Back to Journals » Clinical Ophthalmology » Volume 11
Six-month postoperative outcomes of intraoperative OCT-guided surgical cystotomy for refractory cystoid macular edema in diabetic eyes
Authors Asahina Y, Tachi N, Asahina Y, Yoshimura K, Ueta Y, Hashimoto Y
Received 30 August 2017
Accepted for publication 25 October 2017
Published 23 November 2017 Volume 2017:11 Pages 2099—2105
DOI https://doi.org/10.2147/OPTH.S150385
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Supplementary video showing surgical procedure of cystotomy. Notes: The temporal margin of the cystoid space was vertically incised using a 27-gage needle, followed by an additional horizontal incision and flushing the cystoid space with 50–100 μL ba
Views: 615
Yuichi Asahina, Naoko Tachi, Yumi Asahina, Kayoko Yoshimura, Yoshiki Ueta, Yoshihiro Hashimoto
Eye Center, Shinseikai Toyama Hospital, Imizu, Toyama, Japan
Purpose: This study evaluated the outcomes of surgical cystotomy for recurrent diabetic cystoid macular edema (CME).
Patients and methods: We analyzed 20 eyes with a clinical diagnosis of diabetic retinopathy and refractory CME. Release of vitreoretinal adhesion, epiretinal membrane (ERM) and internal limiting membrane (ILM) peeling and cystotomy guided by intraoperative optical coherence tomography (iOCT) were performed in every patient. Pars plana vitrectomy was also performed in 17 patients, 11 of whom also underwent lensectomy and intraocular lens implantation. Central retinal thickness (CRT), central minimum macular thickness (CMMT), macular volume (MV) and best-corrected visual acuity (BCVA) were compared preoperatively and 1 and 6 months post surgery.
Results: CRT, CMMT and MV significantly improved 1 and 6 months post surgery in each group (P<0.01). Significant improvements in BCVA were only observed 6 months post surgery (P<0.01). No intra- or postoperative complications were observed in all patients.
Conclusion: CRT, CMMT, MV and BCVA significantly improved 6 months following surgical cystectomy. This implies that iOCT-guided cystotomy could be another treatment option for refractory CME in diabetic eyes.
Keywords: diabetic retinopathy, cystoid macular edema, intraoperative OCT, cystotomy
A Letter to the Editor has been received and published for this article.
Introduction
Diabetic macular edema (DME) is a leading cause of visual loss in developed nations.1,2 A number of therapeutic approaches have been tested for refractory DME including focal macular photocoagulation and injections of intravitreal steroids or anti-endothelial growth factor (VEGF) agents, all with satisfactory outcomes.3–6 Vitrectomy has also been tested but has had moderate outcomes and is controversial.7 This surgical approach depends on the assumption that vitreous attachment may adversely affect the course of DME; therefore, relieving vitreomacular traction would be beneficial.8 Vitrectomy increases the oxygen supply to the retina, thereby improving retinal ischemia, and removes inflammatory cells and cytokines residing in the preretinal space and reduces the VEGF concentrations in the vitreous cavity.9–14 As vitreous viscosity is estimated to be 300–2,000 times greater than aqueous viscosity, the diffusion coefficient of intravitreal molecules should increase by a similar magnitude after vitrectomy.15,16
However, some patients still experience recurrent DME even in the absence of vitreomacular traction or vitreous itself because of previous surgical interventions. As vitrectomy would not be effective for these patients, we hypothesized that a direct approach could be beneficial for resolving edema. This involves making an incision in the cyst, which contains dense inflammatory deposits, and cleaning it. This maneuver is delicate and could cause some complications such as retinal tears and macular holes, even though there are no neuronal components on the vitreous side of the macular cysts.17 We were able to perform this technique safely using microscope-integrated intraoperative optical coherence tomography (iOCT). This recently introduced method is a novel imaging modality that provides dynamic feedback of tissue alterations during surgery and seamlessly integrates image acquisition, thereby allowing real-time assessment and safer delicate maneuvers.18,19
Since there are not many reports describing surgical approaches other than vitrectomy for the treatment of refractory DME, we aimed to evaluate the outcomes of cystotomy for this condition under the guidance of iOCT.
Patients and methods
In this observational case study, we retrospectively reviewed 20 eyes with a clinical diagnosis of diabetic retinopathy and refractory cystoid macular edema (CME). All patients had been diagnosed and treated at our institute. Written consent was given by patients for their information to be stored in the hospital database and used for research. This study was performed according to the tenets of the Declaration of Helsinki.
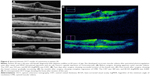
All patients underwent a standard three-port transconjunctival vitrectomy (25 gage or 27 gage) performed by the same surgeon (NT). Triamcinolone acetonide was intravitreously injected during vitrectomy to visualize the vitreous. The epiretinal membrane (ERM) was peeled in the macular region at the discretion of the surgeon. All concomitant internal limiting membrane (ILM) peeling was performed using diluted brilliant Blue G. After these procedures, the temporal margin of the cystoid space was vertically incised using a 27-gage needle, followed by an additional horizontal incision and flushing the cystoid space with 50–100 μL balanced salt solution (BSS). The surgical method for cystectomy is shown in Figure 1. This maneuver is also shown in Video S1. Pars plana vitrectomy (PPV), release of vitreoretinal adhesion, ERM and ILM peeling and cystotomy were all performed under the guidance of iOCT (RESCAN 700; Carl Zeiss Meditec AG, Jena, Germany). Lensectomy and intraocular lens implantation were combined for removing significant cataracts that were present preoperatively or for anticipated postoperative cataract formation. This surgical procedure was performed by corneal 2.2 mm incision, phacoemulsification and aspiration of the cataract and in the bag implantation of a hydrophilic acrylic lens.
The Wilcoxon signed-rank test was used to compare the preoperative and 1- and 6-month postoperative measurements of central retinal thickness (CRT), central minimum macular thickness (CMMT), macular volume (MV) and best-corrected visual acuity (BCVA). CRT, CCMT and MV were evaluated using the Heidelberg Spectralis OCT (Heidelberg Engineering, Heidelberg, Germany) in accordance with the manufacturer’s instructions. BCVA was converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis. All analyses were performed using the R statistical software (R version 3.1.3, released on 2015; R Foundation for Statistical Computing, Vienna, Austria).
Ethics
The research ethics committee of Shinseikai Toyama Hospital approved this study.
Results
The characteristics of patients are presented in Table 1. There were six females and 14 males, with a mean age of 64.1±8.2 years. The mean follow-up period was 10.8±3.6 months. Preoperatively, nine eyes were pseudophakic and 11 were phakic. All patients underwent release of vitreoretinal adhesion, ERM and ILM peeling and cystotomy. Of these, 11 patients with phakic eyes also underwent PPV, phacoemulsification and aspiration (PPV triple + cystotomy group). Six of the nine patients with pseudophakic eyes underwent combined cystotomy with PPV only (PPV + cystotomy group), and the remaining three patients, who had avitreous eyes, did not undergo any additional procedures (cystotomy only group).
At the 6-month postoperative follow-up, six patients in the PPV triple + cystotomy group needed additional treatment, including intravitreal or sub-Tenon’s capsule injection of corticosteroids. Two patients in the PPV + cystotomy group required additional treatment. No intra- or postoperative complications such as macular hole or retinal tear were observed in any of the 20 patients.
The preoperative mean CRT of 461±75 μm, which was significantly decreased to 385±42 μm (P<0.01) 1 month after surgery and to 391±84 μm (P<0.01) 6 months after surgery. There were not significant changes between results at 1 month after surgery and 6 months after surgery (Figure 2). The preoperative CMMT of 392±77 μm also significantly decreased to 265±80 μm (P<0.01) 1 month after surgery and to 268±91 μm (P<0.01) 6 months after surgery. There were not significant changes between results at 1 month after surgery and 6 months after surgery (Figure 3). These significant reductions between preoperative and 6 months after surgery in CRT and CMMV were only seen in the PPV + cystotomy group (P<0.05). The preoperative MV of 10.8±1.4 mm3 was significantly reduced to 10.3±1.0 mm3 (P<0.01) 1 month after surgery and to 9.7±1.0 mm3 (P<0.01) 6 months after surgery. Significant reductions were also seen between results at the 1-month and 6-month follow-ups (P<0.01; Figure 4). There was not a significant improvement between the preoperative BCVA (0.43±0.31) or 1-month postoperative BCVA (0.30±0.25). However, there was a significant improvement between the preoperative and 6-month postoperative (0.25±0.23) BCVA measurements (P<0.01). This improvement was measured in 13 eyes (65%) only. There were no changes in five eyes (25%), and two eyes (10%) presented deterioration (Figure 5).
Discussion
This study shows that cystotomy is an option for the resolution of refractory CME. Although this maneuver is delicate and can potentially cause macular holes or retinal tears, we were able to perform this technique safely under the guidance of iOCT without any serious intraocular complications. The fact that there are no neuronal components on the vitreous side of the macular cysts17 is also an advantage for this technique.
One of the causes of DME is vascular leakage to the retina. This is due to increased vascular permeability, which is caused when the blood–retinal barrier breaks down, and increased numbers of inflammatory cytokines. When dense fluid composed of deposits such as lipoproteins remains in the fovea, the osmotic gradient presumably becomes higher. This hampers fluid drainage by the retinal pigment epithelium and consequently worsens the macular edema. The postoperative photoreceptor status of the fovea and the integrity of the external limiting membrane and the ellipsoid zone on optical coherence tomography (OCT) are positively correlated with visual acuity in eyes with resolved DME after vitrectomy.20–22 Since chronic DME causes permanent photoreceptor dysfunction and disrupts the integrity of the external limiting membrane and the ellipsoid zone, early resolution of DME is crucial. In the clinical real-life, there are some cases of DME in which resolution cannot be achieved with repeated conventional treatment.
In this study, we chose cases that were resistant to conventional treatments such as photocoagulation, corticosteroids and anti-VEGF injection. We managed to resolve macular edema and improve visual acuity, which suggests that our novel technique improves CME pathology by a different mechanism to that used by conventional medical treatments. Possible explanations for the effectiveness of our treatment include injecting BSS into the cystoid space to wash out deposits and cytokines in the fovea, thereby reducing the oncotic pressure and facilitating drainage from the retina to the choroid. Our intraoperative findings also supported this explanation: dense liquids were found floating from the intraretinal space while flushing the cystoid space with BSS. We also noticed a capsule in some cases, which presumably contained dense fluid composed of inflammatory deposits (Figure 1C).
It is also notable that, with the exception of two eyes (patients 11 and 14; Table 1), the beneficial effects on macular edema continued for 6 months with only a few additional treatments required. In some cases, a dramatic improvement in visual acuity and macular edema was achieved, which was still observed 6 months after surgery (Figure 6). This long-term effect could be a benefit of the direct cleaning of dense fluid and cytokines. This not only reduces leakage from the retinal vessels but also allows time for the retina to reattach with the choroid, thereby enabling the choroid to resume supplying nutrients and oxygen to the retina.
A recent meta-analysis showed that vitrectomy produces structural and functional improvements in selected eyes with DME, but the visual gains were not significantly better than those achieved with laser or in an observation group.7 In this era where several medical treatments are available, including anti-VEGF agents, it is clear that surgery would not be the first choice of treatment. However, there are always some cases that are resistant to these conventional treatments. Favorable results have been obtained using additional surgical approaches for DME other than vitrectomy. Avci et al23 excised plaque-like foveal hard exudates from 11 patients with chronic DME and reported a significant long-term improvement in BCVA (logMAR) at the 3-year follow-up. Morizane et al treated diffuse DME in 20 patients using a planned foveal detachment technique. This involved injecting 50–100 μL BSS into the subretinal space using a 38-gage needle. Significant improvements in CRT and BCVA were seen after 6 months in 13 eyes (65%). Six (30%) eyes remained unchanged, and one (5%) eye worsened.24 Similarly, we found a significant decrease in CRT 6 months after surgery. Improvements in BCVA were also seen 6 months after surgery in 13 (65%) eyes. Five (25%) eyes remained unchanged, and two (5%) eyes worsened. Our results are similar to those of prior studies, indicating that the technique that we performed would also be an option for refractory CME.
Limitations of our study include its retrospective nature; therefore, the details of the surgical procedures, follow-up protocols and treatment histories before surgery were not standardized. Moreover, the number of eyes in the cystotomy only group was small compared to those in the other two groups that underwent combined procedures. Further large-scale prospective controlled studies are warranted to validate our findings.
Author contributions
Yuichi Asahina, NT, Yumi Asahina, KY, YU, YH prepared the material. Yuichi Asahina and NT wrote the main manuscript text and prepared figures. Yuichi Asahina, NT, Yumi Asahina, KY, YU and YH reviewed the manuscript. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91(12):1464–1474. | ||
Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119(8):1658–1665. | ||
Photocoagulation for Diabetic Macular Edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–1806. | ||
Haller JA, Kuppermann BD, Blumenkranz MS, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Arch Ophthalmol. 2010;128(3):289–296. | ||
Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113(9):1533–1538. | ||
Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193–1203. | ||
Jackson TL, Nicod E, Angelis A, Grimaccia F, Pringle E, Kanavos P. Pars plana vitrectomy for diabetic macular edema: a systematic review, meta-analysis, and synthesis of safety literature. Retina. 2017;37(5):886–895. | ||
Jackson TL, Nicod E, Angelis A, et al. Vitreous attachment in age-related macular degeneration, diabetic macular edema, and retinal vein occlusion: a systematic review and meta-analysis. Retina. 2013;33(6):1099–1108. | ||
Christoforidis JB, D’Amico DJ. Surgical and other treatments of diabetic macular edema: an update. Int Ophthalmol Clin. 2004;44(1):139–160. | ||
Wilson CA, Benner JD, Berkowitz BA, Chapman CB, Peshock RM. Transcorneal oxygenation of the preretinal vitreous. Arch Ophthalmol. 1994;112(6):839–845. | ||
Stefánsson E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthalmol Scand. 2001;79:435–440. | ||
Stefánsson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51(4):364–380. | ||
Kadonosono K, Itoh N, Ohno S. Perifoveal microcirculation before and after vitrectomy for diabetic cystoid macular edema. Am J Ophthalmol. 2000;130:740–744. | ||
Shamsi HN, Masaud JS, Ghazi NG. Diabetic macular edema: new promising therapies. World J Diabetes. 2013;4(6):324–338. | ||
Lee B, Litt M, Buchsbaum G. Rheology of the vitreous body. Part I: viscoelasticity of human vitreous. Biorheology. 1992;29(5–6):521–533. | ||
Stefánsson E. Physiology of vitreous surgery. Graefes Arch Clin Exp Ophthalmol. 2009;247(2):147–163. | ||
Tachi N, Hashimoto Y, Ogino N. Cystotomy for diabetic cystoid macular edema. Doc Ophthalmol. 1999;97(3–4):459–463. | ||
Ehlers JP, Dupps WJ, Kaiser PK, et al. The prospective intraoperative and perioperative ophthalmic ImagiNg with optical CoherEncE TomogRaphy (PIONEER) study: 2-year results. Am J Ophthalmol. 2014;158(5):999–1007. | ||
Ehlers JP, Goshe J, Dupps WJ, et al. Determination of feasibility and utility of microscope-integrated optical coherence tomography during ophthalmic surgery: the DISCOVER Study RESCAN Results. JAMA Ophthalmol. 2015;133(10):1124–1132. | ||
Harbour JW, Smiddy WE, Flynn HW Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121(4):405–413. | ||
Sakamoto A, Nishijima K, Kita M, Oh H, Tsujikawa A, Yoshimura N. Association between foveal photoreceptor status and visual acuity after resolution of diabetic macular edema by pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1325–1330. | ||
Yanyali A, Bozkurt KT, Macin A, Horozoglu F, Nohutcu AF. Quantitative assessment of photoreceptor layer in eyes with resolved edema after pars plana vitrectomy with internal limiting membrane removal for diabetic macular edema. Ophthalmologica. 2011;226(2):57–63. | ||
Avci R, Inan UU, Kaderli B. Long-term results of excision of plaque-like foveal hard exudates in patients with chronic diabetic macular oedema. Eye (Lond). 2008;22(9):1099–1104. | ||
Morizane Y, Kimura S, Hosokawa M, et al. Planned foveal detachment technique for the resolution of diffuse diabetic macular edema. Jpn J Ophthalmol. 2015;59(5):279–287. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.