Back to Journals » International Medical Case Reports Journal » Volume 16
Sirolimus Induced Toxic Optic Neuropathy
Authors Pakravan P, Miri S, Lam BL
Received 23 September 2022
Accepted for publication 25 May 2023
Published 31 May 2023 Volume 2023:16 Pages 329—332
DOI https://doi.org/10.2147/IMCRJ.S388481
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Parastou Pakravan, Shahnaz Miri, Byron L Lam
Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, Miami, FL, USA
Correspondence: Byron L Lam, Bascom Palmer Eye Institute, 900 NW 17th St, Miami, FL, 33136, USA, Email [email protected]
Objective: To describe a case of optic neuropathy after prolonged sirolimus therapy in the setting of cardiac transplant.
Background: Sirolimus is an immunosuppressant that inhibits Mechanistic Target of Rapamycin (mTOR) and blocks T-cell activation and B-cell differentiation by preventing response to Interleukin-2 (IL-2). Tacrolimus is another immunosuppressive agent, one of the known but uncommon side effects of which is bilateral optic neuropathy years after taking the medication. To the best of our knowledge, this is the first report of sequential optic neuropathy after years of treatment with sirolimus.
Case Presentation: A 69-year-old male with a history of cardiac transplantation presented with progressive, sequential, and painless vision loss. Visual acuity was 20/150 OD and 20/80 OS, with impaired color vision in both eyes (Ishihara 0/10) and bilateral disc pallor and mild optic disc edema in the left eye. Visual field was constricted in both eyes. The patient was on prolonged sirolimus therapy for over 7 years. Orbital MRI revealed bilateral chiasmatic thickness and FLAIR hyperintensity, without optic nerve enhancement post gadolinium. After extensive work up, other etiologies such as infectious, inflammatory, and neoplastic lesions were ruled out. Subsequently, sirolimus was substituted with cyclosporin that led to gradual improvement of vision and visual fields bilaterally.
Conclusion: Optic neuropathy is a rare side effect of tacrolimus, which has been seen as sudden, painless, and bilateral vision loss in post-transplant patients. Other concurrent medications influencing the cytochrome P4503A enzyme complexes may alter the pharmacokinetics of tacrolimus and increase the likelihood of toxicity. Discontinuation of offending agent has been shown to improve visual defects. We presented a rare case of optic neuropathy in a patient on sirolimus, whose visual defects improved upon discontinuation of sirolimus and switching to cyclosporin.
Keywords: sirolimus, optic neuropathy, vision loss, immunosuppressant
Introduction
Organ transplantation is a life-saving procedure for patients with end-stage organ failure. Immunosuppressants are necessary to prevent graft rejection, which can cause various side effects, including ocular complications. Tacrolimus-induced optic neuropathy has been reported as a rare, but serious side effect of tacrolimus, which is a commonly used immunosuppressive drug.1 However, there is little known about sirolimus-induced optic neuropathy, which is another immunosuppressive drug that is increasingly being used in transplantation medicine.2 Therefore, there is a need for more research on the ocular safety and toxicity of sirolimus, especially regarding optic neuropathy, which can lead to permanent vision loss.
We present a case of sirolimus-induced optic neuropathy, which highlights the need for close monitoring of patients on sirolimus therapy for ocular complications, especially those with a preexisting risk for optic neuropathy.
Case Presentation
A 69-year-old man was evaluated for gradually progressive and sequential painless loss of vision. He first experienced painless “fogginess” in his right eye, followed by vision loss in his left eye after a few weeks. His past ocular history was non-contributory except for mild cataracts. Medical history revealed sarcoidosis (pulmonary biopsy proven, inactive since 2011), hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, coronary artery bypass grafting in 2007, and subsequently, a heart transplant in 2012, which resulted in prolonged oral sirolimus 2 mg daily for 93 months as well as mycophenolate 2000 mg and dapsone 100 mg daily.
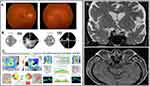
Best-corrected visual acuity (BCVA) was 20/150 OD and 20/80 OS, with impaired color vision in each eye (Ishihara 0/10) and no relative afferent pupillary defect. The anterior segment, ocular motility, and intraocular pressures were normal. Fundus exam showed sector optic disc edema OD and diffuse optic disc edema OS (Figure 1A). Humphrey visual field revealed advanced constriction and depression in both eyes (Figure 1B). Optical coherence tomography (OCT) revealed increased average retinal nerve fiber layer (RNFL) thickness of 104 μm OD and 164 μm OS and decreased average ganglion cell complex (GCC) thickness of 66 μm OD and 60 um OS (Figure 1C). Brain and orbit magnetic resonance imaging (MRI) with contrast revealed T2 hyperintensity and possibly increased thickness of the optic nerves near the chiasm; no post-contrast enhancement in optic nerves or chiasm was observed (Figure 1D). These findings have been previously seen in toxic optic neuropathies rather than ischemic optic neuropathies.2 Laboratory workup was all negative and included Lyme, Toxoplasma, QuantiFERON, Syphilis, COVID-19, Herpes simplex virus, Herpes zoster virus, Varicella-zoster virus, Epstein–Barr virus, Bartonella, Cytomegalovirus, liver function tests, basic metabolic panel, thyroid panel, vitamin B1, B12, folic acid, rheumatoid factors (RF), antinuclear antibodies (ANA), antineutrophil cytoplasmic antibodies (ANCA), and angiotensin converting enzyme (ACE). Spinal tap showed a normal opening pressure of 16.5 cmH2O, normal cerebrospinal fluid analysis, negative culture, and cytology. Given the MRI pattern and exclusion of other etiologies, a toxic etiology was considered. He was on multiple immunosuppressive agents including sirolimus for 8 years, and blood sirolimus levels were always within normal limits. After cardiology consultation, sirolimus was replaced with cyclosporin 50mg, and prednisone 60mg daily initiated during the workup was tapered down to a maintenance dose of 5 mg daily. Over 19 weeks, BCVA gradually improved to 20/70 OD and 20/30 OS, the visual field improved as well (Figure 2A), and the optic disc edema resolved. Repeat OCT after 17 weeks showed an average RNFL thickness of 68 microns OD and 80 microns OS (Figure 2B). Given the improvement in visual acuity and resolution of optic disc edema (although may be non-specific for toxic optic neuropathy), with the exclusion of other optic neuropathy etiologies with comprehensive evaluation, the clinical course is consistent with sirolimus-induced toxic optic neuropathy.
Discussion
Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor that affects cell cycle progression and is used as a potent immunosuppressive agent in solid organ transplant recipients.2 Sirolimus is a substrate for the CYP3A enzyme; therefore, there may be interactions between sirolimus with other drugs that induce or inhibit this enzyme.2 These include non-dihydropyridine calcium channel antagonists, azole antifungals, clarithromycin, and erythromycin, which will increase sirolimus concentrations and should be used only with careful drug monitoring.2 The most common reported side effects of sirolimus are myelosuppression and hyperlipidemia.3
The pathophysiology of toxic optic neuropathy differs among involved toxins, and the involvement may be in the retina, optic nerve, chiasm, or even the optic tracts.4 Patients may have variable presentations ranging from slightly subnormal visual acuity to significant vision loss.4 Most patients report progressive, painless, and bilateral symptoms, where they can initially experience unilateral vision loss but develop bilateral symptoms within days to weeks.4 Relative afferent pupillary defect is usually not present with bilateral involvement.4 Depending on the timing of the examination, optic nerve appearance can vary from normal, edema, or pallor.4 Papillomacular retinal ganglion cell bundle damage, central or cecocentral scotoma, and reduction of color vision are other features of toxic optic neuropathy.4 Although most cases of toxic neuropathy develop symmetric and bilateral symptoms, there have been reports of unilateral tacrolimus-induced optic neuropathy without progression to bilateral symptoms.5 Similarly, in this case, there is some degree of asymmetry in visual acuity, visual field defect, and fundoscopic appearance, which has also been reported in tacrolimus-induced optic neuropathies. Therefore, although rare, it is important to consider sirolimus- or tacrolimus-induced optic neuropathy in patients with unilateral or asymmetric symptoms. Additionally, toxic optic neuropathy is a diagnosis of exclusion and necessary diagnostic procedures are determined on an individual basis.4
The main treatment is prompt discontinuation of the suspected toxic medication if possible.3 Prognosis depends on the dosage and duration of exposure to the offending agent.5 Typically, the vision may improve over several days or weeks after discontinuation of toxin exposure.5 Close follow-up of the patient’s visual acuity, color vision, visual field, pupil reaction, and optic disc examination, and OCT imaging is helpful.3
Conclusion
In summary, we presented a case of optic neuropathy in a patient on sirolimus, whose visual function improved with discontinuation and switching to cyclosporin. The course of disease in this patient was very similar to reported cases of tacrolimus optic neuropathy. To our knowledge, this is the first reported case of sirolimus-induced optic neuropathy in English literature. Awareness of this serious adverse effect underscores the importance of timely assessment of patients with visual loss while on sirolimus even if taken for a long period of time with consideration given to prompt replacement medication if possible. Clinicians should be aware of this potential side effect and carefully monitor patients on sirolimus therapy for visual disturbances. OCT can be a useful tool in diagnosing and monitoring cases with sirolimus-induced visual disturbances.
Acknowledgment
This case was presented at 146th annual meeting of American Neurological Association for which the abstract has been published.
Ethics and Consent
A consent form was obtained from the patient to publish this case report.
This case report is exempt from University of Miami IRB approval since a single case report with no personal identifiers neither requires consent or IRB approval based on University of Miami protocol.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rasool N, Boudreault K, Lessell S, Prasad S, Cestari DM. Tacrolimus optic neuropathy. J Neuroophthalmol. 2018;38(2):160–166. doi:10.1097/WNO.0000000000000635
2. Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67(3):369–391. doi:10.2165/00003495-200767030-00004
3. Nguyen LS, Vautier M, Allenbach Y, et al. Sirolimus and mTOR inhibitors: a review of side effects and specific management in solid organ transplantation. Drug Saf. 2019;42:813–825. doi:10.1007/s40264-019-00810-9
4. Grzybowski A, Zülsdorff M, Wilhelm H, Tonagel F. Toxic optic neuropathies: an updated review. Acta Ophthalmol. 2015;93(5):402–410. doi:10.1111/aos.12515
5. Ascaso FJ, Mateo J, Huerva V, Cristóbal JA. Unilateral tacrolimus-associated optic neuropathy after liver transplantation. Cutan Ocul Toxicol. 2012;31(2):167–170. doi:10.3109/15569527.2011.629325
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.