Back to Journals » Eye and Brain » Volume 12
Spaceflight Associated Neuro-Ocular Syndrome (SANS): A Systematic Review and Future Directions
Authors Martin Paez Y, Mudie LI , Subramanian PS
Received 3 June 2020
Accepted for publication 22 September 2020
Published 19 October 2020 Volume 2020:12 Pages 105—117
DOI https://doi.org/10.2147/EB.S234076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Margaret Wong-Riley
Yosbelkys Martin Paez,1,2 Lucy I Mudie,1 Prem S Subramanian1– 3
1Sue Anschutz-Rodgers/UCHealth Eye Center and Departments of Ophthalmology, Aurora, CO, 80045, USA; 2Neurology, University of Colorado School of Medicine, Aurora, CO 80045, USA; 3Neurosurgery, University of Colorado School of Medicine, Aurora, CO 80045, USA
Correspondence: Prem S Subramanian
University of Colorado School of Medicine, 1675 Aurora Court, Mail Stop F731, Aurora, CO 80045, USA
Tel +1 720 848 2500
Fax +1 720 848 5014
Email [email protected]
Purpose: To present a systematic review of the current body of literature surrounding spaceflight associated neuro-ocular syndrome (SANS) and highlight priorities for future research.
Methods: Three major biomedical databases were searched with the following terms: ((neuro ocular) OR ((brain) AND (eye))) AND ((spaceflight) OR (astronaut) OR (microgravity)) AND (ENGLISH[Language]). Once duplicates were removed, 283 papers were left. Articles were excluded if they were not written in English or conference abstracts only. We avoided including review papers which did not provide any new information; however, two reviews on the pathophysiology of SANS were included for completeness. No limitations on date of publication were used. All included entries were then summarized for their contribution to knowledge about SANS.
Results: Four main themes among the publications emerged: papers defining the clinical entity of SANS, its pathophysiology, technology used to study SANS, and publications on possible prevention of SANS. The key clinical features of SANS include optic nerve head elevation, hyperopic shifts, globe flattening, choroidal folds, and increased cerebrospinal fluid (CSF) volume in optic nerve sheaths. Two main hypotheses are proposed for the pathophysiology of SANS. The first being elevated intracranial pressure and the second compartmentalization of CSF to the globe. These hypotheses are not mutually exclusive, and our understanding of the pathophysiology of SANS is still evolving. The use of optical coherence tomography (OCT) has greatly furthered our knowledge about SANS, and with the deployment of OCT to the International Space Station, we now have ability to collect intraflight data. No effective prevention for SANS has been found, although fortunately, even with persistent anatomic and physiologic neuro-ocular changes, any functional impact has been correctable with spectacles.
Conclusion: This is the first systematic review of SANS. Despite the limitations of studying a syndrome that can only occur in a small, discrete population, we present a thorough overview of the literature surrounding SANS and several key areas important for future research are identified.
Keywords: subarachnoid space, cerebrospinal fluid, idiopathic intracranial hypertension, space flight, optic nerve
Introduction
Spaceflight-associated neuro-ocular syndrome (SANS), previously known as visual impairment and intracranial pressure (VIIP) syndrome, describes a collection of ophthalmologic and neurologic findings in astronauts after prolonged spaceflight. Ocular changes during short-duration spaceflight (<2 weeks) such as hyperopic shift had been observed by National Aeronautics and Space Administration (NASA) for years. Potentially pathologic ocular changes including optic disc swelling in astronauts on longer missions aboard the International Space Station (ISS) led to the descriptive term VIIP, with disc edema creating a presumptive diagnosis of elevated intracranial pressure as the cause. A clinical practice guideline (CPG) was developed for diagnosis of VIIP, and long-duration spaceflight (LDSF) ocular changes were first reported in the scientific literature in a series of seven US astronauts who had completed missions on the ISS.1 There is a vast amount still unknown about SANS; however, we have progressed in our understanding over the past decade, including complete revision of the CPG in recognition of the unproven role of intracranial pressure (ICP) elevation in the observed findings. These advances also are reflected in the increasing number of publications regarding proposed hypotheses for the mechanism of SANS, technology to assist in studying SANS, and possible terrestrial models of SANS. This paper presents a systematic review on SANS, providing a methodical overview of the current body of knowledge with an emphasis on the ocular changes and associated CNS findings and highlighting information gaps.
A valid clinical case definition for SANS has yet to be accepted. Most literature describes a combination of clinical and radiographic findings to define SANS among astronauts returned from flight including optic disc edema, globe flattening, choroidal and retinal folds, hyperopic refractive error shift and/or focal areas of ischemic retina.1,2 For the purposes of this comprehensive review, papers using the term “SANS” or describing a constellation of neuro-ocular changes among post-flight astronauts were included. Since the population at risk for SANS is small and unique, almost all studies are observational in nature, although several papers compare SANS to idiopathic intracranial hypertension (IIH) and other terrestrial pathologies such as radiation associated neuro-ocular changes. Most papers also focus on understanding the mechanism behind SANS; few describe technology that can be used to study SANS, and fewer still report on therapy, prevention or countermeasures for SANS. In creating a thorough and critical summary of current literature on SANS, we aim to guide future directions for research in SANS.
Methods
Three databases, PubMed, EMBASE and Web of Science, were searched using the following terms: ((neuro ocular) OR ((brain) AND (eye))) AND ((spaceflight) OR (astronaut) OR (microgravity)) AND (ENGLISH[Language]). These databases were chosen as the major reference collections of biomedical and aeronautical literature. The search was conducted on 5 August2020, and returned 192 results in PubMed, 200 results in EMBASE and 82 in Web of Science. These results were imported to Zotero (Corporation for Digital Scholarship; Vienna, Virginia, USA) citation manager and duplicates were removed, leaving 283 unique papers for review. These articles were then screened for inclusion by two authors (LIM and PSS), with the third author (YMP) acting as adjudicator when needed. Articles were excluded if they were not written in English or conference abstracts only. We avoided including review papers which did not provide any new information, however two reviews on the pathophysiology of SANS were included for completeness. No limitations on date of publication were used. All included entries were then summarized for their contribution to knowledge about SANS. Figure 1 illustrates the flow of results through our systematic review. Four main themes among the publications emerged during this process; papers defining SANS, those reporting on the pathophysiology of SANS, those describing technology to better evaluate SANS, and publications on possible prevention and/or countermeasures for SANS. The results of this systematic review are presented using these subheadings to illustrate where our current body of knowledge about SANS lies to date.
 |
Figure 1 Flow diagram of search results for systematic review. |
Results
A summary of all papers included in the systematic review are shown in Table 1. Four themes emerged in review of each paper: efforts to define SANS, studies on the pathophysiology of SANS, technology for studying SANS, and the prevention and treatment of SANS. We have used these themes to present our results, with each paper being further described under its theme.
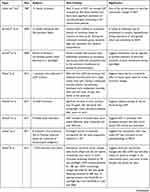 |  |  |  |
Table 1 Summary of All Papers Included in Systematic Review |
Efforts to Define SANS
In 2011, Mader et al1 first described the key clinical and imaging findings in astronauts after LDSF on the ISS. Seven astronauts had complete eye examinations before and after their ISS missions, including cycloplegic and/or manifest refractions and fundus photography. The clinical findings included optic nerve head elevation and swelling, hyperopic refractive error, globe flattening, choroidal and/or retina folds, nerve fiber layer infarcts and increased cerebrospinal fluid (CSF) volume in optic nerve sheaths. These findings have been demonstrated using imaging such as optical coherence tomography (OCT), magnetic resonance imaging (MRI) and computerized tomography (CT) after LDSF, and have been shown to persist for more than two years. Vision changes associated with the hyperopic shift have been shown to correct to 20/20 acuity with refraction, and they also have persisted for years after LDSF. Mader et al1 reported that these hyperopic shift seemed to be more common in astronauts over the age of 40 years, noting that NASA offers astronauts over the age of 40 plus-powered lens “Spaceflight Anticipation Glasses”. In the original cohort of seven astronauts, lumbar punctures (LP) were performed in four individuals (total n=6, as one individual received three LPs). These LPs documented opening pressures (OP) of 22 cmH2O at return +66 days (R+66), 21 cmH2O at R+19, 28 cmH2O at R+12, and 28.5 cmH2O at R+57. In this paper, the authors used a cutoff of 20 cmH2O as the upper limit of normal opening pressure. However the revised diagnostic criteria for IIH from updated guidelines published in 2013 indicates that 25 cmH2O is the better indicator of true elevated opening pressure documented by a LP with manometry.35 To minimize false positive values the patient should be positioned in lateral decubitus with legs stretched before pressure measurements.35 The technique used to measure OP in the initial study by Mader et al1 was not documented, somewhat limiting interpretation of their data. Further, OP as measured by LP is assumed to be the same CSF pressure found in the ventricles and CSF cisterns, which may not be true if CSF pressure within the subarachnoid space does not equalize with lateral ventricular pressures.36
The constellation of refractive error shifts, globe flattening, increased CSF volume in optic nerve sheaths and optic disc swelling with borderline elevated ICP, led to the naming of the condition as the visual impairment and intracranial pressure (VIIP) syndrome. Approximately six years after this initial study, the term spaceflight associated neuro-ocular syndrome (SANS) was adopted in favor of VIIP syndrome given the uncertainty regarding the pathophysiology of SANS and unclear role of intracranial pressure.37,38 Since the initial study, more data has been collected pre, intra and post-spaceflight documenting changes in physiology and anatomy; however, the clinical syndrome is still generally characterized by the findings reported initially by Mader et al1 in 2011.
In a recent prospective pre- and postflight analysis of ocular structural parameters, Macias et al30 presented data from 11 astronauts after LDSF. In addition to changes seen in the optic nerve and retina, both axial length (AL) and anterior chamber depth (ACD) were significantly reduced after LDSF (0.3% and 3.0%, respectively), and these changes persisted for the one year duration of follow-up and resulted in a 0.13D hyperopic shift. This refractive shift is optically significant and although it is usually not considered clinically significant, it may contribute to reports of blurred vision, especially at near. It is not yet clear whether changes in ACD are independent of changes in AL as data were not presented on an individual subject level. AL reduction is more likely related to intraocular changes from choroidal engorgement with chronic remodeling and not radiographic globe flattening, as the observed AL decrease of 0.08 mm would be difficult to see on MRI. For now, anterior segment signs beyond shifts in refractive error are less often considered when defining SANS but may represent an area of future interest.
Early studies with head down tilt (HDT) as a terrestrial model of simulated microgravity reported an elevation in intraocular pressure (IOP) 26% above baseline among healthy volunteers.3 The same study also reported significant alterations of neurophysiological processing in the retinocortical pathway as measured by pattern reversal electroretinograms and pattern reversal visual evoked cortical potentials. However, neither IOP nor neurophysiologic changes in retinocortical pathways are typically included in definitions of SANS and their significance in the pathophysiology of SANS has not been demonstrated to date. Changes in pupil reactivity has also been documented among individuals with IIH (a disease with proposed similar pathophysiology to SANS) during HDT,24 however, once again, the importance of this clinic sign for SANS has not been confirmed.
Finally, it is well documented that astronauts experience positive visual phenomena such as flashes of light during spaceflight. It has been suggested that these phosphenes may occur from heavy ion stimulation of the retina, optic nerve or visual cortex, bypassing the usual photoreceptor activation pathways.20 Although these positive visual phenomena are not typically included in the definition of SANS, they may be indicators of abnormal activation of visual mechanisms during spaceflight and represent an area for further study.
Pathophysiology of SANS
Two main hypotheses have been proposed for the pathophysiology of SANS, the first being related to elevated intracranial pressure, and the second, compartmentalization of CSF to the area behind the globe. We discuss these hypotheses and the studies supporting them separately, but it should be noted that they are not necessarily mutually exclusive.
Given the constellation of optic nerve head edema, flattening of posterior globes and increased CSF volume of optic nerve sheaths, it is not surprising that one of the earliest hypotheses for the pathophysiology of SANS relates to elevated ICP. In this theory, the microgravity environment leads to increased venous outflow resistance from the head, causing increased cerebral venous pressure and decreased CSF resorption. van Ombergen et al15 reported dilated lateral, third and fourth ventricle in 11 astronauts after LDSF, with significant improvement 7 months post-spaceflight, although without returning to previous baseline. The raised ICP is thought to then be transmitted to the globe via the optic nerve sheaths. Lee et al29 reported optic nerve sheath diameter increased 0.5mm in HDT. Alperin and Bagci10 found a strong correlation between orbital CSF volume increases and globe flattening on postflight high-resolution MRI studies in 7 astronauts after LDSF; they did not see similar changes in a cohort of 9 astronauts exposed to short duration flights. They also inferred “moderate” ICP elevation of up to 6 mmHg in 2 subjects by indirect MRI-based calculations.10 Notably, the majority of subjects in both the short- and long-duration spaceflight cohorts were graded as having VIIP per the NASA CPG, highlighting the flaws of that system and its lack of specificity for acute vs. chronic changes. One limitation of studies behind this raised ICP theory is that pre-flight data are not included and using post flight data does not accurately represent ICP during spaceflight. Previous case reports documenting elevated opening pressure in post-flight astronauts were performed only after they were noted to have optic disc swelling.4,8 It also remains to be demonstrated that any measured increase in ventricular volume would have a clinically significant effect and that such a change in volume can be correlated directly with ophthalmic effects such as optic disc edema.18
Arbeille et al5 previously documented a significant increase in cross-sectional area of the jugular and portal veins during LDSF; these findings normalized on return to Earth, suggesting cephalad pooling of venous blood as a contributory mechanism. They also noted that after 150 days of microgravity exposure, choroidal thickness and TRT increased by 50 μm and 26 μm, respectively. Wostyn et al25 reviewed multiple papers regarding the cephalad fluid shift and reported that optic nerve head (ONH) edema may at least in part result from the forcing of perioptic CSF into the ONH along perivascular spaces. Gerlach et al6 reported that on imaging of the optic nerves of healthy volunteers who underwent head down tilt (HDT) for 4.5 hours, fractional anisotropy and axial diffusivity were significantly increased, suggesting increased CSF volume and movement within the optic nerve sheath.
Indirect ICP measurements during parabolic flight have shown a moderate elevation during periods of microgravity,39 while direct measurements have recorded that the normal drop in ICP when subjects go from supine to upright does not occur during this type of simulated microgravity.7 Aside from in vivo measurements, a mathematical model of fluid flow in the eyes and brain embedded into a simplified whole-body circulation model predicted raised ICP and IOP in microgravity.19 This model predicted decreased ocular blood flow to the choroid and ciliary body whereas retinal circulation was found to be less susceptible to microgravity induced changes.
Other studies have reported evidence to the contrary of raised ICP theory. Laurie et al28 reported that the magnitude of edema was different for HDT than occurs after a similar duration of spaceflight, and in their study there was no change in choroidal thickness post-HDT. Despite the structural changes among participants on 30 days of bedrest, they lacked other signs or symptoms of SANS, further highlighting possible differences between astronauts exposed to weightlessness and bedrest subjects exposed to strict head-down tilt for similar periods of time. It has also been noted that the neuro-ocular findings in SANS occur without the classical symptoms of raised intracranial pressure such as diplopia, transient visual obscurations and headaches and can persist for years after return to Earth.1,2 Preflight ICP measurements are not available for astronauts, and postflight LP has been done only for individuals with optic disc edema. Finally, the typical intracranial MRI findings of IIH such as empty sella turcica and transverse sinus stenosis have not been observed widely in SANS.
Another proposed hypothesis for SANS is the compartmentalization of CSF to the globe and optic nerve, in which a one-way valve mechanism traps pressurized CSF within the optic nerve and sheath without requiring elevated intracranial pressure. Several mechanisms have been described to support this hypothesis. The first mechanism is based on reported MRI findings consistent with upward shift of the brain pulling the optic nerve and globe.23 Shinojima et al23 proposed that when cephalad fluid shifts occurred, compensatory decompression mechanisms such as optic nerve sheath expansion may become overwhelmed and CSF may be forced into the optic nerve through perivascular spaces surrounding the central retinal artery. Wåhlin et al34 also provided support for this theory, finding significant optic nerve head displacement (average 0.63 mm) and upward displacement of the optic chiasm among 22 astronauts after both long and short duration spaceflight. The optic nerve head displacement was positively related to length of mission duration. Another mechanism is a fairly new concept of glymphatic flow imbalance. The glymphatic system allows the exchange of CSF with the interstitial fluid. Rasmussen et al32 used near infrared fluorescent lymphatic imaging (NIRFLI) under HDT, sitting, and supine positions and found that lymphatic drainage through pathways shared by CSF outflow are dependent upon gravity and are impaired under short-term HDT. Previous animal models have also shown that microgravity reduces myogenic vasoconstriction and stiffness of cerebral vessels, which could underlie some of the altered fluid exchange dynamics.40 The potential role of these pathways in the long-term development of SANS, in particular the role of compensatory autoregulatory changes over time, remains to be demonstrated.
Other hypotheses have been proposed regarding the unique environment experienced by astronauts. Christian et al41 studied insufficient sleep and mild hypercapnia in healthy volunteers (both sleep deprivation and elevated ambient carbon dioxide (CO2) are experienced by astronauts aboard the ISS). They found these environmental changes predisposed a subset of individuals to the development of total retinal thickening, similar to one finding of SANS. Visible optic disc edema also was reported in 5 of the 11 subjects of the same cohort when fundus photographs were obtained.28 Another study found that IOP and cerebral blood volume pulsatility increased during HDT and this was more pronounced under higher ambient concentrations of carbon dioxide.9 Interestingly, the authors reported no change was seen in noninvasive measurement of ICP under the same study conditions.9 The effect of carbon dioxide on augmenting the increase in IOP during HDT was also reported in a second study by Mekjavic et al31 however as mentioned above the role of elevated IOP in the pathophysiology of SANS is unclear. The precise roles of either sleep changes and/or CO2 levels in the development of SANS-like retinal and optic disc changes cannot be determined from these data. Thus, there is no firm or exact pathophysiology of SANS presented in the literature. As our technology evolves, and our ability to collect more intra-flight data increases, it is hoped that our understanding of the underlying cause of SANS will also expand.
Technology for Studying SANS
Astronauts undergo extensive pre- and post-flight neuro-ocular testing, although immediate post-flight testing has been limited by the need, until recently, for return from orbit to the Russian landing base in Kazakhstan. Several papers have reported on technology that could be utilized for in-flight testing to better understand SANS. OCT has been available on the International Space Station since 2013 and has become the mainstay of detection and early monitoring of SANS.2 OCT technology has evolved dramatically over the past decade, and an upgraded Heidelberg Spectralis (Heidelberg Engineering; Franklin, Massachusetts, USA) “OCT2” is now deployed on the ISS. OCT2 allows for faster imaging and enhanced image quality and is capable of multi-color imaging (MCI), which is reported as equivalent to fundoscopy to detect signs of SANS.2 The scanning protocol for the OCT2 on the ISS is shown in Table 2, adapted from Lee et al.2 Based on data from terrestrial studies of optic neuropathies as well as IIH, evaluation of changes in the peripapillary retinal nerve fiber layer thickness may reveal the earliest sign of SANS, while loss of macular ganglion complex thickness may herald visual damage.
 |
Table 2 OCT2 Scanning Protocol, Adapted from Lee et al.2 |
A study by Masterova et al42 evaluated portable autorefractors to measure inflight refractive error and axial length among astronauts. They found the SVOne (Smart Vision Labs; New York, New York, USA) device was easy to use, could obtain measurements quickly and had excellent intersession reproducibility. Another study by Lerner et al13 reported on ultrasound guided LP technique with remote guidance for possible inflight performance of LP. The found the technique was successful 9 of 11 times and could be performed in both the fetal and seated lordotic positions. Significant barriers to performing LP inflight remain, including concerns about poor sterility aboard the ISS as well as uncertainty as to the ability to measure CSF pressure via standard manometry. In flight ophthalmic ultrasound already is in use on the ISS, and attempts to improve data collected by ultrasound are underway. A study of 11 healthy volunteers suggested 3-dimensional and dynamic measurements could be obtained successfully in HDT,43 potentially allowing quantitative noninvasive measures of ICP such as optic nerve sheath diameter.
Aside from technology that could be used inflight, there are various reports of novel approaches to increase our understanding of SANS. Salerni et al19 reported a mathematical model of fluid flow in the eyes and brain which predicted increased ICP and IOP in microgravity, as well as decreased ocular blood flow in the choroid and ciliary body. As noted above, the lymphatic system has been proposed to play a role in contributing to SANS, and Rasmussen et al32 described a technique that could be used to further our understanding of this. The authors reported subdermal indocyanine green (ICG) injections with near infrared fluorescent lymphatic imaging to visualize deep lymphatic outflow shared by CSF and in a small group of healthy human volunteers showed its disruption during HDT.
Two studies reported on animal models for investigating SANS. Fleischman et al14 published a model of CSF flow in porcine animals using intravenous contrast and rapid sequential CT images with head down tilting to illustrate the role of gravitational forces on CSF. Shen et al22 adapted a murine model of inducible ICP elevation and maintained raised ICP for 2 weeks. The authors used their model of sustained raised ICP to show a loss of retinal ganglion cell (RGC) axons and increased hypoxia-inducible factor (HIF)-1 alpha expression in the RGC. Further development of these models would be required to determine how closely SANS might be recapitulated and studied in the absence of a true microgravity environment.
Prevention and Treatment of SANS
Publications reporting on the prevention of SANS include modifications that could be made on an individual astronaut level as well as to the spaceflight environment. Three studies have examined lower limb thigh cuffs or negative pressure suits to counteract fluid shifts and in turn lower ICP without compromising cerebral perfusion pressure.12,16,17 Petersen et al16,17 found that a lower body negative pressure (LBNP) suit applying 20 mmHg could be used feasibly by astronauts and resulted in a significant increase in cerebral perfusion pressure while in HDT. Artificial gravity has been shown to provide a countermeasure to the cardiovascular effects of spaceflight; however, in a study by Anderson et al11 two models of artificial gravity with short-radius centrifugation did not show any significant impact on intraocular pressure despite expected changes in cardiovascular parameters. The study did not measure intracranial pressure and further work is pending to expand upon the initial results. Scott et al21 evaluated commercially-available swimming goggles and the effect of exercise during HDT. They found that goggle wear could modestly increase IOP by 1.9 mmHg and counteract elevated ICP by reducing the predicted translaminar pressure gradient in HDT and LDSF. More studies are needed to understand the required duration and intensity of this countermeasure to SANS; however, given its simplicity and low-cost strategy, it warrants further investigation.
Two papers reported on spacecraft environmental factors that could be modified to help prevent SANS. Astronauts in spaceflight are also exposed to higher CO2 and radiation levels than on Earth; these environmental factors are under investigation for their role in SANS and may hold a role in future targeting of SANS countermeasures.2 Indeed, the work of Laurie et al28 showed that optic disc edema developed only with the combination of HDT and elevated ambient carbon dioxide levels. However, as noted above, the same researchers found no changes in arterial CO2 pressures and could not definitively demonstrate a pathogenic role for elevated CO2 levels.27 Nonetheless, in response to concerns about the role of CO2 in SANS, NASA has reduced ambient CO2 levels on the ISS.
No studies were found directly evaluating treatment for SANS. In general, studies describing the constellation of signs of SANS report that 20/20 vision can be obtained with refractive correction. The structural changes seen on brain imaging of post-flight astronauts do not have clear functional significance.2 Thus, it seems for now that treatment is aimed at managing complications as they arise. In terms of preventative therapies, we would advocate for using the lowest impact methodology possible and specifically avoiding pharmaceutical prophylaxis, given the complex drug interactions and side effects that may occur with other microgravity-associated physiologic changes. As our understanding of the pathophysiology of SANS and the clinical significance of the structural neuro-ocular changes increases, it is hoped that potential therapies and targets for prevention will grow.
Discussion
Initially called visual impairment and intracranial pressure (VIIP) syndrome, the constellation of neuro-ocular findings such as optic nerve head elevation, hyperopic shifts, globe flattening, choroidal folds, and increased CSF volume in optic nerve sheaths, was later renamed SANS given the uncertainty regarding the pathophysiology and unclear role of intracranial pressure. There is no case definition for SANS, although most studies use the neuro-ocular findings described in the early papers on SANS. A collaborative agreement on a formal definition would benefit the literature. Two major hypotheses exist for SANS: elevated intracranial pressure and compartmentalization of CSF to the globe and optic nerve. These are not mutually exclusive, and more pre-, intra-, and post-spaceflight data are needed to fully understand the pathophysiology of SANS. Advances in technology will be key in these efforts to understand SANS. Improvements in OCT technology and the ability to collect OCT data in space already has contributed immensely; determination of ICP directly or indirectly during LDSF remains a challenge to be met and represents a critical need with respect to understanding SANS pathophysiology and designing interventions. Countermeasures for SANS such as negative pressure lower body suits, artificial gravity and spacecraft environmental modifications have been studied, but none has proven effective, this is an area with significant need for further study, although antecedent to that will be increasing our understanding of the mechanism of SANS.
Both a strength and limitation of this paper is that most of the literature is recent. Despite the benefit of knowing that many works are current, it highlights that our understanding of SANS is still in its infancy. One limitation in studying SANS is that only approximately 12 astronauts per year are launched, meaning that most studies are small and observational. With so few astronauts being exposed to spaceflight, and with a rigorous astronaut selection process, there is limited ability for controlled studies and inherent bias in the study population. Further, even if studies are controlled, they are difficult to mask due to study size and operational concerns about accessing astronaut data. Likewise, data published in the literature often may be limited in details due to concerns that astronauts may be identified. There are also intellectual property and national security concerns that limit publication of some details. Further, the current landing site for astronauts on return to Earth in Kazakhstan is geographically isolated; being far from medical research facilities where pre-flight data was collected limits immediate post-flight data collection. The recent successful SpaceX launch may herald better access to returning astronauts. Finally, due to the difficulty in collecting and publishing original research on SANS, many papers are speculative, expert opinions rather than presentations of original data. Human exposure to LDSF has occurred only in the recent past, and the long-term consequences of SANS-related changes must be studied carefully to guide our response and management of this potentially blinding condition. Furthermore, because of small numbers in both actual LDSF-exposed astronauts and subjects participating in ground-based analog studies, there remains a critical need to develop robust quantitative indicators of SANS onset and severity so that correlation with putative biomarkers can be established.
Conclusion
In this systematic review, we analyze reported data on the ophthalmic and intracranial changes that have been used both to define and to detect development of SANS. A recently published systematic review focused on the potential role of ICP in SANS and concluded that ICP likely plays a causative role but that further studies are needed in valid simulation environments to gather more reliable and robust data.26 The current body of literature regarding the definition and pathophysiology of SANS is presented, as well as a summary of technology used to study SANS and potential countermeasures/treatment for SANS. Despite the limitations of studying a syndrome that can only occur in a small, discrete population, we present a thorough overview of the literature surrounding SANS, and several key areas important for future research are identified.
Abbreviations
CPG, clinical practice guideline; CSF, cerebrospinal fluid; HDT, head down tilt; ICG, indocyanine green; ICP, intracranial pressure; IIH, idiopathic intracranial hypertension; IOP, intraocular pressure; LBNP, lower body negative pressure; LDSF, long-duration spaceflight; NASA, National Aeronautics and Space Administration; NIRFLI, near-infrared fluorescence lymphatic imaging; OCT, optical coherence tomography; SANS, spaceflight associated neuro-ocular syndrome; TRT, total retinal thickness; VIIP, vision impairment intracranial pressure.
Disclosure
Prem S Subramanian reports grants from Research to Prevent Blindness, Inc., during the conduct of the study; In addition, Dr Prem S Subramanian has a patent on novel therapeutics for lowering intracranial pressure pending. The authors report no other potential conflicts of interest in this work. This study was supported in part by an unrestricted grant from Research To Prevent Blindness, Inc. to the Department of Ophthalmology, University of Colorado School of Medicine.
References
1. Mader TH, Gibson CR, Pass AF, et al. Optic disc edema, globe flattening, choroidal folds, and hyperopic shifts observed in astronauts after long-duration space flight. Ophthalmology. 2011;118(10):2058–2069. doi:10.1016/j.ophtha.2011.06.021
2. Lee AG, Mader TH, Gibson CR, et al. Spaceflight associated neuro-ocular syndrome (SANS) and the neuro-ophthalmologic effects of microgravity: a review and an update. NPJ Microgravity. 2020;6(1):7. doi:10.1038/s41526-020-0097-9
3. Linder BJ, Trick GL. Simulation of spaceflight with whole-body head-down tilt: influence on intraocular pressure and retinocortical processing. Aviat Space Environ Med. 1987;58(9 Pt 2):A139–A142.
4. Mader TH, Gibson CRO, Pass AFO, et al. Optic disc edema in an astronaut after repeat long-duration space flight. J Neuroophthalmol. 2013;33(3):249–255. doi:10.1097/WNO.0b013e31829b41a6
5. Arbeille P, Provost R, Zuj K, Vincent N. Measurements of jugular, portal, femoral, and calf vein cross-sectional area for the assessment of venous blood redistribution with long duration spaceflight (vessel imaging experiment). Eur J Appl Physiol. 2015;115(10):2099–2106. doi:10.1007/s00421-015-3189-6
6. Gerlach DA, Marshall-Goebel K, Hasan KM, Kramer LA, Alperin N, Rittweger J. MRI-derived diffusion parameters in the human optic nerve and its surrounding sheath during head-down tilt. NPJ Microgravity. 2017;3:18. doi:10.1038/s41526-017-0023-y
7. Lawley JS, Petersen LG, Howden EJ, et al. Effect of gravity and microgravity on intracranial pressure. J Physiol. 2017;595(6):2115–2127. doi:10.1113/JP273557
8. Mader TH, Gibson CR, Otto CA, et al. Persistent asymmetric optic disc swelling after long-duration space flight: implications for pathogenesis. J Neuroophthalmol. 2017;37(2):133–139. doi:10.1097/WNO.0000000000000467
9. Strangman GE, Zhang Q, Marshall-Goebel K, et al. Increased cerebral blood volume pulsatility during head-down tilt with elevated carbon dioxide: the SPACECOT Study. J Appl Physiol. 2017;123(1):62–70. doi:10.1152/japplphysiol.00947.2016
10. Alperin N, Bagci AM. Spaceflight-induced visual impairment and globe deformations in astronauts are linked to orbital cerebrospinal fluid volume increase. Acta Neurochir Suppl. 2018;126:215–219. doi:10.1007/978-3-319-65798-1_44
11. Anderson AP, Butterfield JS, Subramanian PS, Clark TK. Intraocular pressure and cardiovascular alterations investigated in artificial gravity as a countermeasure to spaceflight associated neuro-ocular syndrome. J Appl Physiol. 2018;125(2):567–576. doi:10.1152/japplphysiol.00082.2018
12. Balasubramanian S, Tepelus T, Stenger MB, et al. Thigh cuffs as a countermeasure for ocular changes in simulated weightlessness. Ophthalmology. 2018;125(3):459–460. doi:10.1016/j.ophtha.2017.10.023
13. Lerner DJ, Chima RS, Patel K, Parmet AJ. Ultrasound guided lumbar puncture and remote guidance for potential in-flight evaluation of VIIP/SANS. Aerosp Med Hum Perform. 2019;90(1):58–62. doi:10.3357/AMHP.5170.2019
14. Fleischman D, Kaskar O, Shams R, et al. A novel porcine model for the study of cerebrospinal fluid dynamics: development and preliminary results. Front Neurol. 2019;10(OCT). doi:10.3389/fneur.2019.01137
15. van Ombergen A, Jillings S, Jeurissen B, et al. Brain ventricular volume changes induced by long-duration spaceflight. Proc Natl Acad Sci. 2019;116(21):10531–10536. doi:10.1073/pnas.1820354116
16. Petersen LG, Lawley JS, Lilja-Cyron A, et al. Lower body negative pressure to safely reduce intracranial pressure. J Physiol. 2019;597(1):237–248. doi:10.1113/JP276557
17. Petersen LG, Hargens A, Bird EM, Ashari N, Saalfeld J, Petersen JCG. Mobile lower body negative pressure suit as an integrative countermeasure for spaceflight. Aerosp Med Hum Perform. 2019;90(12):993–999. doi:10.3357/AMHP.5408.2019
18. Roberts DR, Asemani D, Nietert PJ, et al. Prolonged microgravity affects human brain structure and function. Am J Neuroradiol. 2019;40(11):1878–1885. doi:10.3174/ajnr.A6249
19. Salerni F, Repetto R, Harris A, et al. Biofluid modeling of the coupled eye-brain system and insights into simulated microgravity conditions. PLoS One. 2019;14:8. doi:10.1371/journal.pone.0216012
20. Sannita WG, Narici L, Picozza P. Positive visual phenomena in space: a scientific case and a safety issue in space travel. Vision Res. 2006;46(14):2159–2165. doi:10.1016/j.visres.2005.12.002
21. Scott JM, Tucker WJ, Martin D, et al. Association of exercise and swimming goggles with modulation of cerebro-ocular hemodynamics and pressures in a model of spaceflight-associated neuro-ocular syndrome. JAMA Ophthalmol. 2019;137(6):652–659. doi:10.1001/jamaophthalmol.2019.0459
22. Shen G, Link S, Kumar S, et al. Characterization of retinal ganglion cell and optic nerve phenotypes caused by sustained intracranial pressure elevation in mice. Sci Rep. 2018;8(1):2856. doi:10.1038/s41598-018-21254-8
23. Shinojima A, Kakeya I, Tada S. Association of space flight with problems of the brain and eyes. JAMA Ophthalmol. 2018;136(9):1075–1076. doi:10.1001/jamaophthalmol.2018.2635
24. Soeken TA, Alonso A, Grant A, et al. Quantitative pupillometry for detection of intracranial pressure changes during head-down tilt. Aerosp Med Hum Perform. 2018;89(8):717–723. doi:10.3357/AMHP.4929.2018
25. Wostyn P, Mader TH, Gibson CR, Killer HE. The escape of retrobulbar cerebrospinal fluid in the astronaut’s eye: mission impossible? Eye. 2019;33(10):1519–1524. doi:10.1038/s41433-019-0453-8
26. Elwy R, Soliman MA, Hasanain AA, et al. Visual changes after space flight: is it really caused by increased intracranial tension? A systematic review. J Neurosurg Sci. 2020. doi:10.23736/S0390-5616.20.04927-9
27. Laurie SS, Christian K, Kysar J, et al. Unchanged cerebrovascular CO 2 reactivity and hypercapnic ventilatory response during strict head-down tilt bed rest in a mild hypercapnic environment. J Physiol. 2020;598(12):2491–2505. doi:10.1113/JP279383
28. Laurie SS, Lee SMC, Macias BR, et al. Optic disc edema and choroidal engorgement in astronauts during spaceflight and individuals exposed to bed rest. JAMA Ophthalmol. 2020;138(2):165–172. doi:10.1001/jamaophthalmol.2019.5261
29. Lee C, Rohr J, Sass A, et al. In vivo estimation of optic nerve sheath stiffness using noninvasive MRI measurements and finite element modeling. J Mech Behav Biomed Mater. 2020;110:103924. doi:10.1016/j.jmbbm.2020.103924
30. Macias BR, Patel NB, Gibson CR, et al. Association of long-duration spaceflight with anterior and posterior ocular structure changes in astronauts and their recovery. JAMA Ophthalmol. 2020;138(5):553. doi:10.1001/jamaophthalmol.2020.0673
31. Mekjavic IB, Amoaku W, Mlinar T, Mekjavic PJ. Hypercapnia augments resistive exercise-induced elevations in intraocular pressure in older individuals. Exp Physiol. 2020;105(4):641–651. doi:10.1113/EP088236
32. Rasmussen JC, Kwon S, Pinal A, et al. Assessing lymphatic route of CSF outflow and peripheral lymphatic contractile activity during head-down tilt using near-infrared fluorescence imaging. Physiol Rep. 2020;8(4). doi:10.14814/phy2.14375
33. Serrador JM, Shoemaker JK, Brown TE, Kassam MS, Bondar RL, Schlegel TT. Cerebral vasoconstriction precedes orthostatic intolerance after parabolic flight. Brain Res Bull. 2000;53(1):113–120. doi:10.1016/s0361-9230(00)00315-4
34. Wåhlin A, Holmlund P, Fellows AM, Malm J, Buckey JC, Eklund A. Optic nerve length before and after spaceflight. Ophthalmology. 2020. doi:10.1016/j.ophtha.2020.07.007
35. Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81(13):1159–1165. doi:10.1212/WNL.0b013e3182a55f17
36. Mindermann T. Pressure gradients within the central nervous system. J Clin Neurosci. 1999;6(6):464–466. doi:10.1016/s0967-5868(99)90001-2
37. Lee AG, Mader TH, Gibson CR, Tarver W. Space flight-associated neuro-ocular syndrome. JAMA Ophthalmol. 2017;135(9):992–994. doi:10.1001/jamaophthalmol.2017.2396
38. Mader T, Gibson C, Miller N, Subramanian P, Patel N, Lee A. An overview of spaceflight-associated neuro-ocular syndrome (SANS). Neurol India. 2019;67(8):S206–S211. doi:10.4103/0028-3886.259126
39. Avan P, Normand H, Giraudet F, Gerenton G, Denise P. Noninvasive in-ear monitoring of intracranial pressure during microgravity in parabolic flights. J Appl Physiol. 2018;125(2):353–361. doi:10.1152/japplphysiol.00032.2018
40. Taylor CR, Hanna M, Behnke BJ, et al. Spaceflight-induced alterations in cerebral artery vasoconstrictor, mechanical, and structural properties: implications for elevated cerebral perfusion and intracranial pressure. FASEB J. 2013;27(6):2282–2292. doi:10.1096/fj.12-222687
41. Christian KH, Petitti CS, Ortega-Schwartz KE, et al. VaPER: the development of spaceflight associated neuro-ocular syndrome (SANS) during hypercapnic 6° head-down tilt bed rest; Sans sufficient sleep? FASEB J. 2018;32(1):lb260–lb260.
42. Masterova KS, Anderson AP, Cowan DR, Fellows AM, Zegans ME, Buckey JC. Portable autorefractors for detecting axial length changes in space. Aerosp Med Hum Perform. 2018;89(8):724–730. doi:10.3357/AMHP.5049.2018
43. Dentinger A, MacDonald M, Ebert D, Garcia K, Sargsyan A. Volumetric ophthalmic ultrasound for inflight monitoring of visual impairment and intracranial pressure. Acta Neurochir Suppl. 2018;126:97–101. doi:10.1007/978-3-319-65798-1_21
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
