Back to Journals » Drug Design, Development and Therapy » Volume 9
Sonodynamic effect of hematoporphyrin monomethyl ether on ligature-induced periodontitis in rats
Authors Zhuang DS, Han JL, Bi LJ, Wang YP, Hao YR, Zhou Q, Cao WW
Received 6 February 2015
Accepted for publication 17 March 2015
Published 6 May 2015 Volume 2015:9 Pages 2545—2551
DOI https://doi.org/10.2147/DDDT.S82347
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Deshu Zhuang,1 Jialong Han,1 Liangjia Bi,1 Yueping Wang,1 Yanru Hao,2 Qi Zhou,3 Wenwu Cao3,4
1Department of Stomatology, The Fourth Affiliated Hospital, Harbin Medical University, Harbin, People’s Republic of China; 2Department of Stomatology, The First Affiliated Hospital, Harbin Medical University, Harbin, People’s Republic of China; 3Condensed Matter Science and Technology Institute, Harbin Institute of Technology, Harbin, People’s Republic of China; 4Department of Mathematics and Materials Research Institute, The Pennsylvania State University, University Park, PA, USA
Objectives: The aim of this study was to perform a histological evaluation of sonodynamic therapy (SDT) of hematoporphyrin monomethyl ether (HMME) on artificially induced periodontal disease in rats.
Methods: Submerging ligatures were placed at the subgingival region of the first maxillary molar in rats. Eighty rats were randomly assigned into four groups: group 1 received no treatment; group 2 was subjected to 50 µg/mL HMME alone; group 3 was treated with low-intensity ultrasound alone (1 W/cm2); and group 4 was treated with 50 µg/mL HMME plus ultrasound irradiation (1 MHz, 30 minutes). Ten rats in each group were euthanized at 7 and 15 days, and periodontal tissue samples were taken for histological examination.
Results: The animals treated by SDT showed less bone loss (P<0.05) at all experimental periods than the other three groups. No significant differences were found between the control and HMME groups (P>0.05).
Conclusion: Our results suggest that HMME-mediated SDT can effectively alleviate the periodontal tissue destruction in artificially induced periodontitis in rats. Hence, SDT may have good clinic potential as a noninvasive treatment of periodontal diseases.
Keywords: periodontal tissues, sonodynamic therapy, histology, alveolar bone loss, animal studies
A corrigendum has been published on this paper.
Introduction
Periodontitis is a common chronic inflammatory disease among the adult population. The main cause of periodontal disease is the presence of pathogenic microorganisms, which can interfere with host defenses through various mechanisms.1 The inflammatory cells in gingival tissue are activated by bacterial products, which may stimulate an organism to synthesize lytic enzymes and activate osteoclasts.2 Eventually, the immune response often destroys the periodontal tissue, and alveolar bone appears at different degrees of resorption.3 Therefore, suppression of the number of periodontal pathogens and infectious cells has been a key to the prophylaxis and treatment of periodontal disease.
Successful periodontal therapy is based on the complete suppression of periodontal pathogenic bacteria and the reduction of inflammatory signs. Conventional treatment of periodontopathy involves the application of antibiotics and mechanical debridement (scaling and root planing). Although systemic or local application of antibiotics therapy can reduce pathogenic bacteria and their endotoxins inside the periodontal pockets, the effect is not completely satisfactory, because many bacteria may gain resistance during this antimicrobial process.4 Scaling and root planing has been widely used in clinical treatment for periodontal diseases. It can temporarily remove the dental plaque on the surface of the root.5 However, due to the limitations of periodontal instruments, the mechanical therapy often fails to obliterate the microorganisms in dental plaque that are hiding inside the periodontal tissue and furcation area.6 Therefore, to overcome these deficiencies, new alternative strategies need to be explored.
Sonodynamic therapy (SDT) has a broad application prospect to treat various diseases based on the effect of low-intensity ultrasound combined with a sonosensitizer. Ultrasound has the penetrability to treat the deep tissue without the endoscopy, and the ultrasonic equipment is very simple and cheap. When suitable ultrasound waves reach the specific target of the tissue, they can activate sonosensitizers.7 Several experimental studies showed satisfactory results using SDT to kill inflammatory cells, such as THP-1 and U937.8,9 The lethal sonosensitization of these cells must involve changes in the membranes and/or plasma membrane proteins and DNA damage mediated by reactive oxygen species (ROS).10 However, to date, SDT has not been applied to periodontitis.
Hematoporphyrin monomethyl ether (HMME), a porphyrin-related agent, has been used in SDT research on treating cancers.9 It consists of two positional isomers of 3-(1-methyloxyethyl)-8-(1-hydroxyethyl) deuteroporphyrin IX and 8-(1-methyloxyethyl)-3-(1-hydroxyethyl) deuteroporphyrin IX.11 An experimental study has shown that HMME has higher selective uptake by tumor tissue, stable composition, low phototoxicity to normal tissues, and a relatively short light-avoiding period.12 By ultrasonicactivation, HMME produces ROS to attack the cell mitochondria and inactivate the key antioxidant enzymes, which are considered responsible for the sonodynamic damage of cells. This result is in line with those of other porphyrin sonosensitizers reported by other investigators.9,12,13 These studies suggest that HMME might be a promising sonosensitizer for SDT applications.
Although SDT has been shown effective on cancer cells, inflammatory tissues, and tumors, HMME-mediated SDT on periodontitis has not been reported to date. The purpose of this study was to perform a histological evaluation of the sonodynamic effect of HMME on experimentally induced periodontal disease in rats. We attempt to provide an experimental basis for future clinical application of HMME-mediated SDT in the treatment of periodontal disease in humans.
Materials and methods
Animals
All experimental procedures were in accordance with the Institutional Animal Care and Use Committee of Harbin Medical University (Harbin, People’s Republic of China). The protocol was approved by the Experimental Animal Ethics Committee of Harbin Medical University. The surgery procedures were performed under 10% chloral hydrate anesthesia. Eighty healthy male Wistar rats, weighing an average of 250 g, were used in this study. All animals were artificially fed according to the regulations of the Home Office of the Chinese Government, and were given food and water freely. Rats were purchased from The Animal Facilities, Fourth Affiliated Hospital of Harbin Medical University.
Protocol of experimental periodontal disease
General anesthesia was obtained with 10% chloral hydrate (5 mL/kg) via intraperitoneal injection. A periodontal inflammation model was established through plaque accumulation in the present study. Bilateral first maxillary molars of the rats were immediately ligatured around the subgingival position by cotton ligatures.14 The ligatures were removed after 4 weeks and the rats were randomly divided into four groups (20 rats/group): group 1, no treatment was performed; group 2, rats were subjected to 50 μg/mL HMME alone; group 3, rats were treated with low-intensity ultrasound therapy (1 W/cm2); and group 4, rats were treated with 50 μg/mL HMME plus ultrasound irradiation (1 MHz, 30 minutes). Ten animals from each group were sacrificed at 7 and 15 days. The maxillae were removed and fixed in 10% neutral formalin for 48 hours.
Ultrasound treatment and SDT
The ultrasound generator and power amplifier used in this study was designed and assembled by Harbin Institute of Technology (Harbin, People’s Republic of China), as illustrated in Figure 1. Ultrasonic intensities (W/cm2) were expressed as ISPTP (spatial peak/temporal peak) measured 6 mm away from the ultrasonic transducer radiating surface in the degassed distilled water by a hydrophone (Onda Corp., Sunnyvale, CA, USA). In this study, the ultrasonic transducer (diameter: 6 mm; frequency: 1.0 MHz; duty factor: 10%; pulse repetition frequency: 100 Hz) was placed over the periodontal inflammation regions with a medical ultrasonic coupling agent and held in place with a canvas jacket. HMME was provided by Xianhui Pharmaceutical Co., (Shanghai, People’s Republic of China). An experiment was initiated 4 weeks after establishment of the periodontal inflammation model, and this was considered as day one. In groups 2 and 4, the infection sites of each rat around the first maxillary molar region were subjected to HMME through hypodermic injection of HMME (50 μg/mL) and left in the dark for 2 hours. Then, in group 3 and group 4, the area was exposed to ultrasound for 30 minutes every other day for the experiment. This treatment was repeated eight times: on day 1, 3, 5, 7, 9, 11, 13, and 15, respectively. The temperature of the transducer was 25°C±2°C. During the sonication procedure, temperature increase on the ultrasonic transducer surface was less than 1°C, as measured by a thermocouple.
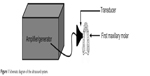  | Figure 1 Schematic diagram of the ultrasound system. |
Laboratory procedures
Following clinical observation, the maxillae were demineralized in a 10% ethylenediaminetetraacetic acid solution for 40 days. After washing, dehydration, and transparency, the samples were embedded in paraffin. Serial 6 μm paraffin sections were made in a mesiodistal direction and stained with hematoxylin and eosin.
Histological and histometric analysis
To establish the bone loss and characteristics of periodontal ligament in the furcation region of first molars, the sections were analyzed by light microscopy. After excluding the first and the last sections where the furcation region was evident, five equidistant sections of each specimen block were selected and captured by a digital camera. The area of bone loss in the furcation region was histometrically determined using an image analysis system (Image Tool; Harbin Medical University).15
Statistical analysis
The hypothesis that there were no differences in bone loss rate in the furcation region between treatment groups was tested using Bioestat 3.0 software (Bioestat, Windows 1995; Sonopress Brazilian Industry, Manaus, Brazil).
After normality testing, the histometric data were analyzed using the Shapiro–Wilk test, and the intragroup and intergroup analyses were performed with a two-way analysis of variance (P<0.05). When the analysis of variance detected a statistical difference, multiple comparisons were performed using Tukey’s test (P<0.05).
Results
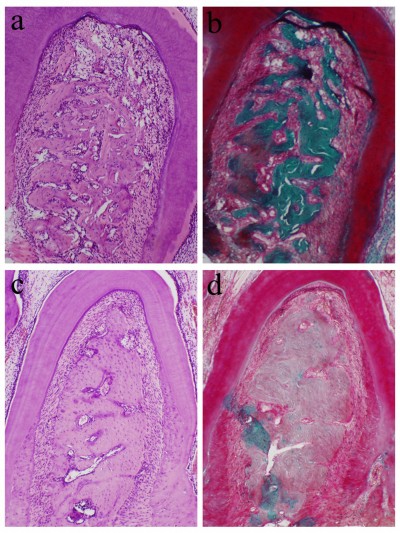
We first analyzed the control and HMME groups (group 1 and 2). At day 7 post-periodontitis treatment, the furcation area showed an evident inflammatory infiltrate composed of polynuclear leukocytes. In addition, an increase of capillaries, disorganization of the connective tissue, and formation of bone trabeculae as a result of the resorption process were observed. At day 15 posttreatment, most specimens in the control (Figure 2A) and HMME (Figure 2B) groups demonstrated some changes. The connective tissue displayed a small number of fibroblasts and bone tissue, with thin bone trabeculae in the areas of coronal furcation and resorption. The periodontal ligament exhibited a mild amount of chronic inflammatory cells.
For the ultrasound group (group 3), at 7 and 15 days (Figure 2C) posttreatment, most specimens demonstrated organized bone and connective tissue with a moderate number of fibroblasts. The inflammatory cells disappeared. The bone trabeculae in bone tissue became thick. The cementum surface in most specimens had areas of resorption.
For the SDT group (group 4), at 7 and 15 days (Figure 2D) posttreatment, the periodontal ligament appeared intact in most specimens. It was possible to observe a small area of well-developed connective tissue with a moderate number of fibroblasts and no inflammatory infiltrate. The bone tissue demonstrated organization with thick bone trabeculae without signs of resorption. The cementum surface did not show areas of resorption.
Histometric analysis
Histometric data are shown in Table 1. Animals in the control group (Figure 3A) and HMME group showed greater bone loss; a statistically significant difference (P<0.05) was evident when compared to the SDT group (Figure 3B) at days 7 and 15 post-periodontitis treatment. The histometric results also demonstrated that there was no statistically significant difference in bone loss between the control and HMME groups across all experimental periods (P>0.05). The furcation areas treated with ultrasound alone also demonstrated significantly decreased bone loss (P<0.05) compared to the control and HMME groups at 15 days. Histometric analysis demonstrated more bone loss with ultrasound-alone treatment compared to SDT treatment at all experimental periods (P<0.05).
Discussion
Periodontal diseases are common chronic inflammatory diseases caused by the presence of periodontal pathogens that invade the organism. Conventional therapeutic strategies for periodontal diseases can provisionally reduce the number of microorganisms in dental plaque, and antimicrobial chemotherapy may further inhibit the periodontal pathogens. However, shortcomings may still exist in those traditional treatments. To overcome these problems, experimental research has become popular in this field.16 SDT is a newly developed therapeutic technique for cancer. In this respect, sonosensitizer accumulates within the special position of the tumor and is activated by ultrasound, which has the ability to penetrate the organism surfaces to tumor tissue.10 Although SDT causes cell-killing effects on cancer cells, it does little damage to the surrounding normal tissues; as a result, SDT has excellent application potential in the treatment of cancer.7,12 Recently, several experimental studies demonstrated that SDT plays an important role in the induction of apoptosis and necrosis of inflammatory cells, such as THP-1 and U937.8,9 However, its applicability for the prevention of periodontitis and feasibility for the treatment of periodontal diseases has rarely been reported. In the present study, we explored the histological evaluation of the sonodynamic efficacy of HMME on experimentally induced periodontal disease in rats.
Histopathological analyses were used to compare the effects of SDT, ultrasound, and HMME treatment in diseased periodontal sites. Our results demonstrated that cotton ligatures around the maxillary right first molar teeth resulted in progressive bone loss at the furcation region (P<0.05). In the control group, significant alveolar bone loss was observed after 7 days; progressive changes continued until the end of the experimental period (day 15). We also observed a mild amount of polynuclear leukocytes and alveolar bone resorption during this time, and there was evidence of a small number of fibroblasts into connective tissue and the periodontal ligament. This is in agreement with previous reports that evaluated periodontal disease in rats.15,17
Some factors may influence SDT, such as the energy input of the sonication and the sonosensitizer concentration.7,10 HMME, a novel second-generation photosensitizer, possesses a stable structure, higher singlet oxygen yield, higher sonoactivity, lower dark toxicity, and faster clearance rate, especially for its cheaper price compared to hematoporphyrin derivative (HPD) (a first-generation photosensitizer), and is a promising sensitizer for photodynamic therapy.13,18 Recently, HMME has also proven to be an effective sonosensitizer for inducing the apoptosis and necrosis of cells in SDT research.9,12,13 In our study, no obvious effects were found in the sonosensitizer-alone group compared with the control group (P>0.05), which means that the isolated sonosensitizer application cannot decrease the areas of chronic inflammation.
Several studies demonstrated the efficacy of low-intensity ultrasound in accelerating tissue repair in damaged areas, with or without sonosensitizing drugs.19,20 Our finding was that, at 15 days, the furcation areas treated with ultrasound alone demonstrated significantly decreased bone loss (P<0.05) compared to the control and HMME groups. This probably is related to the ability of low-intensity ultrasound to promote differentiation of immature cementoblasts, simulation of mature cementoblasts,19,20 angiogenesis during wound healing, and anti-inflammatory effects.21,22
With regard to animals in the SDT group, the furcation areas treated with SDT demonstrated more significantly decreased bone loss (P<0.05) than ultrasound alone compared to the control and HMME groups at days 7 and 15 post-periodontitis treatment. The beneficial effect of SDT for periodontal disease in animals is probably based on preferential uptake and/or retention of sonosensitizers in inflammatory cells and subsequent activation of the drug by ultrasound irradiation.23 In SDT, the activation of sonosensitizers through acoustic cavitation by ultrasound is attributed to the generation of ROS, which are cytotoxic and can induce cell apoptosis.10
SDT can reduce the treatment time, the discomfort of the patient, the need for flaps, and the risk for bacteremia, which occurs routinely after periodontal treatment due to physical damages to the gum tissue. Unequivocal evidence showed a periodontal risk for systemic diseases, such as cardiovascular disease and diabetes.24,25 Because of the noninvasive nature of SDT, it may be a viable alternative treatment for most dental diseases. In addition, as SDT is a local therapy, one can reduce side effects that are associated with the systemic administration of antimicrobial agents.
Conclusion
Our results clearly demonstrate that HMME-mediated SDT can serve as an effective means to alleviate periodontal tissue destruction in rats with artificially induced periodontitis. Hence, HMME-mediated SDT offers a promising clinical method for treating periodontal diseases.
Acknowledgment
Financial support of this work was provided by the National Key Technology Support Program of China, under grant number 2013BAI03B06.
Disclosure
The authors report no conflicts of interest in this work.
References
Kinane DF, Lappin DF. Clinical, pathological and immunological aspects of periodontal disease. Acta Odontol Scand. 2001;59(3):154–160. | ||
Breivik T, Thrane PS, Murison R, Gjermo P. Emotional stress effects on immunity, gingivitis and periodontitis. Eur J Oral Sci. 1996;104(4 (Pt 1)):327–334. | ||
Agnihotri R, Gaur S. Chemically modified tetracyclines: novel therapeutic agents in the management of chronic periodontitis. Indian J Pharmacol. 2012;44(2):161–167. | ||
Walker CB. The acquisition of antibiotic resistance in the periodontal microflora. Periodontol 2000. 1996;10:79–88. | ||
Sbordone L, Ramaglia L, Gulletta E, Iacono V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J Periodontol. 1990;61(9):579–584. | ||
Wasserman B, Hirschfeld L. The relationship of initial clinical parameters to the long-term response in 112 cases of periodontal disease. J Clin Periodontol. 1988;15(1):38–42. | ||
Ninomiya K, Ogino C, Oshima S, Sonoke S, Kuroda S, Shimizu N. Targeted sonodynamic therapy using protein-modified TiO2 nanoparticles. Ultrason Sonochem. 2012;19(3):607–614. | ||
Guo S, Sun X, Cheng J, et al. Apoptosis of THP-1 macrophages induced by protoporphyrin IX-mediated sonodynamic therapy. Int J Nanomedicine. 2013;8:2239–2246. | ||
Su X, Wang P, Wang X, Cao B, Li L, Liu Q. Apoptosis of U937 cells induced by hematoporphyrin monomethyl ether-mediated sonodynamic action. Cancer Biother Radiopharm. 2013;28(3):207–217. | ||
Rosenthal I, Sostaric JZ, Riesz P. Sonodynamic therapy – a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem. 2004;11(6):349–363. | ||
Li P, Sun JG, Huang CR, et al. Development and validation of a sensitive quantification method for hematoporphyrin monomethyl ether in plasma using high-performance liquid chromatography with fluorescence detection. Biomed Chromatogr. 2006;20(12):1277–1282. | ||
Jin H, Zhong X, Wang Z, et al. Sonodynamic effects of hematoporphyrin monomethyl ether on CNE-2 cells detected by atomic force microscopy. J Cell Biochem. 2011;112(1):169–178. | ||
Cheng J, Liang H, Li Q, et al. Hematoporphyrin monomethyl ether-mediated photodynamic effects on THP-1 cell-derived macrophages. J Photochem Photobiol B. 2010;101(1):9–15. | ||
Qin YL, Luan XL, Bi LJ, Sheng YQ, Zhou CN, Zhang ZG. Comparison of toluidine blue-mediated photodynamic therapy and conventional scaling treatment for periodontitis in rats. J Periodontal Res. 2008;43(2):162–167. | ||
de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Bonfante S, Garcia VG. Treatment of experimental periodontal disease by photodynamic therapy in rats with diabetes. J Periodontol. 2008;79(11):2156–2165. | ||
Berakdar M, Callaway A, Eddin MF, Ross A, Willershausen B. Comparison between scaling-root-planing (SRP) and SRP/photodynamic therapy: six-month study. Head Face Med. 2012;8:12. | ||
de Almeida JM, Theodoro LH, Bosco AF, Nagata MJ, Oshiiwa M, Garcia VG. Influence of photodynamic therapy on the development of ligature-induced periodontitis in rats. J Periodontol. 2007;78(3):566–575. | ||
Sun Y, Xing D, Shen L, et al. Bactericidal effects of hematoporphyrin monomethyl ether-mediated photosensitization against pathogenic communities from supragingival plaque. Appl Microbiol Biotechnol. 2013;97(11):5079–5087. | ||
Wang Y, Chai Z, Zhang Y, Deng F, Wang Z, Song J. Influence of low-intensity pulsed ultrasound on osteogenic tissue regeneration in a periodontal injury model: X-ray image alterations assessed by micro-computed tomography. Ultrasonics. 2014;54(6):1581–1584. | ||
Gu XQ, Li YM, Guo J, Zhang LH, Li D, Gai XD. Effect of low intensity pulsed ultrasound on repairing the periodontal bone of Beagle canines. Asian Pac J Trop Med. 2014;7(4):325–328. | ||
Abtahi NS, Eimani H, Vosough A, et al. Effect of therapeutic ultrasound on folliculogenesis, angiogenesis and apoptosis after heterotopic mouse ovarian transplantation. Ultrasound Med Biol. 2014;40(7):1535–1544. | ||
Nakamura T, Fujihara S, Yamamoto-Nagata K, Katsura T, Inubushi T, Tanaka E. Low-intensity pulsed ultrasound reduces the inflammatory activity of synovitis. Ann Biomed Eng. 2011;39(12):2964–2971. | ||
Barati AH, Mokhtari-Dizaji M. Ultrasound dose fractionation in sonodynamic therapy. Ultrasound Med Biol. 2010;36(6):880–887. | ||
Sharma A, Astekar M, Metgud R, Soni A, Verma M, Patel S. A study of C-reactive protein, lipid metabolism and peripheral blood to identify a link between periodontitis and cardiovascular disease. Biotech Histochem. 2014;89(8):577–582. | ||
Soskolne WA, Klinger A. The relationship between periodontal diseases and diabetes: an overview. Ann Periodontol. 2001;6(1):91–98. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.