Back to Journals » Local and Regional Anesthesia » Volume 9
Single-shot lamina thoracic paravertebral block with ketofol for modified radical mastectomy
Authors Rukewe A , Afuwape OO, Ugheoke A, Fatiregun AA
Received 30 June 2016
Accepted for publication 12 September 2016
Published 6 October 2016 Volume 2016:9 Pages 83—86
DOI https://doi.org/10.2147/LRA.S116102
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Stefan Wirz
Ambrose Rukewe,1 Oludolapo O Afuwape,2 Austin Ugheoke,3 Akinola A Fatiregun4
1Department of Anaesthesia & Critical Care, Faculty of Medicine, University of Botswana, Gaborone, Botswana; 2Department of Surgery, College of Medicine, 3Department of Anaesthesia, University College Hospital, Ibadan, 4World Health Organisation, Akure, Ondo State, Nigeria
Abstract: We describe the use of single-shot lamina thoracic paravertebral block (TPVB) with sedation for a 56-year-old female patient who had modified radical mastectomy with axillary clearance. Two years ago, she suffered vocal cord palsy post thyroidectomy, which was managed with tracheostomy. The tracheostomy tube was removed 8 months later, leaving the patient with persistent hoarseness of voice and left vocal cord palsy. She declined general anesthesia and consented for TPVB. The surgery lasted 95 minutes and was successfully completed with TPVB. Her vital signs were stable during the operation. She had low pain scores, minimal opioid use, early alimentation, and no postoperative nausea and vomiting and was discharged early. We present the anesthetic management of this case in our setting, where TPVB under ultrasound guidance and modern drug-delivery systems for sedation are unavailable.
Keywords: anesthesia, breast surgery, lamina, paravertebral, low resource
Introduction
Thoracic paravertebral block (TPVB) remains underutilized for breast cancer surgery in many low-resource centers despite its benefits such as prolonged analgesia, early ambulation and discharge, reduction in postoperative nausea and vomiting (PONV), and reduced cost of treatment. Its low level of acceptance may be due to safety concerns, as the potential for inadvertent vascular puncture, pneumothorax, and block failure exists.1–3 This risk is even greater with multiple thoracic-level injections.1,4,5 Very often, ultrasound, which allows real-time visualization during block placement with improved safety and success, is unavailable. In effect, general anesthesia (GA) remains the predominant anesthetic option for mastectomy. This is accompanied with the challenge of erratic supply of pain-relieving drugs in many sub-Saharan hospitals.6 Sometimes, GA may be contraindicated or undesirable, making the case for regional techniques in our practice environment.4 Our index patient had vocal cord palsy following thyroidectomy, which was managed with tracheostomy for 8 months, 2 years before presenting for modified radical mastectomy. The patient declined GA but consented to TPVB with sedation as an alternative. The surgeon communicated the patient’s request via telephone since this anesthetist now based in Botswana was scheduled to visit the center as faculty for a Regional Anesthesia Workshop and the procedure was scheduled at the time of the Workshop. Our experience with single-shot lamina TPVB with ketofol is presented here for breast cancer surgery, in a setting where echo-guided approach and sophisticated drug-delivery systems are unavailable. The patient gave written informed consent for this publication.
Case report
A 56-year-old female patient, who weighed 66 kg, was 158 cm tall and had American Society of Anesthesiologists’ physical status I, was diagnosed with advanced right breast carcinoma. She presented 4 months prior to the surgery with a history of right breast lump; incisional biopsy revealed invasive ductal carcinoma. She was then commenced on neoadjuvant chemotherapy. Two years prior to this, she had total thyroidectomy under GA for simple multinodular goiter complicated with stridor, hypoxia, and hoarseness of voice, secondary to vocal cord palsy, which was managed with tracheostomy. The tracheostomy tube was removed 8 months later, leaving the patient with a persistent hoarseness of voice and defective left vocal cord. She was worked up for surgery after completion of neoadjuvant chemotherapy. Her serum biochemistry, hematology, electrocardiogram (ECG), and chest X-ray results were normal. She was instructed on the visual analog scale for pain intensity, where 0 meant no pain and 10 meant the worst pain imaginable. The anesthetic plan was explained to and discussed with the patient.
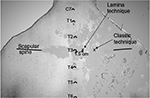
In the theater, intravenous (IV) access was established with 18 G cannula on the left hand, and 500 mL normal saline solution was set up. Using the Classic-120 Multiparameter® monitor (Health-Care Equipment and Supplies Co. Ltd, Surrey, UK), noninvasive blood pressure (BP), pulse oximetry, and continuous ECG were monitored. Her baseline pulse rate was 83 beats/min (bpm), BP was 146/86 mmHg, and arterial oxygen saturation (SaO2) was 98%, breathing room air. Verbal contact was maintained throughout the block placement and surgery. With the patient sitting up on the operating table, a right-sided TPVB using Jüttner et al’s1 modification of Pfeiffer et al’s7 lamina technique was performed. The C7–T6 vertebral spinous processes were palpated and marked. Her T3 spinous process was identified using the scapular spine as a landmark, and 1.5 cm from the midline on the right side was marked as the needle entry point (Figure 1). Povidone iodine (10%) solution was used for skin preparation, followed by infiltration of the injection site using 2 mL 1% lidocaine with 1:200,000 epinephrine. An 18 G Tuohy needle (Portex; Smiths Medical, Ashford, UK) was inserted and advanced in a paramedian sagittal plane at 45° to the skin in the cranial direction until contact was made with the vertebral lamina at a depth of 4 cm. With a negative test aspiration of air, blood, and cerebrospinal fluid, 6 mL 1% lidocaine with 1:200,000 epinephrine was injected to facilitate insertion of the 20 G epidural catheter (Portex) 3.0 cm beyond the needle tip into the paravertebral space. The catheter was secured, and the patient was placed supine on the operating table. Test dose comprising 5 mL 1% lidocaine with 1:200,000 epinephrine was administered via the catheter while monitoring the patient’s pulse rate, BP, respiration, and consciousness to exclude pneumothorax, epidural, or subarachnoid injection. Thereafter, 7 mL 1% lidocaine with 1:200,000 epinephrine and 20 mL 0.5% bupivacaine with 1:200,000 epinephrine were injected over 10 minutes. The block was completed within 28 minutes (catheter was removed after the local anesthetic [LA] injection) and was deemed adequate in 16 minutes by loss-of-cold and pinprick sensation (by the surgeon) over the operative area. The patient was observed to be shivering, but she was reassured and received IV fentanyl 50 μg and midazolam 2 mg. Then a mixture of propofol 150 mg and ketamine 100 mg (ketofol) in 500 mL normal saline solution was infused at 2–4 mL/min to achieve a Ramsey sedation score of 3–4. Supplemental oxygen at 4 L/min via nasal prongs was commenced. The surgical incision was elliptical, which included the nipple–areolar complex and the underlying 14 cm ×16 cm breast mass. The incision at the sternal border elicited pain, for which 10 mL 0.5% lidocaine with 1:200,000 epinephrine was infiltrated by the surgeon. Superior and inferior skin flaps were raised to the level of the clavicle and the lower coastal margins, respectively. The breast tissue was dissected off and the axilla was accessed through the same incision. The skin was closed over a drain. The duration of surgery was 95 minutes. A total of 245 mL ketofol was employed for conscious sedation. The blood loss was 150 mL; intraoperative fluid administered was 500 mL normal saline and 500 mL Gelofusine. The patient’s vital signs throughout surgery were as follows: pulse rate 60–82 bpm, BP 90/50–130/80 mmHg, and SaO2 100% at 4 L/min oxygen via nasal prongs.
At the end of surgery, the patient was transferred awake to the recovery room, and IV paracetamol 600 mg was administered. She was commenced on oral feeding 8 hours after surgery with good effect. On the ward in the first 24 hours, she had IV paracetamol 1 g, IV pentazocine 30 mg, and oral Celebrex 200 mg; her pain score was 2–3. In the next 24 hours, the pain score remained low; analgesics via oral route comprised Celebrex 400 mg and paracetamol 3 g in divided doses. She was discharged home on the sixth day. Both the surgeon and patient described the anesthesia and pain control as satisfactory.
Discussion
We successfully employed single-shot lamina TPVB as an adjunct to GA,8 and this case report demonstrates that, combined with sedation, it can provide satisfactory operating conditions for patients who require a modified radical mastectomy. To the best of our knowledge, this is the first time TPVB was used as the sole anesthetic for mastectomy in our practice environment. Many authors have posited that regional anesthesia gives good intraoperative conditions and prolonged postoperative analgesia.2,4,5,9–11 Much of the literature agrees that regional anesthesia should be a key component of the surgical management of malignant breast disease because of superior perioperative pain relief despite reduced requirement of opioids, early ambulation, decreased incidence of chronic pain syndromes, and favorable immunomodulatory effects, and hence reduced tumor recurrence.12–16
This patient’s anxiety about anesthesia was understandable; the vocal cord paralysis she suffered could have resulted from thyroidectomy and/or GA. Other relative contraindications to GA are comorbidities, cardiac or respiratory compromise, and advancing age.4,5,10,17 The patient’s motivation for the block was remarkable, as there was no need for sedation prior to instituting TPVB, so she remained clear-headed, cooperative, and communicative. The good working relationship that existed between the surgeon and anesthetist allowed setting up the operation during the fifth University College Hospital, Ibadan, Regional Anesthesia Course when this anesthetist with experience in lamina TPVB was visiting that center as faculty.
Our ketofol (ketamine 100 mg and propofol 150 mg) combination in 500 mL normal saline solution infused to achieve Ramsay sedation score 3–4 should be readily available in hospitals that cannot provide modern drug-delivery systems. Over the course of the surgery, which lasted 95 minutes, the total ketamine and propofol administered were 49 and 73.5 mg, respectively. With this combination, communication was maintained as much as possible, and the patient’s vital signs were stable. There was no apnea, no emergence delirium, and no PONV. Low-dose ketamine employed in our ketofol combination is known to provide analgesia via inhibition of the N-methyl-d-aspartate receptor, interaction with the opioid, monoamine, and cholinergic receptor systems, as well as the anti-inflammatory effects via reduced cytokine production.18,19
Lack of expertise and safety concern in needling and local anesthetic dosing generally discourage the practice of nerve-blocking techniques in many hospitals in Nigeria.4,20 The lamina approach with a needle entry point at 1.5 cm lateral to the T3 spinous process using contact with the vertebra lamina as an end-point of insertion was developed by Pfeiffer et al.7 Our technique was described as a safer, highly effective, and easy-to-adopt modification compared to the classic landmark approach.1 The tunneling of the catheter into the paravertebral space engendered sufficient craniocaudal spread of LA, and our initial injection of 1% lidocaine with 1:200,000 epinephrine improved the onset time for surgery to begin 16 minutes after block completion, which is comparable with the incision time by Nikam et al.11 The appeal for lamina TPVB in settings such as ours is that a single injection of high LA volume with epinephrine to delay systemic absorption can reliably produce effective anesthesia and analgesia for mastectomies with axillary dissection.1,11 LA infiltration of the medial part of the breast occasioned by pain sensation during incision might be due to the insufficient time allowed for the block to take. Contralateral innervation of the breast has been identified as a possible reason for LA top-ups.5,10
Shivering was the only side effect observed in our patient, and her discharge from the hospital after 6 days can be adjudged early given that patients’ average hospital stay following mastectomies was 10 days from a previous study.21 The quality of anesthesia and analgesia was evident in low pain scores, minimal opioid use, and early alimentation.
Conclusion
Single-shot lamina TPVB with sedation was successfully used for a breast cancer surgery in a low-resource setting.
Disclosure
The authors report no conflicts of interest in this work.
References
Jüttner T, Werdehausen R, Hermanns H, et al. The paravertebral lamina technique: a new regional anesthesia approach for breast surgery. J Clin Anesth. 2011;23(6):443–450. | ||
Schnabel A, Reichl SU, Kranke P, Pogatzki-Zahn EM, Zahn PK. Efficacy and safety of paravertebral blocks in breast surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2010;105(6):842–852. | ||
Naja Z, Lönnqvist PA. Somatic paravertebral nerve blockade. Incidence of failed block and complications. Anaesthesia. 2001;56(12):1184–1188. | ||
Kolawole IK, Adesina MD, Olaoye IO. Intercostal nerves block for mastectomy in two patients with advanced breast malignancy. J Natl Med Assoc. 2006;98(3):450-453. | ||
Simpson J, Ariyarathenam A, Dunn J, Ford P. Breast surgery using thoracic paravertebral blockade and sedation alone. Anesthesiol Res Pract. 2014;2014:127467. | ||
Harding R, Powell RA, Kiyange F, Downing J, Mwangi-Powell F. Provision of pain- and symptom-relieving drugs for HIV/AIDS in sub-Saharan Africa. J Pain Symptom Manage. 2010;40(3):405–415. | ||
Pfeiffer G, Oppitz N, Schöne S, Richter-Heine I, Höhne M, Koltermann C. Analgesie der Achselhöhle durch Paravertebralkatheter in Laminatechnik [Analgesia of the axilla using a paravertebral catheter in the lamina technique]. Anaesthesist. 2006;55(4):423–427. German. | ||
Rukewe A, Fatiregun A, Ademola AF, Ugheoke A. Single-shot lamina technique of paravertebral block as an adjunct to general anesthesia for modified radical mastectomy – a case report. Niger J Clin Pract. 2015;18(3):429–431. | ||
Klein SM, Bergh A, Steele SM, Georgiade GS, Greengrass RA. Thoracic paravertebral block for breast surgery. Anesth Analg. 2000;90(6):1402–1405. | ||
Terheggen MA, Wille F, Borel Rinkes IH, Ionescu TI, Knape JT. Paravertebral blockade for minor breast surgery. Anesth Analg. 2002;94(2):355–359. | ||
Nikam S, Marghade P, Paliwal N, Lawhale S. Thoracic paravertebral block for breast surgery in a patient with ischemic heart disease. Anaesth Pain Intensive Care. 2014;18(3):280–281. | ||
Kairaluoma PM, Bachmann MS, Rosenberg PH, Pere PJ. Preincisional paravertebral block reduces the prevalence of chronic pain after breast surgery. Anesth Analg. 2006;103(3):703–708. | ||
Vila H Jr, Liu J, Kavasmaneck D. Paravertebral block: new benefits from an old procedure. Curr Opin Anaesthesiol. 2007;20(4):316–318. | ||
Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105(4):660–664. | ||
Sessler DI, Ben-Eliyahu S, Mascha EJ, Parat MO, Buggy DJ. Can regional analgesia reduce the risk of recurrence after breast cancer? Methodology of a multicenter randomized trial. Contemp Clin Trials. 2008;29(4):517–526. | ||
Green JS, Tsui BC. Impact of anesthesia for cancer surgery: continuing professional development. Can J Anaesth. 2013;60(12):1243–1269. | ||
Kozanhan B, Basaran B, Kutlucan L, Ozmen S. Paravertebral block combined with sedation for a myasthenic patient undergoing breast augmentation. Case Rep Anesthesiol. 2015;2015:593282. | ||
Hirota K, Lambert DG. Ketamine: new uses for an old drug? Br J Anaesth. 2011;107(2):123–126. | ||
De Kock M, Loix S, Lavand’homme P. Ketamine and peripheral inflammation. CNS Neurosci Ther. 2013;19(6):403–410. | ||
Obasuyi BI, Alagbe-Briggs OT, Echem RC. Choice of anaesthesia for orthopaedic surgeries in a developing country: how appropriate? J Med Med Sci. 2013;4(3):101–106. | ||
Ogundiran TO, Ayandipo OO, Ademola AF, Adebamowo CA. Mastectomy for management of breast cancer in Ibadan, Nigeria. BMC Surg. 2013;13:59. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.