Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Skin Manifestation Induced by Immune Checkpoint Inhibitors
Authors Yamamoto T
Received 26 February 2022
Accepted for publication 21 April 2022
Published 10 May 2022 Volume 2022:15 Pages 829—841
DOI https://doi.org/10.2147/CCID.S364243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Toshiyuki Yamamoto
Department of Dermatology, Fukushima Medical University, Fukushima, Japan
Correspondence: Toshiyuki Yamamoto, Department of Dermatology, Fukushima Medical University, Hikarigaoka 1, Fukushima, 960-1295, Japan, Email [email protected]
Abstract: In accordance with recent therapeutic progress of immune checkpoint inhibitors for certain cancers, various disorders are induced as immune-related adverse events (irAEs) affecting the skin, gut, thyroid gland, lung, and liver. Among such irAEs, mucocutaneous manifestation is the most common. Cutaneous manifestations are categorized into several groups, ie, inflammatory reactions, immunobullous reactions, alterations of epidermal keratinocytes, and alterations of epidermal melanocytes; however, there are additionally various cutaneous toxicities, unclassified into those groups. Blocking of programmed cell death 1 (PD-1)/programmed cell death ligand 1(PDL1) can lead to the induction of autoimmune reaction, via activation of cytotoxic T cells, inhibition of regulatory T cell function, and alteration of cytokine balance. Similarly, blockade of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) reduces the suppressive function of regulatory T cells. Due to those mechanisms, various autoimmune conditions can be induced, in addition to nonspecific drug eruptions. Dermatologists should be aware of various types of those mucocutaneous manifestations, either common or rare, as well as the management of such conditions. Herein, various mucocutaneous manifestations of irAEs and cases involving Japanese patients have been described, based on a single institute’s experience.
Keywords: skin rash, PD-1, CTLA-4
Introduction
Recent progress has been brought about by immune checkpoint inhibitors (ICIs) for therapy of various advanced cancers. ICIs target cell-surface immune checkpoint proteins such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA4) and programmed cell death 1 (PD-1)/programmed cell death ligand 1(PDL1). PD-1 is an immune checkpoint receptor expressed on antigen stimulated T-cells. A fully human anti-PD-1 antibody (Ab), nivolumab, was initially approved in Japan for patients with unresectable melanoma, and thereafter, therapies with ICIs have been extended to various solid cancers as well as Hodgkin lymphoma. By contrast, CTLA-4 is an inhibitory receptor constitutively expressed on regulatory T (Treg) cells. To date, anti-PD-1 Ab (nivolumab, pembrolizumab, and cemiplimab), anti-PDL1 Ab (avelumab, atezolizumab, and durvalumab), and anti-CTLA4 Ab (ipilimumab and tremelimumab) have been made available. Tumor-specific T-cells can be expanded and stimulated to carry out anti-tumor functions by inhibition of PD-1 and/or CTLA-4 checkpoints.1 In parallel with increased use of ICIs, various immune-related adverse events have occurred, among which skin rashes are most commonly observed,2 and thus management of ICI-induced skin toxicity has been proposed.3 Pruritus, non-specific maculopapular eruptions, as well as severe drug eruptions and various autoimmune skin diseases are widely induced, and the prevalence of cutaneous lesions has been reported to be up to around 50% of the patients.4–8
The frequency of irAEs is much higher during treatment with anti-CTLA-4 Ab compared to anti-PD-1 and PD-L1 inhibitors. Possible mechanisms of the development of cutaneous irAEs include the production of autoreactive CD4+/CD8+ T-cells, stimulation of humoral immunity and B-cells, increased release of proinflammatory cytokines that are involved in immune-related damage in specific tissues/organs, and potential exposure of host antigens from tumor cells due to cytotoxic attacks.9 Blockade of PD-1 enhances exhausted T-cell effector function, reduces the suppressive ability of Tregs, and enhances B-cell and natural killer cell activity, and similarly, blockade of CTLA-4 may result in the reduction of suppressive ability of Tregs. Moreover, PD-1 blockade shifts the immune balance toward a T helper cell (Th)1/Th17 response.
In a real-world setting, patients with any grade irAEs showed a significantly high progression-free survival and overall survival.10 Although the most prevalent cutaneous irAEs under treatment with ICIs are pruritus, rash, and vitiligo mostly grade 1 and grade 2,11 dermatologists should be aware of various irAEs, not only when treating melanoma patients using ICIs, but also when consulted for cutaneous adverse events by other departments that treat non-melanoma cancers. In this paper, various cutaneous irAEs of ICIs are described, which were searched by PubMed, and also Japanese cases experienced in a single institute are introduced.
Various Mucocutaneous Disorders
Eczematous Conditions
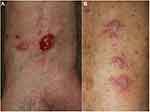
Xerosis is often observed in patients under treatment with anti-PD-1 antibody. Nummular eczema is often induced due to dry skin, especially in winter season. Therefore, anti-PD-1 antibody-induced asteatotic conditions may lead to nummular eczema (Figure 1A). Patients are encouraged to moist their body by emollient cream. Seborrheic dermatitis/seborrheic dermatitis-like scaly erythema is observed on the face and trunk, as well as intertriginous areas such as the axilla (Figure 1B). Topical corticosteroids, calcineurin inhibitors, anti-fungal ointment, and metronidazole gel are used in these cases.
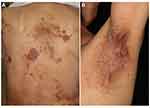 |
Figure 1 (A) Nummular eczema on the back under nivolumab therapy. (B) Seborrheic dermatitis in the axilla under nivolumab therapy. |
Maculopapular or Morbilliform Eruptions
Maculopapular or morbilliform eruptions are commonly observed in around 20% of patients receiving PD-1 inhibitors and up to 55% of patients receiving both PD-1 and anti-CTLA-4 therapy.3–7 Erythematous macules and papules are extensively observed in the face, trunk, and extremities. Skin rashes occur around 1 month after treatment. Maculopapular or morbilliform eruptions may progress into severe drug eruptions, as discussed later, and patients should be carefully followed for several days to a week after the occurrence of such eruptions.
Psoriasis/Psoriasiform Eruption
Psoriasis or psoriasiform scaly erythema is occasionally induced, affecting the trunk and extremities.12 Some cases present with typical psoriatic plaques with well-circumscribed scaly erythema, while other cases present with ill-defined scaly erythemas on the trunk and extremities (Figure 2A and B). In some cases, palms/soles are affected, and small-sized lesions presenting as guttate type psoriasis.13 Histopathological features show parakeratosis, irregular acanthosis of the epidermis, and infiltration of inflammatory cells in and below the epidermis. Subcorneal neutrophilic microabscesses are usually absent, but may be observed (Figure 3). Apoptotic keratinocytes are occasionally seen. In addition to de novo occurrence of psoriasis/psoriasiform eruptions, exacerbation of pre-existing psoriasis is also reported.14 According to the data of the European Network for Cutaneous Adverse Event to Oncologic Drugs, among 115 patients with anti-PD-1/PD-L1-induced psoriasis, 30% had a previous history of psoriasis while 70% of the patients developed psoriasis de novo.15 The patients were treated with either anti-PD-1 (86.1%) or anti-PD-L1 (13.9%). Regarding clinical types of psoriasis, plaque-type psoriasis was the most common (42.6%), followed by palmoplantar psoriasis (12.2%), pustular psoriasis (7%) and guttate psoriasis (7%). Interval between the initiation of ICIs and the onset of psoriasis/psoriasiform eruptions is a few months, which is longer than that of maculopapular eruptions.15
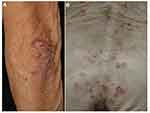 |
Figure 2 Psoriasis plaques showing scaly keratotic erythemas on the elbow (A) and back (B) under nivolumab therapy. |
 |
Figure 3 Histopathological features of psoriasiform eruption showing parakeratosis, partially irregular acanthosis of the epidermis, and infiltration of mononuclear cells below the epidermis. |
Psoriasis is immunologically mediated by aberrant, skin-directed T-cells. Recent progress shows that Th1/Th17 type T-cell subsets play a distinct role in the pathogenesis of psoriasis. IL-23 released from dendritic cells (DC) (TNF-α and inducible nitric oxide synthase (iNOS)-producing DC; Tip-DC) leads to activation and proliferation of Th17 cells. The Th17 subsets release IL-17A and IL-22, which promote neutrophil recruitment and keratinocyte hyperproliferation, respectively. As PD-1 acts as an immune modulating receptor expressed on antigen stimulated T-cells that down-regulates T-cell activity, inhibition of the PD-1 pathway can be presumed to result in overactivation of T-cell function in response to various stimuli.16 Indeed, blockade of the immune checkpoint receptors, such as PD-1 and CTLA-4, by their Abs has been demonstrated to augment Th1 and Th17 cell activities.17,18 The number of reported cases of newly-onset psoriasis or exacerbation of pre-existing psoriasis during anti-PD-1 therapies is gradually increasing. A recent paper collected 21 cases of psoriasis/psoriasiform eruptions during anti-PD-1 therapies. Among them, 20 patients developed plaque psoriasis, 6 of whom also developed guttate psoriasis.19 However, in these 6 patients who developed plaque/guttate psoriasis, 5 showed exacerbation of pre-existing psoriasis and only 1 developed de novo psoriasis. Inhibition of the PD-1 pathway has been suggested to result in overactivation of T-cell function including augmentation of Th1 and Th17 cell activities, which may induce psoriasis. In a study using an imiquimod-induced murine psoriasis model, either PD-1 genetic deficiency or PD-1 blockade by a monoclonal Ab exacerbated psoriasiform dermatitis.20 The PD-1 axis downregulates the Th1/Th17 pathways, and thus blockade of PD-1 could promote a secondary overexpression of proinflammatory cytokines mediated by Th17 cells.
In addition to cutaneous psoriasis, psoriatic arthritis is rarely induced.21,22 Inflammatory arthritis may be induced by inflammatory cytokines due to nivolumab-induced Th17 upregulation. By contrast, cases of palmoplantar pustulosis during the use of ICIs are very few.23
Lichen Planus/Lichen Planus-Like Eruption
As compared with psoriasis/psoriasiform eruptions, lichen planus (LP) is not common during ICI therapy. LP or LP-like eruptions have occurred as purple-colored, slightly keratotic plaques on the trunk and extremities (Figure 4), with a mean time of 6 to 12 weeks after the therapy initiation.5
There are a number of reports on LP or lichenoid dermatitis.24–26 In a single institution cohort study, lichenoid reactions were observed in 17% of 82 patients with metastatic melanoma who received anti-PD-1 therapy.24 Purple-colored keratotic plaques appear on the lower legs after the initiation of nivolumab. Mucous and genital involvement was rarely reported.25 Nail involvement presenting with subungual hyperkeratosis, dystrophy, and ridging has been reported. Rare phenotypes such as hyperkeratotic LP,27,28 bullous LP,29–31 and lichen nitidus32 have been observed. Bullous LP is commonly seen on the lower extremities. Histopathologically, irregular hypertrophic acanthosis of the epidermis and lichenoid interface dermatitis, subepidermal edema, bullous formation, and rarely absence of the epidermis are observed (Figure 5). The degree of interface dermatitis and epidermal changes are variable. Bulla formation may be due to the extensive liquefaction and vacuolation of the basal layer.
 |
Figure 5 (A) Histopathology of bullous lichen planus, showing bullous lesions below the elongated epidermis. (B) Histopathology of ulcerative lichen planus, showing the absence of the epidermis. |
It has been suggested that the pathogenesis of LP is due to epidermal damage caused by autoreactive cytotoxic CD8+ T-cells, mediated by interferon-γ (IFN-γ). In murine LP models, prominent expression of PD-L1 in keratinocytes is suggested to play a protective role against cytotoxic CD8+ T-cells.33 In addition, in vitro studies showed that administration of anti-PD-1 antibodies induced increased production of IFN-γ from peripheral blood mononuclear cells of patients with oral LP.34 Komori et al recently reported a case which focally developed LP in an irradiated area, suggesting Koebner phenomenon.35 They speculated a close relationship between anti-PD-1 therapy plus radiotherapy and the development of LP. Another recent report showed an increased mRNA expression of granzyme B and IFN-γ after nivolumab treatment.36 Inhibition of PD-1 may induce epidermal basal layer damage with prominent edema leading to bullous LP, mediated by IFN-γ and other molecules.
Autoimmune Bullous Diseases
Bullous pemphigoid (BP) is occasionally induced during ICI therapy occurring between a few weeks and several months after the therapy initiation.4 Tense blisters, bullae, and erosions with erythemas are observed on the trunk and extremities (Figure 6). Serum Abs against BP180 Nc16a IgG are detected. The occurrence of BP is associated with favorable tumor response to anti-PD-1 therapy and improved survival,37 while other studies could not support the correlation.38 Lopez et al39 recently reported a literature review of 21 published cases of BP associated with ICIs, and Siegel et al40 also reported 9 cases of bullous disorders, including 7 BP cases, that developed during ICI therapy at their institute. In both reports, melanoma and non-small cell lung cancer were the most frequent underlying malignancies. The median time between the initiation of ICI therapy and the occurrence of BP was approximately 6 months, which is longer than that of most irAEs. Non-specific pruritic eruptions preceded blister formation in approximately half of the cases.39
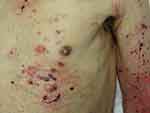 |
Figure 6 Bullous pemphigoid showing tense blisters, erosions and erythema in a patient with lung cancer under nivolumab. |
Blockade of the PD-1/PD-L1 pathway has been shown to enhance B-cell activation, which may lead to production of autoantibodies and inflammatory cytokines. In addition, the development of BP may be due to Ab recognition of a common antigen which targets the basement membrane of the skin (cross-reactivity), and the production of autoantibodies against different epitopes, which can be induced by lichenoid reaction, may be attributed to epitope-spreading phenomena.38 B cell-mediated Ab production needs longer period until the onset of associated diseases, compared with T cell-mediated responses. Occasionally, BP exhibits severe erosive erythemas mimicking toxic epidermal necrolysis (TEN).41 In addition, as rare variants, lichen planus pemphigoides,42–45 and mucous membrane pemphigoid46 have been reported.
Acantholytic dermatosis (Grover’s disease) or Grover disease-like lesions are rarely reported,47–49 which present with pruritic papulovesicular eruptions on the erythema involving the trunk and extremities. Their histopathological characteristic is acantholysis of the epidermis, with or without dyskeratosis. Direct immunofluorescence reveals negative deposition of immunoglobulins and complements.
Vitiligo
Vitiligo is frequently induced by specific autoimmunity against melanocytes during ICIs therapy, especially in patients with advanced melanoma (Figure 7A). By contrast, cases occurring in patients with cancers other than melanoma, such as lung adenocarcinoma, cholangiocarcinoma, renal cell carcinoma, squamous cell carcinoma, transitional cell carcinoma, and hematologic malignancies (Figure 7B), are less commonly reported.7 Occurrence of depigmentation during ICI therapy is significantly associated with a favorable prognosis such as higher survival rates.50 Vitiligo is more frequently induced during anti-PD-1 therapy than during anti-CTLA-4 therapy. During ICIs therapy, CD8+ cytotoxic T-cells are activated against melanoma-associated antigens, such as MART-1, GP100, tyrosinase-related proteins 1 and 2, and tyrosinase, shared by melanocytes and melanoma cells, resulting in depigmentation.51
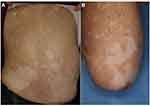 |
Figure 7 Vitiligo in patients with melanoma under nivolumab therapy (A) and lung cancer under pembrolizumab therapy (B). |
Scleroderma
Scleroderma is rarely induced during the use of ICIs, especially anti-PD-1 agents (Figure 8A and B). According to a recent literature review by Terrier et al,52 6 cases of systemic sclerosis (2 limited and 4 diffuse type) and 4 cases of morphea (2 localized and 2 generalized type) were reported from 2015 to 2019. Regarding internal organ involvement, interstitial lung disease (n = 2), gastroesophageal reflux disease (n = 2), and scleroderma renal crisis (n = 1) were observed. Serum anti-nuclear antibody was negative (n = 7), positive (n = 2), and unknown (n = 1). The authors speculate that the inhibition of PD-1/PD-L1 induces profibrotic M2 type macrophages, which may promote fibroblast activation and excessive production of extracellular matrix protein. Recent studies have shown that, in addition to Th2, Th17 is also involved in systemic sclerosis.53 Although both in vitro and in vivo data on the role of IL-17A in fibrosis are still controversial, upregulation of Th17 cell activities provoked by ICIs may have a role on the development of fibrotic disorders. In addition to scleroderma, cases of scleroderma-like syndrome54 and acral ischemia55 induced by ICIs have been reported.
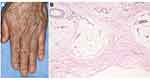 |
Figure 8 (A) Scleroderma showing edematous fingers of an oral melanoma patient under nivolumab therapy. (B) Histopathology showing thickened collagen bundles in the lower dermis. |
Granulomatous Skin Lesions
Sarcoidosis or sarcoidosis-like lesions are induced 4.5 to 6 months, on average, after the initiation of ICIs. According to a recent review, 80 patients developed sarcoidosis/sarcoid-like reactions in association with ICI treatment. Mediastinal/hilar lymph nodes were most commonly affected (56/80), followed by the skin (40/80). By contrast, exclusive cutaneous involvement was observed only in 10% (8/80).56 Clinically, subcutaneous erythematous nodules, papulonodules, plaques, and annular erythematous lesions are presented (Figure 9A and B). Some cases such as infiltrative erythema do not fit into any type of cutaneous sarcoidosis, and are reported under the term of sarcoidosis-like, sarcoid reaction, or sarcoidal granulomatous dermatitis. Furthermore, sarcoid-like reactions can appear on tattoos.57 TNF-α plays an important role in sarcoidosis, and recent studies suggest that IL-17 is involved in the pathogenesis of sarcoidosis, especially in the lung.58 CD163+ histiocytes are abundantly detected in immunotherapy-associated reactions.
Other granulomatous diseases induced by ICIs include granuloma annulare,59 and interstitial granulomatous dermatitis.60 ICIs inhibit Th1 cells but activate macrophages, which may lead to granulomatous skin diseases. ICI-induced granuloma formation may be caused by the activation of the innate immune system, dysfunction of Tregs, and expansion of Th17 cells.
Erythema Nodosum
Panniculitis is rarely induced by ICIs. Erythema nodosum is a representative panniculitis which presents with reddish tender erythematous nodules mainly on the extremities, predominantly on the lower legs. It is generally considered to be a benign and self-limiting hypersensitivity reaction to various inciting factors. Erythema nodosum may be either idiopathic or triggered by infection, drugs, or other disorders including inflammatory bowel disease.61 Histologically, early onset of erythema nodosum is characterized by a neutrophilic inflammatory infiltrate in the septa of the subcutaneous tissue. Sometimes, neutrophilic abscesses are observed in the subcutaneous tissues. CD8-positive T-cells infiltrate into the subcutaneous tissues. Although erythema nodosum is frequently induced during treatment with BRAF inhibitors, cases of ICI-induced erythema nodosum are rarely reported.62–64
Stevens-Johnson Syndrome/TEN
Severe drug eruptions such as Stevens-Johnson syndrome (SJS) and TEN due to ICIs have been reported. Such severe mucocutaneous reactions develop extensive erosions on the trunk and extremities, as well as oral mucosa (Figure 10A and B). According to previous reports, SJS/TEN occurred after a mean of 11 weeks of nivolumab use and after a mean of 3 weeks of pembrolizumab use.65
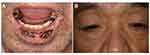 |
Figure 10 Mucosal involvement of the mucous membrane of the lip (A) and eyelid conjunctiva (B) under nivolumab therapy. |
The mechanism is speculated that checkpoint inhibition interferes with maintenance of peripheral tolerance and protective function of keratinocytes from injury, which leads to a tendency for severe drug eruptions affecting mucocutaneous regions. Also, blockade of the PD-1/PD-L1 signaling pathway allows autoreactive CD8+ T-cells targeting keratinocytes to proliferate and be activated. Treatment with anti-PD-1 increases the expression of PD-L1 in epidermal keratinocytes, leading to the targeting and apoptosis of keratinocytes by activated cytotoxic CD8+ T-cells.
Patients may develop diffuse erythema multiforme-like erythemas with or without targetoid lesions on the trunk and extremities (Figure 11A and B). Biopsy reveals liquefaction degeneration of the basement membrane of the epidermis, vacuolar interface alteration, and epidermal necrosis. Such cases require prompt systemic treatment in order not to develop into SJS/TEN. Thus, it should be kept in mind that severe skin toxicities can appear some weeks to months after the therapy initiation. Other types of severe drug eruptions such as acute generalized exanthematous pustulosis and drug-induced hypersensitivity syndrome (DIHS)/drug reaction with eosinophilia and systemic symptoms (DRESS) are rarely reported.66
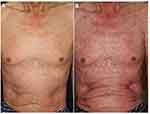 |
Figure 11 Erythema multiforme-like slight reddish erythemas on the trunk under nivolumab therapy (A), which worsened with targetoid lesions 2 days later (B). |
Pyoderma Gangrenosum
Pyoderma gangrenosum is a disease characterized by refractory sterile deep ulcers predominantly in the extremities of middle-aged individuals. Although innate immunity plays an important role in the pathogenesis of pyoderma gangrenosum, acquired immunity of Th1/Th17 types also plays a role.67,68 Pyoderma gangrenosum is rarely induced by drugs, such as iodide, bromide, isotretinoin, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor,69,70 and ICIs can be included as possible candidate drugs. In addition to pyoderma gangrenosum, other neutrophilic dermatoses such as Sweet’s syndrome71 and hidradenitis suppurativa72 have also been reported.
Acquired Reactive Perforating Collagenosis
Prurigo-like lesions are sometimes seen on the lower extremities or buttocks. Also, the occurrence of acquired reactive perforating collagenosis (ARPC) is observed during ICIs therapy (Figure 12). Clinically, a number of umbilicated papules and nodules with central keratotic plugs develop on the trunk and extremities. ARPC is histologically characterized by necrobiotic basophilic collagen bundles which run transversely through the epidermis (transepidermal elimination of altered collagen). Pruriginous eruption may be induced by scratching due to severe pruritus. Such scratching may cause microtrauma and necrobiosis of the connective tissues. Insufficient blood supply as a result of vasculopathy may be associated as an underlying factor in ARPC. ARPC is seen in association with various systemic disorders in susceptible patients, and in particular, it is frequently associated with uncontrolled severe diabetes, as well as other disorders such as chronic renal failure, liver dysfunction, viral infection, lung fibrosis, and thyroid dysfunction. Pruritus is considered as a most common symptom induced by ICIs. Scratching is the main cause of ARPC, but other factors are also highly involved.
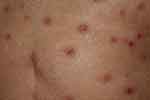 |
Figure 12 Pruriginous nodules of acquired reactive perforating collagenosis with central umbilical keratosis under nivolumab therapy. |
Other Skin Manifestations
Non-scarring alopecia presenting with alopecia areata or diffuse alopecia,73 and nail changes such as nail dystrophy, onychomadesis, onychoschizia, and paronychia have been observed.74 Vasculitis (cutaneous leukocytoclastic vasculitis), rheumatic diseases (systemic and cutaneous lupus erythematosus (acute, subacute, and chronic), dermatomyositis, Sjogren's syndrome, and autoimmune fasciitis), and rare forms of skin lesions mimicking erythema annulare centrifugum or pityriasis rubra pilaris, have been reported.6,7 Both benign and malignant skin tumors, such as keratoacanthoma, porokeratosis, actinic keratosis, squamous cell carcinoma, and pseudolymphoma, have been reported.6 Eruptive keratoacanthoma showed spontaneous regression following nivolumab treatment.75
Conclusions
ICIs are now approved for many advanced malignancies. In accordance with an increase in both the use of ICIs and the number of cancers for which ICI therapy was approved, the diversity of AEs associated with ICIs is growing. In general, the induction of adverse events may correlate with the antitumor immune response. Cutaneous irAE can be classified into several types, ie, i) inflammatory reactions, ii) immunobullous reactions, iii) keratinocyte alterations, and iv) melanocytic alterations (Table 1). There are variations in time interval between the initiation of ICIs and development of those mucocutaneous lesions. Of note, different types of mucocutaneous lesions can be developed in the same individuals, at a different timing depending on the type of lesions.
 |
Table 1 Proposed Classification of Cutaneous irAEs |
Many cutaneous irAEs do not always require interruption of ICIs, because the majority of the cases are classified as grade 1 and grade 2. Although rare, severe lesions such as SJS/TEN and DIES/DRESS should be carefully managed with the Grading systems.5 Thus, the role of dermatologists is important to control skin rash tolerated as long as the rash does not reach grade 3. Furthermore, prompt treatment is necessary for severe cases of grade 4. Additionally, combined treatment with anti-CTLA-4 and anti-PD-1 therapy results in the development of more frequent cutaneous irAEs with greater severity and an earlier onset than monotherapy with ICIs.76 Resumption of ICIs may induce both the same and different cutaneous lesions,77 with an escalated grade. Therefore, careful and long-term monitoring, as well as management of cutaneous irAEs are required.
Informed-Consent Statement
Written informed consent was obtained from all patients for accompanying images to be published in this paper.
Funding
There is no funding to report.
Disclosure
The author declares no conflicts of interest related to this work.
References
1. Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors I melanoma. Lancet. 2021;398:1002–1014. doi:10.1016/S0140-6736(21)01206-X
2. Jin KT, Wang SB, Ying XJ, et al. Immune-mediated adverse effects of immune checkpoint inhibitors and their management in cancer. Immunol Lett. 2020;221:61–71. doi:10.1016/j.imlet.2020.02.008
3. Kamińska-Winciorek G, Cyulska-Stopa B, Lugowska I, et al. Principles of prophylactic and therapeutic management of skin toxicity during treatment with checkpoint inhibitors. Adv Dermatol Allergol. 2019;36:382–391. doi:10.5114/ada.2018.80272
4. Ellis SR, Vierra AT, Millsop JW, et al. Dermatologic toxicities to immune checkpoint inhibitor therapy: a review of histopathologic features. J Am Acad Dermatol. 2020;83:1130–1143. doi:10.1016/j.jaad.2020.04.105
5. Geisler AN, Phillips GS, Barrios DM, et al. Immune checkpoint inhibitor-related dermatologic adverse events. J Am Acad Dermatol. 2020;83:1255–1268. doi:10.1016/j.jaad.2020.03.132
6. Quach HT, Johnson DB, LeBoeuf NR, et al. Cutaneous adverse events caused by immune checkpoint inhibitors. J Am Acad Dermatol. 2021;85:956–966.
7. Malviya N, Tattersall IW, Leventhal J, Alloo A. Cutaneous immune-related adverse events to checkpoint inhibitors. Clin Dermatol. 2020;38:660–678.
8. Bhardwaj M, Chiu MN, Sah SP. Adverse cutaneous toxicities by PD-1/PD-L1 immune checkpoint inhibitors: pathogenesis, treatment, and surveillance. Cutan Ocul Toxicol. 2022;20:1–18.
9. Apalla Z, Rapoport B, Sibaud V. Dermatologic immune-related adverse events: the toxicity spectrum and recommendations for management. Int J Womens Dermatol. 2021;7:625–635.
10. Bai R, Chen N, Chen X, et al. Analysis of characteristics and predictive factors of immune checkpoint inhibitor-related adverse events. Cancer Biol Med. 2021;18:1118–1133. doi:10.20892/j.issn.2095-3941.2021.0052
11. Garrett NF, da Costa ACC, Ferreira EB, et al. Prevalence of dermatological toxicities in patients with melanoma undergoing immunotherapy: systematic review and meta-analysis. PLoS One. 2021;16:e0255716. doi:10.1371/journal.pone.0255716
12. Ohtsuka M, Miura T, Mori T, Ishikawa M, Yamamoto T. Occurrence of psoriasiform eruption during Nivolumab therapy for a patient with mucosal melanoma. JAMA Dermatol. 2015;151:797–799. doi:10.1001/jamadermatol.2015.0249
13. Yamamoto T. Anti-programmed cell death-1-induced plaque and guttate psoriasis. Indian J Dermatol. 2018;63:88–89. doi:10.4103/ijd.IJD_46_17
14. Matsumura N, Ohtsuka M, Kikuchi N, Yamamoto T. Exacerbation of psoriasis during nivolumab therapy for metastatic melanoma. Acta Derm Venereol. 2016;96:259–260. doi:10.2340/00015555-2212
15. Nikolaou V, Sibaud V, Fattore D, et al. Immune checkpoint-mediated psoriasis: a multicenter European study of 115 patients from the European Network for Cutaneous Adverse Event to Oncologic Drugs (ENCADO) group. J Am Acad Dermatol. 2021;84:1310–1320. doi:10.1016/j.jaad.2020.08.137
16. Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi:10.1007/82_2010_116
17. Dulos J, Carven GJ, van Boxtel SJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35:169–178. doi:10.1097/CJI.0b013e318247a4e7
18. Sarnaik AA, Yu B, Yu D, et al. Extended dose ipilimumab with a peptide vaccine: immune correlates associated with clinical benefit in patients with resected high-risk stage IIIc/IV melanoma. Clin Cancer Res. 2011;17:896–906. doi:10.1158/1078-0432.CCR-10-2463
19. Bonigen J, Raynaud-Donzel C, Hureaux J, et al. Anti-PD-1-induced psoriasis: a study of 21 patients. J Eur Acad Dermatol Venereol. 2017;31:e254–e257. doi:10.1111/jdv.14011
20. Imai Y, Ayithan N, Wu X, et al. PD-1 regulates imiquimod-induced psoriasiform dermatitis through inhibition of IL-17A expression by innate γδ-low T cells. J Immunol. 2015;195:421–425. doi:10.4049/jimmunol.1500448
21. Law-Ping-Man S, Martin A, Briens E, et al. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology. 2016;55:2087–2089. doi:10.1093/rheumatology/kew281
22. Jatwani K, Kaur H, Chugh K, Jatwani S. Nivolumab-induced psoriatic arthritis in a patient with advanced small cell lung cancer. J Clin Rheumatol. 2021;27:e162–e163. doi:10.1097/RHU.0000000000001301
23. Furuta H, Kato S, Masago K, Hida T. Palmoplantar pustulosis caused by immune checkpoint inhibitors. Clin Lung Cancer. 2021;22:e829–e832. doi:10.1016/j.cllc.2021.04.002
24. Hwang SJE, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455–461. doi:10.1016/j.jaad.2015.10.029
25. Shi VJ, Rodic N, Gettinger S, et al. Clinical and histologic features of lichenoid mucocutaneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128–1136. doi:10.1001/jamadermatol.2016.2226
26. Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-a therapy. Cancer Immunol Res. 2015;3:18–22. doi:10.1158/2326-6066.CIR-14-0134
27. Niesert AC, Guertler A, Schutti O, et al. Ulcerative lichen planus after adjuvant use of programmed cell death-1-inhibitor: a case report and systematic review of the literature. Acta Derm Venereol. 2021;101:adv00472. doi:10.2340/00015555-3840
28. Maarouf M, Alexander C, Shi VY. Nivolumab reactivation of hypertrophic lichen planus, a case report and review of published literature. Dermatol Online J. 2018;24:9.
29. Hanami Y, Yamamoto T. Bullous and non-bullous lichen planus during nivolumab therapy for lung cancer. Actas Dermosifiliogr. In press 2022.
30. Wakade DV, Carlos G, Hwang SJE, Chou S, Hui R, Fernandez-Peñas P. PD-1 inhibitors induced bullous lichen planus-like reactions: a rare presentation and report of three cases. Melanoma Res. 2016;26:421–424. doi:10.1097/CMR.0000000000000263
31. Biolo G, Caroppo F, Salmaso R, Alaibac M. Linear bullous lichen planus associated with nivolumab. Clin Exp Dermatol. 2019;44:67–68. doi:10.1111/ced.13700
32. Komatsu-Fujii T, Nakajima S, Iwata M, et al. Upregulated programmed death ligand 1 expression in nivolumab-induced lichen nitidus: a follow-up report with an immunohistochemical analysis. J Dermatol. 2020;47:e319–e320. doi:10.1111/1346-8138.15480
33. Okiyama N, Katz SI. Programmed cell death (PD-1) regulates the effector function of CD8 T cells via PD-L1 expressed on target keratinocytes. J Autoimmun. 2014;53:1–9. doi:10.1016/j.jaut.2014.06.005
34. Zhou G, Zhang J, Ren XW, et al. Increased B7-H1 expression on peripheral blood T cells in oral lichen planus correlated with disease severity. J Clin Immunol. 2012;32:794–801. doi:10.1007/s10875-012-9683-2
35. Komori T, Honda T, Irie H, et al. Lichen planus in irradiated skin during nivolumab treatment. Acta Derm Venereol. 2017;97:391–392. doi:10.2340/00015555-2545
36. Anegawa H, Otsuka A, Kaku Y, et al. Upregulation of granzyme B and interferon-γ mRNA in responding lesions by treatment with nivolumab for metastatic melanoma: a case report. J Eur Acad Dermatol Venereol. 2016;30:e231–e232. doi:10.1111/jdv.13567
37. Nelson CA, Singer S, Chen T, et al. Bullous pemphigoid after anti-PD-1 therapy: a retrospective case-control study evaluating impact on tumor response and survival outcomes. J Am Acad Dermatol. 2020. doi:10.1016/j.jaad.2019.12.068
38. Tsiogka A, Bauer JW, Patsatsi A. Bullous pemphigoid associated with anti-programmed cell death protein 1 and anti-programmed cell death ligand 1 therapy: a review of the literature. Acta Derm Venereol. 2021;101:
39. Lopez AT, Khanna T, Antonov N, et al. A review of bullous pemphigoid associated with PD-1 and PD-L1 inhibitors. Int J Dermatol. 2018;57:664–669. doi:10.1111/ijd.13984
40. Siegel J, Totonchy M, Damsky W, et al. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: a retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. J Am Acad Dermatol. 2018;79:1081–1088. doi:10.1016/j.jaad.2018.07.008
41. Qiu C, Shevchenko A, Hsu S. Bullous pemphigoid secondary to pembrolizumab mimicking toxic epidermal necrolysis. JAAD Case Rep. 2020;6:400–402. doi:10.1016/j.jdcr.2020.03.003
42. Strickley JD, Vence LM, Burton SK, Callen JP. Nivolumab-induced lichen planus pemphigoides. Cutis. 2019;103:224–226.
43. Sato Y, Fujimura T, Mizuashi M, Aiba S. Lichen planus pemphigoides developing from patient with non-small-cell lung cancer treated with nivolumab. J Dermatol. 2019;46:e374–e375.
44. Okada H, Kamiya K, Murata S, et al. Case of lichen planus pemphigoides after pembrolizumab therapy for advanced urothelial carcinoma. J Dermatol. 2020;47:e321–e322. doi:10.1111/1346-8138.15461
45. Yoshida S, Shiraishi K, Yatsuzuka K, et al. Lichen planus pemphigoides with antibodies against the BP180C-terminal domain induced by pembrolizumab in a melanoma patient. J Dermatol. 2021;48:e449–e451. doi:10.1111/1346-8138.16006
46. Durmus Ö, Gulseren D, Akdogan N, Gokoz O. Mucous membrane pemphigoid in a patient treated with nivolumab for Hodgkin’s lymphoma. Dermatol Ther. 2020;33:e14109. doi:10.1111/dth.14109
47. Munoz J, Guillot B, Girard C, et al. First report of ipilimumab-induced Grover disease. Br J Dermatol. 2014;171:1236–1237. doi:10.1111/bjd.13058
48. Uemura M, Fa’ak F, Haymaker C, et al. A case report of Grover’s disease from immunotherapy: a skin toxicity induced by inhibition of CTLA-4 but not PD-1. J Immunother Cancer. 2016;4:55. doi:10.1186/s40425-016-0157-6
49. Koelzer VH, Buser T, Willi N, et al. Grover’s –like drug eruption in a patient with metastatic melanoma under ipilimumab therapy. J Immunother Cancer. 2016;4:47. doi:10.1186/s40425-016-0151-z
50. Lommerts J, Bekkenk MW, Luiten RM. Vitiligo induced by immune checkpoint inhibitors in melanoma patients: an expert opinion. Expert Opin Drug Saf. 2021;20:883–888. doi:10.1080/14740338.2021.1915279
51. Gault A, Anderson AE, Plummer R, et al. Cutaneous immune-related adverse events in patients with melanoma treated with checkpoint inhibitors. Br J Dermatol. 2021;185:263–271. doi:10.1111/bjd.19750
52. Terrier B, Humbert S, Preta LH, et al. Risk of scleroderma according to the type of immune checkpoint inhibitors. Autoimmun Rev. 2020;19:102596. doi:10.1016/j.autrev.2020.102596
53. Kurasawa K, Hirose K, Sano H, et al. Increased interleukin-17 production in patients with systemic sclerosis. Arthritis Rheum. 2000;43:2455–2463. doi:10.1002/1529-0131(200011)43:11<2455::AID-ANR12>3.0.CO;2-K
54. Cho M, Nonomura Y, Kaku Y, et al. Scleroderma-like syndrome associated with nivolumab treatment in malignant melanoma. J Dermatol. 2019;46:e43–e44. doi:10.1111/1346-8138.14492
55. Suenaga A, Ito T, Eto A, Furue M. Acral ischemia induced by nivolumab: a case report. J Dermatol. 2021;48:e223–e224. doi:10.1111/1346-8138.15830
56. Apalla Z, Kemanetzi C, Papageorgiou C, et al. Challenges in sarcoidosis and sarcoid-like reactions associated to immune checkpoint inhibitors: a narrative review apropos of a case. Dermatol Ther. 2021;34:e14618. doi:10.1111/dth.14618
57. Kluger N. Tattoo reactions associated with targeted therapies and immune checkpoint inhibitors for advanced cancers: a brief review. Dermatology. 2019;235:522–524.
58. Talreja J, Talwar H, Bauerfeld C, et al. HIF-1α regulates IL-1β and IL-17 in sarcoidosis. eLife. 2019;8:e44519.
59. Wu J, Kwong BY, Martires KJ, et al. Granuloma annulare associated with immune checkpoint inhibitors. J Eur Acad Dermatol Venereol. 2018;32:e124–e126.
60. Trinidad C, Nelson KC, Oliva ICG, et al. Dermatologic toxicity from immune checkpoint blockade therapy with an interstitial granulomatous pattern. J Cutan Pathol. 2018;45:504–507.
61. Spagnuolo R, Dastoli S, Silvestri M, et al. Anti-interleukin 12/23 in the treatment of erythema nodosum and Crohn disease: a case report. Dermatol Ther. 2019;32:e12811.
62. Tetzlaff MT, Jazaeri AA, Torres-Cabala CA, et al. Erythema nodosum-like panniculitis mimicking disease recurrence: a novel toxicity from immune checkpoint blockade therapy. Report of 2 patients. J Cutan Pathol. 2017;44:1080–1086.
63. Choi ME, Lee KH, Won CH, et al. A case of erythema nodosum-like panniculitis induced by nivolumab in a patient with oesophageal cancer. Australas J Dermatol. 2019;60:154–156.
64. Pach J, Moody K, Ring N, et al. Erythema nodosum-like panniculitis associated with immune checkpoint inhibitor therapy: two cases reporting a rare cutaneous adverse event. JAAD Case Rep. 2021;13:118–120.
65. Maloney NJ, Ravi V, Cheng K, et al. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: a systematic review. Int J Dermatol. 2020;59:e183–e188.
66. Coleman EL, Olamiju B, Leventhal JS. The life-threatening eruptions of immune checkpoint inhibitor therapy. Clin Dermatol. 2020;38:94–104.
67. Antiga E, Maglie R, Volpi W, et al. T helper type 1-related molecules as well as interleukin-15 are hyperexpressed in the skin lesions of patients with pyoderma gangrenosum. Clin Exp Immunol. 2017;189:383–391.
68. Caproni M, Antiga E, Volp W, et al. The Treg/Th17 cell ratio is reduced in the skin lesions of patients with pyoderma gangrenosum. Br J Dermatol. 2015;173:275–278.
69. Wang JY, French LE, Shear NH, et al. Drug-induced pyoderma gangrenosum: a review. Am J Clin Dermatol. 2018;19:67–77.
70. Wu BC, Patel ED, Ortega-Loayza AG. Drug-induced pyoderma gangrenosum: a model to understand the pathogenesis of pyoderma gangrenosum. Br J Dermatol. 2017;177:72–83.
71. Ravi V, Maloney N, Worswick S. Neutrophilic dermatoses as adverse effects of checkpoint inhibitors: a review. Dermatol Ther. 2019;32:e13074.
72. Maillard A, Pastor D, Merat R. Anti-PD-1-induced hidradenitis suppurativa. Dermatopathology. 2021;8:37–39.
73. Antoury L, Maloney N, Bach DQ, et al. Alopecia areata as an immune-related adverse event of immune checkpoint inhibitors: a review. Dermatol Ther. 2020;33:e14171.
74. Sibaud V. Dermatologic reactions to immune checkpoint inhibitors. Am J Clin Dermatol. 2018;19:345–361.
75. Fujimura T, Lyu C, Tsukada A, et al. Eruptive keratoacanthoma with spontaneous regression arising from a cervical squamous cell carcinoma patient treated with nivolumab. J Dermatol. 2019;46:e177–e178.
76. Yamazaki N, Kiyohara Y, Uhara H, et al. Efficacy and safety of nivolumab in Japanese patients with previously untreated advanced melanoma: a Phase II study. Cancer Sci. 2017;108:1223–1230.
77. Dolladille C, Ederhy S, Sassier M, et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6:865–871.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.