAT plans. Dose-volume metrics of PTV, total lung, heart, esophagus and spinal cord were recorded. The quality score was used to evaluate organs at risk (OAR) protection for two type prescription plans.
Results: Conformal coverage of the targets (PGTV/PTV) by 95% of the prescription dose was well achieved in radiation plans. SIB-VMAT plans achieved significantly higher quality score than C-VMAT plans (Mean: 68.15± 13.32 vs 49.15± 13.35, P< 0.001). More plans scored above sixty in SIB-VMAT group compared to C-VMAT group (72.5% vs 20%, P< 0.001). Notable reductions in mean dose, V30, V40 and V50 of total lung were observed in SIB-VMAT plans compared to C-VMAT plans, with median decreased proportions of 6.5%, 8.7%, 19.6% and 32.1%, respectively. Statistically significant decrease in heart V30 and V40 was also achieved in SIB-VMAT plans, with median decreased proportions of 26.1% and 38.8%. SIB-VMAT plans achieved significant reductions in the maximum doses to both esophagus and spinal cord. Patients with CTV/(GTV+GTVnd) ≥ 8.6 showed more notable decrease in total lung V50 (median, 33.6% vs 28.8%, P=0.001) in SIB-VMAT plans compared to those with the ratio being less than 8.6.
Conclusion: SIB-VMAT technique could lead to a substantial sparing of normal organs, including lung, heart, esophagus and cord, mainly through reducing high and inter-median dose exposure. Patients with CTV/(GTV+GTVnd) ≥ 8.6 might benefit more from SIB-VMAT.
Keywords: lung cancer, simultaneous integrated boost, radiotherapy, automatic planning, organ at risks
Background
Definitive radiotherapy combined with chemotherapy remains the standard regimen for inoperable locally advanced non-small cell lung cancer (LA-NSCLC),1 with median overall survival (OS) ranging from 20 to 30 months.2–4 However, in clinical practice, certain patients with a large tumor, extensive lymph node metastasis or poor pulmonary function often failed to tolerate definitive radiotherapy because of high risk of treatment-related toxicities. Pneumonitis induced by radiation (RP) remained the most important dose-limiting toxicity, which might result in treatment interruption or lead to inferior survival outcomes. Continuous efforts have been made to develop an effective alternative treatment regimen for patients who are not candidates for radical radiotherapy, but the results are not satisfying. Nawrocki et al5 evaluated concurrent chemotherapy and palliative radiotherapy (30Gy/10f) in stage III NSCLC patients who were not eligible for surgery or definitive radiotherapy, and the median OS was only 12.9 months.5 In the Phase III trial conducted by the Norwegian Lung Cancer Study Group, a palliative regimen of 42Gy in 15 fractions was applied in stage III NSCLC patients with large tumors, poor performance status or weight loss. Despite that the survival and quality-of-life benefits were observed compared to chemotherapy alone, the survival outcome remained poor, with a median OS of 12.6 months.6
In recent years, increasing interest in reducing radiation treatment volumes has emerged, with the purpose of reducing toxicity while maintaining local control. In the era of intensity-modulated radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT), simultaneous integrated boost (SIB) strategy was chosen and applied in the treatment of LA-NSCLC. According to our knowledge, SIB technique was applied in two ways in lung cancer radiotherapy. Firstly, it could be used for dose-escalation, by conferring a radical dose (60Gy) to PTV while a higher dose (≥66Gy) to PGTV. Despite the negative result of RTOG 0617 trial, much interest remained in dose-escalation for LA-NSCLC.4 A retrospective analysis of 33,566 patients with stage III NSCLC revealed that patients with a dose of ≥66Gy had better OS than those with dose of 59.4–60Gy (median: 21.1 vs 18.8 months, P<0.001).7 SIB-IMRT seemed to be an effective tool for dose-escalation, in which the increased dose was only delivered to GTV/PGTV. Secondly, SIB technique could be used for dose-reduction, by reducing PTV dose (45–54Gy) while maintaining a radical dose (60Gy) to PGTV. The second strategy resulted in improved normal-tissue sparing and treatment tolerance, which expanded the applicable people for radical radiotherapy. Liu et al8 found that a prescribed dose of 54Gy to the elective nodal regions among small cell lung cancer patients could result in reduced radiation-induced toxicities without compromising local-regional control and overall survival.8 Our previous study indicated that reducing the dose to clinical target volume based on SIB technique (PTV 50.4Gy/28f; PGTV: 59.92Gy/28f) offered LA-NSCLC patients with large tumor volume the chance to receive definitive radiotherapy.9 Other studies also confirmed that reducing CTV dose with SIB technique was effective and well tolerated for patients with LA-NSCLC.10,11 However, no study has clarified the dosimetric advantages of SIB-based dose-reduction regimen for NSCLC.
With the method of deep machine learning, automatic treatment planning has been applied in the generation of radiation plans. The mdaccAutoPlan system was developed based on our clinical protocol, with authorization from developer Zhang’s team,12 and the technique improved the consistency and quality of plans and reduced treatment planning time. It has been proved that the VMAT plans with high quality can be automatically generated for most stage III/IV NSCLC patients treated with curative radiotherapy.13,14
In this study, we implemented an automatic planning method to generate VMAT plans and aimed to quantify the dose-sparing benefits of SIB-VMAT compared to C-VMAT plans.
Materials and Methods
Patients
Forty patients with stage III NSCLC in our hospital between 2014 and 2016 were retrospectively included. The major inclusion criteria were histologically/cytologically confirmed NSCLC, older than 18 at the time of diagnosis, stage III disease (American Joint Committee on Cancer, 7th edition), receiving thoracic radiotherapy and having complete image material of simulated CT. The patient characteristics are summarized in Table 1. A total of 21 (52.5%) tumors were located in the left lung and 19 (47.5%) in the right lung. Overall, 8 (20%) patients had stage IIIA and 32 (80%) had stage IIIB. Every patient was retrospectively optimized using automated VMAT planning methods. This study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (Approval No. 19–048/1833).
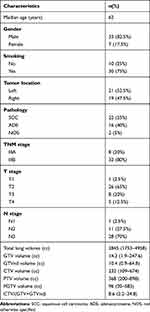 |
Table 1 The Characteristics of Patients |
Immobilization and Simulation
The patients were immobilized in the supine position with a thermoplastic custom-made mask (including head-neck-shoulder mask and chest mask). The computed tomographic (CT) scan at 5-mm intervals with contrast enhancement for each patient was obtained using a CT simulator. Four-dimensional CT (4DCT) was used to handle respiratory movements. The scanned regions extended from the laryngeal prominence to the bottom of the L2 vertebral body. These CT images were transferred to Pinnacle 9.10 system (Version 9.10, Philips Radiation Oncology System, Fitchburg, WI, USA) for planning.
Target Volume and Organs at Risk Delineation
The Radiotherapy and Oncology Group (RTOG) guidelines served as a reference for the delineation of target volumes and organs at risk (OARs). The gross tumor volume (GTV) involved the primary lesions and positive lymph nodes, which were defined as those with a short-axis diameter of at least 1 cm on CT images or less than 1 cm but having high fluorodeoxyglucose (FDG) uptake on positron emission tomography (PET)-CT images. The clinical target volume (CTV) was generated by expanding GTV by 0.6–0.8 cm, covering the involved hilum and mediastinal nodal stations. The planning target volume (PTV) was created by a uniform expansion of 0.5 cm surrounding the CTV. The planning gross tumor volume (PGTV) was generated by expanding GTV by 0.5 cm. The lungs, heart, esophagus and cord were contoured as the dose constraint OARs.
Prescribed Dose and Dose Constraints
The prescribed dose was 50.4Gy in 28 fractions to PTV and 59.92Gy in 28 fractions to PGTV in SIB-VMAT plans, with 60Gy in 30 fractions to PTV in C-VMAT plans. The dose should be prescribed to cover ≥95% of the PTV/PGTV volume. The maximum dose should be less than 110% of the prescribed dose. The dose constraints of OARs were referred to the values summarized in Table 2.
 |
Table 2 Descriptions of Each Plan Quality Metric (PQM) |
Automatic Treatment Planning
Both SIB-VMAT and C-VMAT plans were designed with 160-leaf MLC VersaHD LINAC (Elekta, AB, Stockholm, Sweden). Each plan was designed with the same optimization parameter by the mdaccAutoPlan system and evaluated quantitatively for each patient. Plans could be generated by one button click in the mdaccAutoPlan system which was a plug-in to the Pinnacle3 TPS. The mdaccAutoPlan system was developed based on our clinical protocol, with authorization from developer Zhang’s team.12 The quality of radiation plans mainly depended on the experience and skill level of radiation physicists, thus the manual plans made by different physicists might be of variable quality.15 The use of automated planning decreased inter-operator variability and guaranteed high quality VMAT treatment plans in our study. VMAT plans were calculated using 6MV photons, with a maximum variable dose rate of 600 MU/min. Double-arcs with coplanar arcs of 360° shared the same iso-center, using opposite rotation (clockwise and counter-clockwise). The collimator was always rotated to 10° and 350°, respectively, in two arcs, to avoid a tongue and groove effect. The gantry angle spacing was 4°. The calculation voxel size was isotropic and 4 mm.
Plan Comparison
Dose coverage of the PGTV/PTV by plans was evaluated using the endpoint of Vp (volume receiving at least the prescribed dose). The following dosimetric parameters were recorded to evaluate tissue-sparing: total lung; mean dose (MLD) and volume minus GTV receiving 5Gy (V5), 10Gy (V10), 20Gy (V20), 30Gy (V30), 40Gy (V40) and 50Gy (V50), esophagus; maximum dose (Dmax), mean dose and volume receiving 40G (V40) and 50Gy (V50), heart; maximum dose, mean dose and volume receiving 5Gy (V5), 30Gy (V30) and 40Gy (V40), spinal cord; maximum dose, spinal cord PRV; maximum dose.
Plan quality was evaluated by plan scores which was introduced by the ESTRO QUASIMODO group.16 The QUASIMODO (Quality Assurance of Intensity-Modulated beams in Radiation Oncology) network consisted of 15 radiotherapy institutions from nine European countries. The organization introduced the plan scores to quantitatively compare different radiation plans against the same dose objectives. This scoring system has been proved to be accurate and objective for plan comparison in an inter-institutional study conducted by Nelms et al.17 As there were more predefined structures in this analysis than that in the one by Bohsung et al,16 the dosimetric data was extracted from the collected data sets and compared to the corresponding dose objectives listed in Table 2. Plan scores were composed of the Plan Quality Metric (PQM) components. The Plan Quality Algorithm (PQA) was employed here as an objective method to quantify a plan’s quality, particularly in terms of meeting clear and specific treatment-plan goals. PQM removed any ambiguity from the plan objectives and provided a fair comparison of plan results.17,18 There were 10 components of the PQM for the cases, with a full score of 100. Each plan got a score based on a unique PQA, of which the PQM value function was used to calculate a point value. Descriptions of each PQM are shown in Table 2. VnGy (%) was the percentage of the organ volume receiving ≥n Gy.18 Dmax and Dmean were the maximum and average absorbed doses delivered to each OAR, respectively.19 Taking V5 as an example, the score would be zero with V5≥75%, and ten with V5≤40%. If V5 was equal to 60%, the score was calculated as follows: Score = (75%-60%)/(75%-40%)*10 = 4.2857. We should note that the optimal objectives of each PQM component were stricter than the metric used in clinical routine in order to distinguish the quality of plans.
Statistical Analysis
Statistical analysis was performed using the software of SPSS (Version 22.0, IBM Corporation, Armonk, NY, USA). Dosimetric parameters are presented as median value with range. Plan quality scores are presented as mean ± standard deviation. Continuous variables were compared using the Mann–Whitney U-test. Comparisons of categorical variables were performed using Chi-square or Fisher’s test. All tests were two-sided, and P<0.05 was considered statistically significant.
Results
Dose Coverage of PGTV and PTV
Conformal coverage of the targets (PGTV/PTV) by 95% of the prescription dose was well achieved in radiation plans. For SIB-VMAT plans, the median Vp (volume receiving at least 59.92Gy) of PGTV was 95.06% (94.8–95.5%), and the median Vp (volume receiving at least 50.4Gy) of PTV was 98.87% (95–99.92%). For C-VMAT plans, the median Vp (volume receiving at least 60Gy) of PTV was 95.03% (94.86–95.4%). Figure 1 showed the typical isodose distributions of the PTV/PGTV and the OARs from one patient. Both SIB-VMAT and C-VMAT plans achieved good conformity to the prescription isodose line of the PGTV/PTV. A visibly reduced volume of normal tissue exposed to the 60Gy dose was observed in the SIB-VMAT plan.
Plan Quality Score
The quality scores of SIB-VMAT plans were significantly higher than that of C-VMAT plans (Mean: 68.15±13.32 vs 49.15±13.35, P<0.001). It demonstrated that the SIB strategy had better OAR sparing. The scores above 80 were obtained in 22.5% of SIB-VMAT plans, but in none of the C-VMAT plans (0%). More plans scored above 60 in the SIB-VMAT group compared to the C-VMAT group (72.5% vs 20%, P<0.001).
Pulmonary Dose
The median total lung volume was 2845 cc (1753–4958 cc). As illustrated in Table 3, several dosimetric objectives in lung have exclusively reduced as following: 1) The total lung V30 decreased from 19.2% (12.4–26.2%) in C-VMAT plans to 17.08% (10.21–25.4%) in SIB-VMAT plans (P=0.037), with median decreased proportion of 8.7% (0.2–24.6%); 2) The MLD was 14.7Gy (11.32–19.44Gy) in C-VMAT plans compared to 13.8Gy (10.6–18.1Gy) in SIB-VMAT (P=0.045), with median decreased proportion of 6.5% (3.7–14.5%); 3) The significant reduction in the total lung V40 (P=0.002) was also achieved in the SIB-VMAT group, with median decreased proportion of 19.6% (1.6–31%); 4) In lung V50, SIB-VMAT plans got a sharp reduction compared to C-VMAT plans (median, 4.79% vs 7.16%, P<0.001), and the median decreased proportion was 32.1% (17.9–45.5%). Other dosimetric parameters, such as lung V5 (P=0.366), V10 (P=0.965) and V20 (P=0.95) were comparable between the SIB-VMAT and C-VMAT plans.
 |
Table 3 Comparison of Dosimetric Metrics Between SIB-VMAT and C-VMAT Plans |
Heart Dose
Compared to the C-VMAT plans, statistically significant reductions in heart V30 (median, 10.34% vs 15.4%, P=0.049) and V40 (median, 4.36% vs 6.93%, P=0.005) were observed in SIB-VMAT plans, with decreased proportions of 26.1% (0–53.7%) and 38.8% (0–67.2%). The maximum doses of heart were 66.21Gy (8.18–70Gy) and 61.47Gy (7.02–65.1Gy) for C-VMAT and SIB-VMAT plans, respectively (P<0.001). Box-plots of dosimetric metrics for SIB-VMAT vs C-VMAT plans were shown in Figure 2. SIB-VMAT plans achieved lower heart Dmax, V30 and V40 than C-VMAT plans.
Esophagus Dose
A large decrease in the maximum dose of esophagus was observed in SIB-VMAT plans, from 66.84Gy (61.06–70.63Gy) to 62.82Gy (51.73–65.28Gy) (P<0.001). The median decreased proportion was 7.2% (1.6–19.9%). SIB-VMAT plans achieved a slight decrease in esophagus mean dose compared to C-VMAT plans, but without statistical significance (median, 24.97Gy vs 28.08Gy, P=0.119).
Spinal Cord Dose
The SIB-VMAT plans achieved significant reductions in maximum doses of spinal cord (median, 32.55Gy vs 38.77Gy, P<0.001) and cord PRV (median, 37.74Gy vs 44.78Gy, P<0.001) compared to C-VMAT plans. All plans got acceptable maximum doses to spinal cord using the SIB-VMAT approach, which ranged from 30.1 to 39Gy.
Predictors of Dose Reductions
The correlations between volume parameters and dose reductions of OAR in SIB-VAMT plans were analyzed. Based on the ratio of CTV to (GTV+GTVnd), 40 patients were divided into two groups: patients with CTV/(GTV+GTVnd) being less than 8.6 (the median value) were classified as group A, otherwise they were classified as group B [CTV/(GTV+GTV)≥8.6]. Patients in group B showed more notable reduction in total lung V50 (median, 33.6% vs 28.8%, P=0.001) in SIB-VMAT plans than those in group A. Besides, a greater decrease was also observed in total lung V40 (median, 21.9% vs 17.8%, P=0.157) and esophagus mean dose (median, 11.5% vs 8.2%, P=0.072), despite neither of them being statistically significant. Detailed information is available in Table 4. Neither PTV nor PGTV volume was found to be predictive of the dose reductions of OARs in SIB-VAMT plans.
 |
Table 4 The Correlation Between CTV/(GTV+GTVnd) and Dose Reductions of OARs in SIB-VAMT Plans |
Discussion
This is a unique study focusing on the dosimetric advantages of SIB-VMAT in LA-NSCLC. Moreover, the inter-operator variability was reduced in the plan comparison by using the automated planning approach.
In this study, a SIB-based dose-reduction regimen was applied, with a prescribed dose of 50.4Gy in 28 fractions to PTV and 59.92Gy in 28 fractions to PGTV. The dose regimen has been confirmed to be effective and increases the number of applicable people for definitive radiotherapy in our previous study.9,20 Patients with large PTV or poor lung function benefit a lot from the SIB-based dose-reduction regimen. We conducted this treatment planning study to further quantify the dosimetric advantage of this dose regimen. In the trial of RTOG 0617, the prescribed dose delivered to gross tumor volume (GTV) and clinical target volume (CTV) were the same in the control group, with a total dose of 60Gy in 30 fractions.4 Similarly, this dose regimen has been widely used in our hospital and confirmed to be safe and effective.21 Therefore, we applied this dose regimen in C-VMAT plans in this study. The automated planning method was used to exclude the influence caused by subjective factors. This approach could be straightforwardto implement in future clinical practice, saving human labor and guaranteeing the consistency and quality of VMAT plans. The results showed that SIB-VMAT plans yielded full protection for normal structures compared to C-VMAT plans, with significant reductions in the doses to total lung, heart, esophagus, and spinal cord. SIB-VMAT plans obtained higher quality scores than C-VMAT plans, indicating a superior normal-tissue sparing for SIB-VMAT approach.
Radiation-induced pneumonitis (RP) was the most common dose-limiting complication of LA-NSCLC treated by thoracic radiotherapy. Numerous studies indicated that dosimetric parameters, such as mean lung dose (MLD), V5, V10, V20, V30, V40, V50, were associated with the occurrence of RP.22–25 All metrics above were evaluated in our study. According to the study conducted by Sheng et al,25 total lung V5, V20, V30 and mean dose were all correlated with grade ≥2 RP, furthermore, lung V30 was the independent risk factor.25 Patients with high lung V30 (exceeding 14.2%) suffered 2.92-fold increased risk of RP compared to those with low V30 (no more than 14.2%). Another two studies also considered lung V30 as an independent predictor for the occurrence of symptomatic RP.24,26 In our study, the SIB-VMAT plans achieved a sharp reduction in lung V30, with median decreased proportion of 8.7%, which would benefit a lot in the reduction of lung toxicities. Xia et al27 compared the SIB-IMRT with C-IMRT plans, and found that the SIB-IMRT plans got lower mean dose, V5 and V20 of total lung.27 According to the study conducted by Xhaferllari et al,28 VMAT was dosimetrically advantageous in treating early-stage NSCLC with SABR compared to fixed-beam IMRT, and provided significantly shorter treatment times.28 Moreover, they pointed out that no significant difference was observed in the two VMAT techniques (SmartArc (SA) and RapidArc (RA)). No studies have looked into the SIB and conventional prescription VMAT plans. Since it was widely known that VMAT was superior to IMRT in dosimetric aspects,28 our study focused on these two type of prescriptions in the VMAT plans. SIB-VMAT plans achieved significant reductions in mean dose, V30, V40 and V50 of total lung compared to C-VMAT plans, but lung V5 (P=0.366), V10 (P=0.965) and V20 (P=0.95) were comparable between the two groups. The advantages of SIB-VMAT mainly rested on the reduction of high and inter-median dose exposure in the pulmonary, but not on the decrease of low dose exposure.
The cardiac doses have been proved to correlate with the outcomes of LA-NSCLC, including both radiation-induced toxicities and overall survival. According to a systematic review conducted by Zhang et al,29 the heart dose-volume parameters of V5 and V30 were independent predictors for both cardiac events and overall survival among NSCLC patients.29 Similarly, Wang et al30 found that heart V30 was significantly correlated with cardiac toxicity, including pericardial, ischemic and arrhythmic events.30 The secondary analysis of RTOG 0617 trial also indicated that heart V40 was significantly associated with OS for LA-NSCLC (HR 1.012, P<0.001).31 Our study observed obvious dosimetric advantages in heart V30 and V40 in SIB-VMAT plans compared to C-VMAT plans, with decreased proportions of 26.1% and 38.8%. It suggested that SIB-VMAT plan displayed better performance in heart protection, especially in terms of the reduction of heart volumes exposed to high and inter-median radiation doses.
Esophagus toxicity was also a common complication when radiotherapy was delivered to the thorax. Numerous studies have correlated esophagus toxicity with dose-volumetric data for lung cancer patients treated with radiotherapy, including the maximum dose, mean dose and the volume of esophagus receiving 20–70Gy.32 However, the best predictors remained unclear. According to the model made by the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) group, the incidence rate of acute esophagitis was supposed to surpass 30% when V50 exceeded 40%.33 Other studies also reported that the maximum dose above 58 or 60Gy was significantly associated with the increased risk of grade 3–5 esophagus injury.34,35 The SIB-VMAT plans achieved significant reductions in maximum dose of esophagus, which is of great benefit in the prevention of severe esophagitis.
In order to identify patients who could benefit more from SIB-VMAT, we analyzed the correlations between dose reductions of OAR and volume parameters, including PTV, PGTV and CTV/(GTV+GTVnd). Only the variable, CTV/(GTV+GTVnd), was found to have predictive value. Considering that a reduced dose was delivered to CTV regions in SIB-VMAT plans, rather than GTV/GTVnd areas, patients with large CTV volume but relatively limited GTV/GTVnd volume might achieve more significant advantage from the technique of SIB-VMAT. Our study revealed that patients with CTV/(GTV+GTVnd)≥8.6 achieved greater decrease in lung and esophagus doses, which provided evidence for this view. Due to the limited size of sample, only total lung V50 was observed to be statistically significant. Future studies with large sample sizes are warranted to confirm the predictive value of CTV/(GTV+GTVnd).
The technique of automated planning was used in VMAT plan design in the present study, and conformal coverage of the PGTV/PTV by the 95% of the prescription dose was well achieved. 22.5% of SIB plans scored above 80 and 72.5% scored above 60. It indicated that the quality of automatic plans was promising. The mdaccAutoPlan system used in our study has been proved to guarantee high quality of VMAT plans by Prof. Zhang and Prof. Liao from MD Anderson Cancer Center.14 They evaluated the quality of VMAT and IMRT plans generated by the mdaccAutoPlan system for patients with stage III lung cancer. Independent blind reviews by five experienced radiation oncologists and dosimetric measures showed that the mdaccAutoPlan system was capable of generating high-quality VMAT and IMRT plans for stage III lung cancer. In our study, several plans with very large volume of PTV (>600 cc) exceeded the OAR constraints, therefore manual intervention in plan design should be provided for particular patients with large target volume. After manual adjustment by experienced radiation physicists, the SIB-VAMT plans with large PTV volume could achieve acceptable OAR doses. For example, by sacrificing the conformity or homogeneity of targets, the doses to OAR could be decreased. At this point, an automated plan may serve as a benchmark for planners (dosimetrists or medical physicists) and radiation oncologists when clinical decisions are made. At present, one research study is being carried out to further optimize the autoplan system, in order to satisfy more functions and demands. It should be emphasized that the manual adjustment was not involved in our treatment planning study, to ensure the objective comparison of plan quality. The manual adjustment may bring the bias in this plan comparison when planners put more efforts on one plan.
There are several limitations in the present study. Firstly, as a study conducted in a single center with small sample size, the results may be affected by potential confounding factors. Secondly, treatment-related toxicities of patients receiving the proposed SIB-VMAT strategy were not reported in this study. Therefore, further studies are still needed to present the reduced toxicity of SIB-VMAT technique in clinic practice.
Conclusions
In summary, we demonstrated that the SIB technique could lead to substantial sparing of OAR, including lung, heart, esophagus and spinal cord, mainly through reducing high and inter-median dose exposure. The technique of automated planning method was firstly implemented in the VMAT plan design and comparison for LA-NSCLC. Patients with CTV/(GTV+GTVnd) ≥8.6 might benefit more from SIB-VMAT. Future studies are warranted to explore whether the dosimetric advantages can be translated into improved toxicity outcomes.
Abbreviations
SIB, simultaneous integrated boost; VMAT, volumetric-modulated arc therapy; IMRT, intensity-modulated radiation therapy; NSCLC, non-small cell lung cancer; GTV, gross tumor volume; CTV, clinical target volume; CT, computed tomography; PTV, planning target volume; PGTV, planning gross tumor volume; OS, overall survival; PFS, progression-free survival; OAR, organs at risk; MLD, mean lung dose; Dmax; the maximum dose; PRV, planning organ at risk volume.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences (Approval No. 19-048/1833). Written informed consent for scientific usage of clinical data was obtained from all patients.
Disclosure
This study was supported by the Capital's Funds for Health Improvement and Research (grant nubmer 2020-2-4022). The authors report no conflicts of interest in this work.
References
1. Bezjak A, Temin S, Franklin G, et al. Definitive and adjuvant radiotherapy in locally advanced non-small-cell lung cancer: American Society of Clinical Oncology clinical practice guideline endorsement of the American Society for Radiation Oncology evidence-based clinical practice guideline. J Clin Oncol. 2015;33:2100–2105. doi:10.1200/JCO.2014.59.2360
2. Liang J, Bi N, Wu S, et al. Etoposide and cisplatin versus paclitaxel and carboplatin with concurrent thoracic radiotherapy in unresectable stage III non-small cell lung cancer: a multicenter randomized phase III trial. Ann Oncol. 2017;28:777–783. doi:10.1093/annonc/mdx009
3. Senan S, Brade A, Wang LH, et al. PROCLAIM: randomized Phase III trial of pemetrexed-cisplatin or etoposide-cisplatin plus thoracic radiation therapy followed by consolidation chemotherapy in locally advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2016;34:953–962. doi:10.1200/JCO.2015.64.8824
4. Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial Phase 3 study. Lancet Oncol. 2015;16:187–199.
5. Nawrocki S, Krzakowski M, Wasilewska-Tesluk E, et al. Concurrent chemotherapy and short course radiotherapy in patients with stage IIIA to IIIB non-small cell lung cancer not eligible for radical treatment: results of a randomized Phase II study. J Thorac Oncol. 2010;5:1255–1262. doi:10.1097/JTO.0b013e3181e15d33
6. Strom HH, Bremnes RM, Sundstrom SH, et al. Concurrent palliative chemoradiation leads to survival and quality of life benefits in poor prognosis stage III non-small-cell lung cancer: a randomised trial by the Norwegian Lung Cancer Study Group. Br J Cancer. 2013;109:1467–1475. doi:10.1038/bjc.2013.466
7. Brower JV, Amini A, Chen S, et al. Improved survival with dose-escalated radiotherapy in stage III non-small-cell lung cancer: analysis of the National Cancer Database. Ann Oncol. 2016;27(10):1887–1894. doi:10.1093/annonc/mdw276
8. Liu Z, Wang J, Yuan Z, et al. Preliminary results about application of intensity-modulated radiotherapy to reduce prophylactic radiation dose in limited-stage small cell lung cancer. J Cancer. 2018;9:2625–2630. doi:10.7150/jca.24976
9. Wang D, Bi N, Zhang T, et al. Comparison of efficacy and safety between simultaneous integrated boost intensity-modulated radiotherapy and conventional intensity-modulated radiotherapy in locally advanced non-small-cell lung cancer: a retrospective study. Radiat Oncol. 2019;14:106. doi:10.1186/s13014-019-1259-3
10. Ji K, Zhao LJ, Liu WS, et al. Simultaneous integrated boost intensity-modulated radiotherapy for treatment of locally advanced non-small-cell lung cancer: a retrospective clinical study. Br J Radiol. 2014;87:20130562. doi:10.1259/bjr.20130562
11. Fondevilla Soler A, Lopez-Guerra JL, Dzugashvili M, et al. Outcome and toxicity of intensity modulated radiotherapy with simultaneous integrated boost in locally advanced non-small cell lung cancer patients. Clin Transl Oncol. 2017;19(12):1469–1477. doi:10.1007/s12094-017-1689-z
12. Zhang X, Li X, Quan EM, et al. A methodology for automatic intensity-modulated radiation treatment planning for lung cancer. Phys Med Biol. 2011;56:3873–3893. doi:10.1088/0031-9155/56/13/009
13. Della Gala G, Dirkx MLP, Hoekstra N, et al. Fully automated VMAT treatment planning for advanced-stage NSCLC patients. Strahlenther Onkol. 2017;193:402–409. doi:10.1007/s00066-017-1121-1
14. Quan EM, Chang JY, Liao Z, et al. Automated volumetric modulated arc therapy treatment planning for stage III lung cancer: how does it compare with intensity-modulated radio therapy? Int J Radiat Oncol Biol Phys. 2012;84:e69–76. doi:10.1016/j.ijrobp.2012.02.017
15. Batumalai V, Jameson MG, Forstner DF, et al. How important is dosimetrist experience for intensity modulated radiation therapy? A comparative analysis of a head and neck case. Pract Radiat Oncol. 2013;3:e99–e106. doi:10.1016/j.prro.2012.06.009
16. Bohsung J, Gillis S, Arrans R, et al. IMRT treatment planning:- a comparative inter-system and inter-centre planning exercise of the ESTRO QUASIMODO group. Radiother Oncol. 2005;76:354–361. doi:10.1016/j.radonc.2005.08.003
17. Nelms BE, Robinson G, Markham J, et al. Variation in external beam treatment plan quality: an inter-institutional study of planners and planning systems. Pract Radiat Oncol. 2012;2:296–305. doi:10.1016/j.prro.2011.11.012
18. Chen J, Fu G, Li M, et al. Evaluation of MLC leaf transmission on IMRT treatment plan quality of patients with advanced lung cancer. Med Dosim. 2018;43:313–318. doi:10.1016/j.meddos.2017.10.008
19. Hodapp N The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT). 2012.
20. Wang D, Bi N, Chen D, Wang L. Complete remission after hypofractionated radiotherapy for a patient with inoperable adenoid cystic carcinoma of bronchus: a case report. Medicine (Baltimore). 2018;97:e13463. doi:10.1097/MD.0000000000013463
21. Wang J, Zhou Z, Liang J, et al. Intensity-modulated radiation therapy may improve local-regional tumor control for locally advanced non-small cell lung cancer compared with three-dimensional conformal radiation therapy. Oncologist. 2016;21:1530–1537. doi:10.1634/theoncologist.2016-0155
22. Ueyama T, Arimura T, Takumi K, et al. Risk factors for radiation pneumonitis after stereotactic radiation therapy for lung tumours: clinical usefulness of the planning target volume to total lung volume ratio. Br J Radiol. 2018;91:20170453. doi:10.1259/bjr.20170453
23. Madani I, De Ruyck K, Goeminne H, et al. Predicting risk of radiation-induced lung injury. J Thorac Oncol. 2007;2(9):864–874. doi:10.1097/JTO.0b013e318145b2c6
24. Hernando ML, Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi:10.1016/S0360-3016(01)01685-6
25. Sheng L, Cui X, Cheng L, et al. Risk factors of grade >/= 2 radiation pneumonitis after gemcitabine induction chemotherapy for patients with non-small cell lung cancer. Radiat Oncol. 2019;14:229. doi:10.1186/s13014-019-1440-8
26. Zhao Y, Chen L, Zhang S, et al. Predictive factors for acute radiation pneumonitis in postoperative intensity modulated radiation therapy and volumetric modulated arc therapy of esophageal cancer. Thorac Cancer. 2015;6:49–57. doi:10.1111/1759-7714.12142
27. Xia F, Zhou L, Yang X, et al. Is a clinical target volume (CTV) necessary for locally advanced non-small cell lung cancer treated with intensity-modulated radiotherapy? -a dosimetric evaluation of three different treatment plans. J Thorac Dis. 2017;9(12):5194–5202. doi:10.21037/jtd.2017.10.147
28. Xhaferllari I, El-Sherif O, Gaede S. Comprehensive dosimetric planning comparison for early-stage, non-small cell lung cancer with SABR: fixed-beam IMRT versus VMAT versus TomoTherapy. J Appl Clin Med Phys. 2016;17(5):329–340. doi:10.1120/jacmp.v17i5.6291
29. Zhang TW, Snir J, Boldt RG, et al. Is the importance of heart dose overstated in the treatment of non-small cell lung cancer? A systematic review of the literature. Int J Radiat Oncol Biol Phys. 2019;104(3):582–589. doi:10.1016/j.ijrobp.2018.12.044
30. Wang K, Pearlstein KA, Patchett ND, et al. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for Stage III non-small-cell lung cancer. Radiother Oncol. 2017;125(2):293–300. doi:10.1016/j.radonc.2017.10.001
31. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35:56–62. doi:10.1200/JCO.2016.69.1378
32. Bar-Ad V, Ohri N, Werner-Wasik M. Esophagitis, treatment-related toxicity in non-small cell lung cancer. Rev Recent Clin Trials. 2012;7:31–35. doi:10.2174/157488712799363235
33. Werner-Wasik M, Yorke E, Deasy J, et al. Radiation dose-volume effects in the esophagus. Int J Radiat Oncol Biol Phys. 2010;76:S86–93. doi:10.1016/j.ijrobp.2009.05.070
34. Qiao WB, Zhao YH, Zhao YB, Wang RZ. Clinical and dosimetric factors of radiation-induced esophageal injury: radiation-induced esophageal toxicity. World J Gastroenterol. 2005;11:2626–2629. doi:10.3748/wjg.v11.i17.2626
35. Ahn SJ, Kahn D, Zhou S, et al. Dosimetric and clinical predictors for radiation-induced esophageal injury. Int J Radiat Oncol Biol Phys. 2005;61:335–347. doi:10.1016/j.ijrobp.2004.06.014
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


