Back to Journals » Journal of Pain Research » Volume 8
Single- and multiple-dose pharmacokinetics of biphasic immediate-release/extended-release hydrocodone bitartrate/acetaminophen (MNK-155) compared with immediate-release hydrocodone bitartrate/ibuprofen and immediate-release tramadol HCl/acetaminophen
Authors Devarakonda K, Kostenbader K, Giuliani MJ, Young J
Received 24 February 2015
Accepted for publication 20 April 2015
Published 30 September 2015 Volume 2015:8 Pages 647—656
DOI https://doi.org/10.2147/JPR.S83416
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Robinson
Krishna Devarakonda,1 Kenneth Kostenbader,2 Michael J Giuliani,3 Jim L Young4
1Department of Clinical Pharmacology, Mallinckrodt Pharmaceuticals, 2Mallinckrodt Pharmaceuticals, 3Research and Development, Mallinckrodt Pharmaceuticals, 4Department of Clinical Affairs and Program Management, Mallinckrodt Pharmaceuticals, Hazelwood, MO, USA
Objective: To characterize the single-dose and steady-state pharmacokinetics (PK) of biphasic immediate-release/extended-release hydrocodone bitartrate/acetaminophen (IR/ER HB/APAP), IR HB/ibuprofen, and IR tramadol HCl/APAP.
Methods: In this single-center, open-label, randomized, four-period crossover study, healthy participants received four treatments under fasted conditions: 1) a single dose of two IR/ER HB/APAP 7.5/325 mg tablets (15/650 mg total dose) on day 1, followed by two tablets every 12 hours (q12h) beginning on day 3; 2) a single dose of IR HB/ibuprofen 15/400 mg (divided as one 7.5/200 mg tablet at hour 0 and 6), followed by one tablet every 6 hours (q6h) beginning on day 3; 3) a single dose of IR tramadol HCl/APAP 75/650 mg (divided as one 37.5/325 mg tablet at hour 0 and 6), followed by one tablet q6h beginning on day 3; and 4) a single dose of three IR/ER HB/APAP 7.5/325 mg tablets (22.5/975 mg total dose) on day 1, a three-tablet initial dose at 48 hours followed by two-tablet doses q12h beginning on day 3. Hydrocodone and APAP single-dose and steady-state PK were assessed. Adverse events were monitored.
Results: The PK analysis was carried out on 29 of 48 enrolled participants who completed all treatment periods. Single-dose hydrocodone exposure was similar for IR/ER HB/APAP 22.5/975 mg and IR HB/ibuprofen 15/400 mg; time to maximum observed plasma concentration was shorter and half-life was longer for IR/ER HB/APAP (22.5/975 mg and 15/650 mg) vs IR HB/ibuprofen. Single-dose APAP exposure was similar for IR/ER HB/APAP 15/650 mg and IR tramadol HCl/APAP 75/650 mg. Steady-state hydrocodone and APAP exposures were similar between treatments. Adverse events were similar for each treatment and typical of low-dose combination opioid analgesics. With dosing q12h, IR/ER HB/APAP had half as many concentration peaks and troughs as the comparators treated q6h.
Conclusion: With dosing q12h, IR/ER HB/APAP provided similar peak and total steady-state hydrocodone and APAP exposure vs IR comparators.
Keywords: acetaminophen, extended release, hydrocodone, ibuprofen, immediate release, tramadol
Introduction
Recognizing that most pain involves multiple underlying mediators, analgesics that incorporate multiple mechanisms of action may improve effectiveness.1 Fixed-dose combinations (FDCs) of analgesics with complementary mechanisms of action can potentially provide additive analgesic effects while allowing a reduction in the total dose of each component drug, which could lower the risk of dose-related adverse events (AEs) compared with a higher-dose monotherapy with one of the constituents.2,3 Current FDC opioid analgesics indicated for acute moderate to severe pain include immediate-release (IR) hydrocodone bitartrate (HB)/acetaminophen (APAP), IR HB/ibuprofen, IR tramadol HCl/APAP, IR oxycodone (OC)/APAP, and biphasic IR/extended-release (ER) OC/APAP.4–8
Immediate-release opioid formulations are frequently prescribed for acute pain to provide rapid onset of pain relief.9 In comparison, ER opioid formulations provide stable plasma drug concentrations over a prolonged period, more sustained analgesic effects, and reduced dosing frequency.10 ER opioids have been attributed with other putative advantages, such as more consistent pain relief, improved sleep quality, and fewer AEs, but supportive evidence is lacking.11–13
A biphasic delivery system has been developed, integrating an IR component for rapid onset and an ER component for stable plasma drug concentrations over a prolonged duration of action.10 Biphasic IR/ER HB/APAP 7.5/325 mg tablets (MNK-155; Mallinckrodt Pharmaceuticals, Hazelwood, MO, USA) are designed to provide IR of 25% of the HB and 50% of the APAP for rapid onset of effect and ER of 75% of the HB and 50% of the APAP for extended analgesia over a 12-hour dosing interval.
This study examined the single-dose and steady-state pharmacokinetic (PK) profile of IR/ER HB/APAP compared with two IR FDC analgesics available for the treatment of acute pain, IR HB/ibuprofen (Vicoprofen®; Halo Pharmaceutical Inc., Whippany, NJ, USA) and IR tramadol HCl/APAP (Ultracet®; PriCare Division of Janssen Ortho Pharmaceuticals Inc., Gurabo, PR, USA), in healthy volunteers under fasted conditions. Tramadol is listed by the US Department of Justice – Drug Enforcement Administration as a schedule IV drug,14 whereas FDC products containing hydrocodone are schedule II.15 Comparative evaluation of these two products was required by the US Food and Drug Administration (FDA) because the most similar FDC formulation (ie, IR HB/APAP) has no recent clinical data for comparison. However, these IR FDC products are also of interest because of their clinical utility for acute pain. Because hydrocodone is a substrate for the hepatic enzyme cytochrome P450 (CYP) 2D6, the FDA also required that all participants were genotyped to determine CYP2D6 metabolizer status, which could help to understand any outlier PK values.
An analysis of two studies comparing the single-dose and steady-state PK characteristics of IR/ER HB/APAP with IR HB/APAP have been published elsewhere.16 One notable difference in the present study is that the steady-state PK of IR/ER HB/APAP 7.5/325 mg tablets was examined with either an initial three-tablet dose or two-tablet dose before twice-daily, two-tablet repeated dosing. The three-tablet initial dose was intended to achieve relatively higher concentrations after the first dose. This is also the planned commercial dosing regimen.17
Methods
Study design
This was a Phase I single-center, open-label, randomized, four-period crossover study conducted in the US between June 21, 2012, and August 30, 2012. As a Phase I study, it was not registered with ClinicalTrials.gov. Participants were randomized to receive four study treatments taken orally with 8 ounces of room-temperature water under fasted conditions. Participants underwent a 14-day washout before the first treatment and a ≥14-day washout before subsequent treatments per FDA recommendations that washouts between treatment periods be >5 times the half-life of the drug.18 The treatments were 1) a single dose of two IR/ER HB/APAP 7.5/325 mg tablets (15/650 mg total dose) on day 1 (hour 0), followed by two tablets every 12 hours (q12h) beginning on day 3 (hour 48) and ending on day 7 (hour 144) for a total of nine doses; 2) a single dose of IR HB/ibuprofen 15/400 mg (divided as one 7.5/200 mg tablet at hour 0 and 6), followed by one tablet every 6 hours (q6h) beginning on day 3 (hour 48) and ending on day 7 (hour 150) for a total of 18 doses; 3) a single dose of IR tramadol HCl/APAP 75/650 mg (divided as one 37.5/325 mg tablet at hour 0 and 6), followed by one tablet q6h beginning on day 3 (hour 48) and ending on day 7 (hour 150) for a total of 18 doses; and 4) a single dose of three IR/ER HB/APAP 7.5/325 mg tablets (22.5/975 mg total dose) on day 1, a three-tablet initial dose on day 3 (hour 48), followed by two-tablet doses q12h beginning on day 3 (hour 60) and ending on day 7 (hour 144) for a total of nine doses.
The study protocol was approved by IntegReview, Ethical Review Board (Austin, TX, USA), and the study was conducted in accordance with the International Conference on Harmonisation of Good Clinical Practice and the US clinical research regulations and guidelines. All participants provided informed, written consent.
Study population
Healthy men and nonpregnant women aged 18–55 years with a body mass index of 19–30 kg/m2 and a minimum weight of 59 kg (men) or 45 kg (women) were eligible for participation. Individuals with a positive urine test for substances of abuse, nicotine use in the past 6 months, prescription or over-the-counter drug use in the past 14 days, gastric bypass or gastric band surgery, conditions known to interfere with the PK of the study drugs, or allergy or intolerance to study drugs were excluded from enrollment.
Plasma sampling and assessments
Plasma samples were collected by venipuncture at regular intervals for up to 156 hours after the initial dose (single-dose phase: hours 0−48; multiple-dose phase: hours 48−156). On day 1, samples were collected predose and at 15, 30, and 45 minutes and 1, 2, 3, 4, 6, 8, 10, 12, 16, and 18 hours postdose, with additional samples collected 30 minutes after the dose administered at 6 hours for the IR HB/ibuprofen and IR tramadol HCl/APAP treatments. Samples were also obtained at 24 and 36 hours (day 2), before the morning dose at 48 and 60 hours (day 3), at 72 hours (day 4), at 96 hours (day 5), and at 120 hours (day 6). On day 7, samples were collected predose and at 15, 30, and 45 minutes after the dose administered at 144 hours and at 145, 146, 147, 148, 150, 152, 154, and 156 hours. Additional samples were collected 30 minutes after the dose was administered at 150 hours for the IR HB/ibuprofen and IR tramadol HCl/APAP treatments.
The collected blood sample tubes were placed in an ice bath/cryoblock immediately after collection. Samples were centrifuged at approximately 4°C, according to the study site’s established procedures. Centrifugation yielded approximately 2 mL of plasma. Each plasma fraction was withdrawn by a pipette and divided into equal halves into two labeled polypropylene screw-cap tubes and frozen at −70°C, or below. Hydrocodone and APAP concentrations were determined using a high-performance liquid chromatography/tandem mass spectrometry assay at PPD Bioanalytical Lab, Middleton, WI, USA. The method was developed and validated over a calibration range of 0.100–50 ng/mL for hydrocodone and 100–15,000 ng/mL for APAP, using 300 μL of plasma containing dipotassium ethylenediaminetetraacetic acid as an anticoagulant. The method used a liquid-liquid extraction procedure followed by chromatographic separation and tandem mass spectrometry detection of the analytes, using hydrocodone-d6 and APAP-d4 as the internal standards. Data were collected using the Micromass MassLynx version 4.1 (Waters Corp, Milford, MA, USA) and PPD Assist LIMS version 5 (PPD, Richmond, VA, USA). The lower limit of quantitation of the assay was 0.100 ng/mL for hydrocodone and 100 ng/mL for APAP. The assay was validated for linearity, precision, accuracy, ruggedness, recovery, and specificity. Short-term stability and long-term storage stability were also established.
The average recovery of drug was 98.0% for hydrocodone, 78.5% for APAP, and 99.5% and 74.4% for the hydrocodone and APAP internal standards, respectively. The quality control (QC) intraday precision range was 1.38%–17.3% for hydrocodone and 1.86%–11.5% for APAP, and the QC intraday accuracy range was 1.25%–17.8% for hydrocodone and −10.5% to 12.3% for APAP. The QC interday precision range was 2.34%–10.7% for hydrocodone and 3.93%–11.2% for APAP, and the QC interday accuracy range was 4.06%–16.5% for hydrocodone and −0.0845% to 2.39% for APAP.
PK parameters were calculated using standard noncompartmental methods. The PK parameters included peak (Cmax) plasma concentration, area under the concentration-time curve from 0 to infinity (AUC0−∞) and from 0 to time t (AUC0−t), time to maximum observed plasma concentration (tmax), lag time (tlag), half-life (t½), AUC from time 0–12 hours at steady state (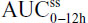 ), average steady-state plasma drug concentration during multiple-dose administration at steady state (
), average steady-state plasma drug concentration during multiple-dose administration at steady state (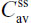 ), Cmax at steady state (
), Cmax at steady state (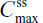 ), minimum plasma drug concentration at steady state (
), minimum plasma drug concentration at steady state (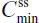 ), and tmax at steady state (
), and tmax at steady state (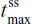 ).
).
Genotype evaluation
Genotyping was performed using the INFINITI™ CYP2D6I assay (Autogenomics, Vista, CA, USA). One of four CYP2D6 phenotypes was assigned based on CYP2D6 genetic variants: extensive metabolizer (ie, the normative metabolic state), intermediate metabolizer (ie, one active CYP2D6 allele), poor metabolizer (ie, no active alleles), or ultrarapid metabolizer. The Grubb test and likelihood distance test were used to identify PK outliers. Analyses of outliers were intended to determine whether CYP2D6 genotype explained outlier status.
Safety assessment
Adverse events were assessed throughout the study and coded using the Medical Dictionary for Regulatory Activities version 14.1. Additional safety measures were clinical laboratory tests, vital signs, pulse oximetry, physical examination, and electrocardiogram; any clinically significant changes were reported as an AE.
Statistical methods
The PK analysis set consisted of participants who completed all four treatment periods of the single- and multiple-dose components of the study. For single-dose PK, analysis of variance was performed using the SAS® version 9.1.3 mixed-effects linear model procedure (SAS Institute, Inc., Cary, NC, USA) with the natural log-transformed dose-normalized PK parameters Cmax, AUC0–t, and AUC0–∞ and untransformed t½ as dependent variables; sequence, treatment, and period as fixed effects; and participants nested within sequences as a random effect. For steady-state PK, the SAS® mixed-effects linear model procedure was used to perform analysis of variance with the natural log-transformed dose-normalized PK parameters (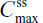 ,
, 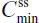 ,
, 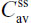 , and
, and 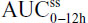 ) as the dependent variables; sequence, treatment, and period as fixed effects; and participants nested within sequences as a random effect. A 90% CI (confidence interval) of the geometric least squares (LS) mean ratio fully contained within 80%–125% was defined as no difference between treatments. The Wilcoxon signed-rank test was used to compare tmax, tlag, and
) as the dependent variables; sequence, treatment, and period as fixed effects; and participants nested within sequences as a random effect. A 90% CI (confidence interval) of the geometric least squares (LS) mean ratio fully contained within 80%–125% was defined as no difference between treatments. The Wilcoxon signed-rank test was used to compare tmax, tlag, and 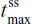 ; statistically significant differences were defined as P≤0.05.
; statistically significant differences were defined as P≤0.05.
The safety analysis set consisted of all enrolled participants who received ≥1 dose of study drug. Safety data were analyzed using descriptive statistics.
Results
Participant disposition and demographics
Forty-eight participants enrolled in the study and 29 (60.4%) completed all single- and multiple-dose treatment periods (Table 1). Of the 18 participants (37.5%) who discontinued early, 15 (31.3%) did so because of AEs, mostly vomiting (n=14). A single participant discontinued because of ectopic pregnancy. Demographic and baseline characteristics are summarized in Table 2; the mean age of participants was 30.9 years, most were white (62.5%), and there was an equal representation of men and women.
  | Table 1 Participant disposition |
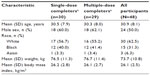  | Table 2 Demographics and baseline characteristics |
Hydrocodone pharmacokinetics
Single-dose pharmacokinetics
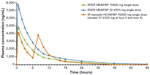
Hydrocodone plasma concentrations after single-dose administration of IR/ER HB/APAP and IR HB/ibuprofen are shown in Figure 1, and PK parameters are shown in Table 3. For both the two- and three-tablet single doses of IR/ER HB/APAP, the mean hydrocodone t½ was longer (two-tablet dose, 7.32 hours; three-tablet dose, 7.08 hours; IR HB/ibuprofen, 5.74 hours) and the mean hydrocodone tmax was shorter (two-tablet dose, 3 hours; three-tablet dose, 3 hours; IR HB/ibuprofen, 8 hours) compared with IR HB/APAP. The hydrocodone tmax for IR HB/ibuprofen occurred 2 hours after dose administration at hour 6 because a higher hydrocodone concentration followed the hour 6 dose than the hour 0 dose. Mean hydrocodone Cmax was approximately 50% greater with three vs two tablets of IR/ER HB/APAP but similar between three tablets of IR/ER HB/APAP and IR HB/ibuprofen. Although overall hydrocodone exposure (AUC0–∞) was approximately 50% greater after three vs two tablets of IR/ER HB/APAP and approximately 40% greater after three tablets of IR/ER HB/APAP vs after IR HB/ibuprofen, there were no significant differences based on the 90% CIs of the geometric LS mean ratios.
Steady-state pharmacokinetics
Steady-state hydrocodone plasma concentrations are shown in Figure 2, and steady-state hydrocodone PK parameters are shown in Table 4. Desired steady state for hydrocodone was reached by 48 hours for all treatments. Steady-state hydrocodone exposure (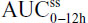 ,
, 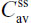 ,
, 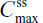 ) was similar for IR/ER HB/APAP (with a three- or two-tablet initial dose) and IR HB/ibuprofen, but the median
) was similar for IR/ER HB/APAP (with a three- or two-tablet initial dose) and IR HB/ibuprofen, but the median 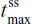 was shorter for both doses of IR/ER HB/APAP (initial three-tablet dose, 3 hours; initial two-tablet dose, 2.02 hours) compared with IR HB/APAP (8 hours; P=0.008 vs IR/ER HB/APAP, initial three-tablet dose; P=0.006 vs IR HB/APAP, initial two-tablet dose). There were twice as many hydrocodone peaks and troughs for IR HB/ibuprofen compared with IR/ER HB/APAP (Figure 2), consistent with dosing q6h vs q12h.
was shorter for both doses of IR/ER HB/APAP (initial three-tablet dose, 3 hours; initial two-tablet dose, 2.02 hours) compared with IR HB/APAP (8 hours; P=0.008 vs IR/ER HB/APAP, initial three-tablet dose; P=0.006 vs IR HB/APAP, initial two-tablet dose). There were twice as many hydrocodone peaks and troughs for IR HB/ibuprofen compared with IR/ER HB/APAP (Figure 2), consistent with dosing q6h vs q12h.
Acetaminophen pharmacokinetics
Single-dose pharmacokinetics
Acetaminophen plasma concentrations after single-dose administration of IR/ER HB/APAP and IR tramadol HCl/APAP are shown in Figure 3, and PK parameters are summarized in Table 5. The t½ was significantly longer for IR/ER HB/APAP (two tablets, 7.93 hours; three tablets, 8.26 hours) compared with IR tramadol HCl/APAP (4.37 hours), and the tmax was longer for two tablets of IR/ER HB/APAP (0.63 hours) compared with IR tramadol HCl/APAP (0.53 hours; P=0.035). Although mean APAP Cmax and overall exposure (AUC0–∞) were more than 50% greater after three tablets of IR/ER HB/APAP than after two tablets of IR/ER HB/APAP or IR tramadol HCl/APAP, only the difference in Cmax between three tablets of IR/ER HB/APAP and IR tramadol HCl/APAP was significantly different based on the 90% CIs of the geometric LS mean ratios.
Steady-state pharmacokinetics
Steady-state APAP plasma concentrations are shown in Figure 4, and steady-state PK parameters are summarized in Table 6. Steady state for APAP was achieved in 24 hours after IR/ER HB/APAP with an initial two-tablet dose and in 48 hours after IR/ER HB/APAP with an initial three-tablet dose and IR tramadol HCl/APAP. Steady-state APAP exposure (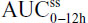 ,
, 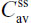 , and
, and 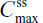 ) was similar for IR/ER HB/APAP (with a three- or two-tablet initial dose) and IR tramadol HCl/APAP. However, there were twice as many APAP concentration peaks and troughs for IR tramadol HCl/APAP compared with IR/ER HB/APAP (Figure 4), consistent with dosing q6h vs q12h.
) was similar for IR/ER HB/APAP (with a three- or two-tablet initial dose) and IR tramadol HCl/APAP. However, there were twice as many APAP concentration peaks and troughs for IR tramadol HCl/APAP compared with IR/ER HB/APAP (Figure 4), consistent with dosing q6h vs q12h.
Genotype evaluation
Of 48 participants who were genotyped for CYP2D6 status, 29 were determined to be intermediate metabolizers, 17 extensive metabolizers, one ultrarapid metabolizer, and a poor metabolizer. No clinically relevant effects of genotype were found in any study participant. No PK outliers were identified using the Grubb test after single- or multiple-dose administration. A single outlier for AUCss0–12h was identified using the likelihood distance test. This subject was neither a poor nor an ultrarapid metabolizer; hence, his PK results were not attributed to genetic factors. The subject completed treatment and was included in the PK analyses.
Safety and tolerability
Forty-one of 48 participants (85.4%) reported ≥1 treatment-emergent AE (TEAE), and 38 (79.2%) experienced ≥1 TEAE that was considered by investigators to be related to treatment. The types of TEAEs were similar across treatments. TEAEs occurring in ≥10% of participants were nausea (n=25 [52.1%]), vomiting (16 [33.3%]), somnolence (15 [31.3%]), pruritus (12 [25.0%]), constipation (eleven [22.9%]), dizziness (ten [20.8%]), headache (ten [20.8%]), and euphoric mood (five [10.4%]). All TEAEs were considered mild to moderate in severity. There was one serious AE, ectopic pregnancy, considered not related to treatment. No deaths occurred during the study. There were no clinically significant changes in mean clinical laboratory test results, electrocardiogram findings, or vital signs, and physical examination results remained unchanged for all participants.
Discussion
In healthy participants under fasted conditions, single-dose IR/ER HB/APAP exhibited an early Cmax (ie, short tmax) for hydrocodone and APAP similar to IR HB/ibuprofen and IR tramadol HCl/APAP, respectively, consistent with its biphasic design. Additionally, IR/ER HB/APAP provided prolonged plasma concentrations with a longer t½ for hydrocodone and APAP than the comparator IR formulations, which supports dosing q12h vs q6h. Total exposure to hydrocodone (based on dose-normalized AUC) after single-dose administration was not significantly different between two tablets of IR/ER HB/APAP, three tablets of IR/ER HB/APAP, and one tablet of IR HB/ibuprofen. Similarly, total exposure to APAP after single-dose administration was not significantly different between two tablets of IR/ER HB/APAP, three tablets of IR/ER HB/APAP, and one tablet of IR tramadol HCl/APAP. In addition, after multiple doses, peak and total steady-state exposure to hydrocodone and APAP were similar with IR/ER HB/APAP (with a three- or two-tablet initial dose) and IR HB/ibuprofen and IR tramadol HCl/APAP, respectively. Participant genotyping and outlier analyses revealed no association between hydrocodone PK values and CYP2D6 genotype.
The safety and tolerability of single and repeated doses of IR/ER HB/APAP (with a three- or two-tablet initial dose) were consistent with those observed with low-dose combination opioid analgesics.19–27 In PK studies of low-dose combination opioids enrolling healthy adult volunteers, a discontinuation rate of 20%–40% due to vomiting is typical.21,28,29 In acute pain studies, short-term multidose regimens of IR HB/APAP and/or IR OC/APAP have reported nausea and vomiting in 14%–42% and 4%–24% of patients, respectively.19,22,26,27 Similar results have been observed with opioid/ibuprofen and tramadol/APAP combination therapies.22,24–26
These findings are concordant with those from two studies comparing the single- and multiple-dose PK of IR/ER HB/APAP with IR HB/APAP in healthy volunteers.16 In those studies, IR/ER HB/APAP demonstrated similar single- and multiple-dose PK outcomes and tolerability compared with IR HB/APAP. The comparison of IR/ER HB/APAP with IR HB/ibuprofen and IR tramadol/APAP provides important data for prescribers. In addition to its opioid receptor–agonist properties, tramadol acts as a weak inhibitor of norepinephrine and an inhibitor of serotonin reuptake,7 differentiating tramadol/APAP combinations from opioid/APAP combinations. From a tolerability standpoint, ibuprofen has been associated with gastrointestinal effects typical of the nonsteroidal anti-inflammatory drug class,30 whereas the serotonergic mechanism of tramadol may create tolerability issues in patients taking other serotonergic drugs.7 Thus, it is important to characterize the PK and safety profiles of these drugs because of their frequent use in acute pain indications and examine tolerability because of the need for careful drug selection in patients receiving different concurrent medications or in patients with different drug sensitivities.
This study, unlike the studies comparing IR/ER HB/APAP to IR HB/APAP,16 compared the PK of IR/ER HB/APAP with an initial three-tablet and two-tablet dose, then maintained with two tablets administered every 12 hours throughout the course of study. Although there was no difference in the time to achieve steady-state concentrations for the three-tablet and two-tablet initial dose regimens, the similar safety profiles support the administration of either a three-tablet or two-tablet initial dose.
A limitation of this study is that study medications were administered under fasted conditions, leaving potential food effects unexamined. However, a study comparing the single-dose PK of IR/ER HB/APAP showed minimal effects of food.31 Also, the study groups were small and did not include pediatric or geriatric individuals, the substantially underweight or overweight, or individuals with comorbidities.
Conclusion
In this open-label trial, single and multiple doses of IR/ER HB/APAP 7.5/325 mg tablets exhibited a shorter time to achieve maximum drug concentrations similar to that of IR HB/ibuprofen 7.5/200 mg tablets and IR tramadol/APAP 37.5/325 mg tablets, but also provided sustained drug exposure with 12-hour dosing. AEs were consistent with those expected during treatment with low-dose opioid-combination therapies.
Acknowledgments
Mallinckrodt Pharmaceuticals (Hazelwood, MO, USA) sponsored this research and funded editorial support provided by Jeffrey Coleman, MA, and Robert Axford-Gatley, MD, of C4 MedSolutions, LLC (Yardley, PA, USA), a CHC Group company.
Disclosure
All authors are employees of Mallinckrodt; Dr Kostenbader is a paid contract employee of Mallinckrodt. The authors report no other conflicts of interest in this work.
References
Raffa RB, Tallarida RJ, Taylor R Jr, Pergolizzi JV Jr. Fixed-dose combinations for emerging treatment of pain. Expert Opin Pharmacother. 2012;13(9):1261–1270. | |
Raffa RB. Pharmacology of oral combination analgesics: rational therapy for pain. J Clin Pharm Ther. 2001;26(4):257–264. | |
Beaver WT. Combination analgesics. Am J Med. 1984;77(3A):38–53. | |
Percocet® (oxycodone and acetaminophen) tablets [prescribing information]. Malvern, PA: Endo Pharmaceuticals; 2013. | |
Vicoprofen® (hydrocodone bitartrate and ibuprofen) tablets [prescribing information]. Whippany, NJ: AbbVie Inc.; 2014. | |
Xartemis™ XR (oxycodone hydrochloride and acetaminophen) extended-release tablets [prescribing information]. Hazelwood, MO: Mallinckrodt Brand Pharmaceuticals, Inc.; 2014. | |
Ultracet® (tramadol hydrochloride and acetaminophen) tablet [prescribing information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; 2014. | |
Norco™ (hydrocodone bitartrate and acetaminophen) tablets [prescribing information]. Parsippany, NJ: Actavis Pharma, Inc.; 2014. | |
McCarberg BH, Barkin RL. Long-acting opioids for chronic pain: pharmacotherapeutic opportunities to enhance compliance, quality of life, and analgesia. Am J Ther. 2001;8(3):181–186. | |
de Leon-Casasola O. A review of the literature on multiple factors involved in postoperative pain course and duration. Postgrad Med. 2014;126(4):42–52. | |
Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–130. | |
Pedersen L, Borchgrevink PC, Riphagen II, Fredheim OM. Long- or short-acting opioids for chronic non-malignant pain? A qualitative systematic review. Acta Anaesthesiol Scand. 2014;58(4):390–401. | |
Argoff CE, Silvershein DI. A comparison of long- and short-acting opioids for the treatment of chronic noncancer pain: tailoring therapy to meet patient needs. Mayo Clin Proc. 2009;84(7):602–612. | |
Drug Enforcement Administration, Department of Justice. Schedule of controlled substances: placement of tramadol into schedule IV. Final rule. Fed Regist. 2014;79(127):37623–37630. | |
Drug Enforcement Administration, Department of Justice. Schedules of controlled substances: rescheduling of hydrocodone combination products from schedule III to schedule II. Final rule. Fed Regist. 2014;79(163):49661–49682. | |
Devarakonda K, Cheruvu NPS, Kostenbader K, Giuliani M, Young JL. Single- and multiple-dose pharmacokinetics of 1 and 2 tablets of extended-release hydrocodone bitartrate/acetaminophen (MNK-155) compared with immediate-release hydrocodone bitartrate/acetaminophen. Presented at: American Pain Society 33rd Annual Scientific Meeting; April 30–May 3, 2014, Tampa, FL. | |
Waife SO, Gruber CM Jr, Rodda BE, Nash JF. Problems and solutions to single-dose testing of analgesics: comparison of propoxyphene, codeine, and fenoprofen. Int J Clin Pharmacol Biopharm. 1975;12(1–2):301–304. | |
US Food and Drug Administration. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products/general considerations. Rockville, MD: Center for Drug Evaluation and Research, US Food and Drug Administration; 2003. | |
Chang DJ, Desjardins PJ, Bird SR, et al. Comparison of rofecoxib and a multidose oxycodone/acetaminophen regimen for the treatment of acute pain following oral surgery: a randomized controlled trial. Curr Med Res Opin. 2004;20(6):939–949. | |
Chang DJ, Desjardins PJ, King TR, Erb T, Geba GP. The analgesic efficacy of etoricoxib compared with oxycodone/acetaminophen in an acute postoperative pain model: a randomized, double-blind clinical trial. Anesth Analg. 2004;99(3):807–815, table of contents. | |
Devarakonda K, Morton T, Margulis R, Giuliani M, Barrett T. Pharmacokinetics and bioavailability of oxycodone and acetaminophen following single-dose administration of MNK-795, a dual-layer, biphasic, IR/ER combination formulation, under fed and fasted conditions. Drug Des Devel Ther. 2014;8:1125–1134. | |
Hewitt DJ, Todd KH, Xiang J, Jordan DM, Rosenthal NR; CAPSS-216 Study Investigators. Tramadol/acetaminophen or hydrocodone/acetaminophen for the treatment of ankle sprain: a randomized, placebo-controlled trial. Ann Emerg Med. 2007;49(4):468–480, 480. e1–e2. | |
Korn S, Vassil TC, Kotey PN, Fricke JR Jr. Comparison of rofecoxib and oxycodone plus acetaminophen in the treatment of acute pain: a randomized, double-blind, placebo-controlled study in patients with moderate to severe postoperative pain in the third molar extraction model. Clin Ther. 2004;26(5):769–778. | |
Litkowski LJ, Christensen SE, Adamson DN, Van Dyke T, Han SH, Newman KB. Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double-blind, placebo-controlled, single-dose, parallel-group study in a dental pain model. Clin Ther. 2005;27(4):418–429. | |
Bourne MH, Rosenthal NR, Xiang J, Jordan D, Kamin M. Tramadol/acetaminophen tablets in the treatment of postsurgical orthopedic pain. Am J Orthop. 2005;34(12):592–597. | |
Palangio M, Morris E, Doyle RT Jr, Dornseif BE, Valente TJ. Combination hydrocodone and ibuprofen versus combination oxycodone and acetaminophen in the treatment of moderate or severe acute low back pain. Clin Ther. 2002;24(1):87–99. | |
Marco CA, Plewa MC, Buderer N, Black C, Roberts A. Comparison of oxycodone and hydrocodone for the treatment of acute pain associated with fractures: a double-blind, randomized, controlled trial. Acad Emerg Med. 2005;12(4):282–288. | |
Devarakonda K, Morton T, Giuliani M, Kostenbader K, Barrett T. Steady-state pharmacokinetics of MNK-795, an extended-release oxycodone and acetaminophen combination analgesic: results from 2 active comparator studies. J Bioequiv Availab. 2014;6(2):53–60. | |
Devarakonda K, Morton T, Giuliani M, Kostenbader K, Barrett T. Single-dose pharmacokinetics of MNK-795, an extended-release oxycodone and acetaminophen combination analgesic: results from 2 active comparator studies. J Bioequiv Availab. 2014;6(2):38–45. | |
Hippisley-Cox J, Coupland C, Logan R. Risk of adverse gastrointestinal outcomes in patients taking cyclo-oxygenase-2 inhibitors or conventional non-steroidal anti-inflammatory drugs: population based nested case-control analysis. BMJ. 2005;331(7528):1310–1316. | |
Cheruvu NPS, Kostenbader K, Giuliani M, Young JL, Devarakonda K. Single-dose pharmacokinetics of 2 or 3 tablets of extended-release hydrocodone bitartrate/acetaminophen (MNK-155) under fed and fasted conditions. Presented at: PAINWeek; September 2–6, 2014, Las Vegas, NV. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.