Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Simple Lymphangioma of the Scrotum: A Case Report
Authors Deng D, Niu M, Yang J , Deng L
Received 19 May 2022
Accepted for publication 30 August 2022
Published 22 September 2022 Volume 2022:15 Pages 2017—2020
DOI https://doi.org/10.2147/CCID.S375428
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Dongmei Deng,1,* Mu Niu,1,* Jie Yang,1 Lijun Deng2
1Department of Cosmetic Dermatology, The Fifth People’s Hospital of Hainan Province, Haikou, People’s Republic of China; 2Department of Dermatovenereology, Wuzhong People’s Hospital, Suzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Yang, Department of Cosmetic Dermatology, The Fifth People’s Hospital of Hainan Province, Haikou, People’s Republic of China, Email [email protected] Lijun Deng, Department of Dermatovenereology, Wuzhong People’s Hospital, Suzhou, 215128, People’s Republic of China, Email [email protected]
Abstract: As scrotal simple lymphangioma is a rare benign proliferation of lymphatic vessels in the scrotum, a few reports were documented. A 68-year-old man gradually developed vesicles on the scrotum for ten years and easily ruptured due to friction. Physical examination showed diffuse millet-sized vesicles on the scrotum with milky white fluids and chyle-like exudates. Histopathological examination revealed proliferating and dilated lymphatic vessels with various sizes of lumens in the dermis. An immunohistochemical study showed positive staining for D2-40 and CD31. Therefore, the patient was diagnosed with scrotal lymphangioma and received carbon dioxide laser therapy. After the treatment, the vesicles markedly decreased, and no apparent exudates were observed. During one year of the follow-up, no relapse, scars, or other complications occurred.
Keywords: lymphangioma, scrotum, carbon dioxide laser
Case Report
A 68-year-old man presented with generalized blisters in the scrotum for more than ten years. The patient developed several pale blisters, ranging from needle tip to grain size, with a smooth surface on the scrotum without obvious cause. He had no apparent symptoms and was diagnosed with “scrotal eczema” at a local hospital. Although he was given a glucocorticoid ointment (unspecified), the symptoms did not resolve. In the last three years, blisters increased significantly and spread over the entire scrotal surface without itching and pain. Some blisters fused into a bulla, and some blisters ruptured and exuded. Therefore, he came to our hospital for treatment. He had no history of chronic diseases such as hypertension, diabetes, coronary heart disease, and cerebral infarction and no history of infection such as tuberculosis and malaria. Similarly, he reported no history of drug or food allergies. The patient’s parents, younger brother, and sister did not show similar skin lesions.
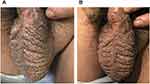
Physical examination revealed no superficial lymph node enlargement in the groin. The thickened skin and the deepened folds on both sides of the scrotum were observed. The scrotal surface was covered with numerous blisters without peculiar smell and tenderness. The blister wall was thick with a clear boundary. The blister was filled with colorless or reddish serous exudates. Some blisters fused into a bulla (Figure 1A and B). No similar lesions were observed in other parts of the body.
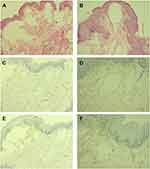
All laboratory results were normal. The acetowhite test was also negative. The abdominal CT and B-ultrasound showed no abnormalities. Histopathological examination of the skin revealed that the cystic spaces of dermal cells were mainly filled with light red serous fluid, and a few inflammatory cells were observed around the cystic spaces, which was consistent with the pathological diagnosis of lymphangioma (Figure 2A and B). Immunohistochemistry showed positive CD31 (Figure 2C and D) and D2-40 protein (Figure 2E and F). Therefore, the patient was diagnosed with a simple-acquired lymphangioma of the scrotum. Thus, the patient received a carbon dioxide laser (CO2 laser) to individually cauterize the blisters at the skin lesions and remove the blisters until normal tissue was visible. The patient was also instructed to apply potassium permanganate solution (1:8000) to the wounds apart from laser therapy once a day and fusidic acid ointment twice a day. The patient was followed up continuously, and during one year of the follow-up, no relapse, scars, or other complications occurred.
Discussion
Lymphangioma (LA) is a benign proliferation of lymphatic vessels.1 The LA can be divided into three types: simple lymphangioma, cavernous lymphangioma, and cystic lymphangioma.2 The lacunae of simple lymphangioma are usually located in the upper dermis. They are manifested as clustered, deep, and tension blisters, which can occur in any part of the skin and mucous membrane, including the armpit, shoulder, neck, proximal extremities, tongue, vulva, and buccal mucous membrane. However, skin lesions in the scrotum are sporadic.3 Cavernous lymphangioma vesicle is the commonest LA located in the subcutaneous tissue and contains large and thin lymphatic vessels. The clinical manifestation of spongiform lymphangioma blister is spongy subcutaneous tissue mass or diffuse swelling of subcutaneous tissue and more invasions of the head and neck skin. Cystic lymphangioma lesions are often located deep in the dermis and contain large cystic lumens characterized by multilocular and extensive subcutaneous tissue masses. The lesions are progressively enlarged and may cause dyspnea and death in severe cases.
In our case, the patient presented thick-walled, transparent, round, or flat white blisters in the scrotum with thickened skin on both sides. According to the history, clinical manifestations, and histopathological and immunopathological tests, the patient’s symptoms were consistent with the diagnosis of congenital simple lymphangioma. Clinically, the disease should be differentiated from hemangioma, angiokeratoma, verruca vulgaris, molluscum contagiosum, herpes zoster, herpes simplex, and verrucous nevus and other infections.4 Therefore, in addition to the patient’s medical history, careful physical examination and histopathologic examination should be performed for diagnosis. The histopathological features include highly dilated lymphatic vessels with a layer of flat epithelial cells in the tubes. The sac contains much lymphatic fluid and a few lymphocytes. However, there is no standard treatment for lymphangioma. Currently, treatments include freezing, surgical resection, electric cauterization, laser therapy, and microwave therapy. Unfortunately, no one treatment can treat all types of lymphangioma. Therefore, different treatment methods should be adopted for each patients.5
In this case, due to scattered skin lesions with minimal area involvement in the elder, we used a CO2 laser to remove the blisters of lymphangioma, followed by applying potassium permanganate solution by wet compression, and obtained a satisfactory healing effect. Although surgical resection and reconstruction is the gold standard of treatment,6,7 it is mainly used for treating deep lesions such as scrotal cavernous lymphangioma and cystic hygroma. Due to the enormous scope of surgical resection, the influence of surgical resection on the structure and function of the perineal area cannot be ignored. It has been reported that photodynamic therapy (PDT) has achieved good efficacy in the treatment of head and neck lymphangioma and female vulvar lymphangioma.8,9 The patient was treated with a carbon dioxide laser in our hospital. The skin lesions did not recur after 1-year follow-up. Simple lymphangioma is common, but the skin lesions on the scrotum are rare. This special case provided a reference for other similar symptoms. Accurate diagnosis of the pathological type of blisters is helpful to better symptomatic treatment and improve the therapeutic effect.
Consent Statement
Informed consent for publication of the case details and associated images was obtained from the patient, and all procedures were performed in accordance with the Helsinki Declaration. Institutional approval was not required to publish the case details.
Funding
This work was supported by Hainan Province Clinical Medical Center.
Disclosure
Dongmei Deng and Mu Niu are co-first authors for this study. The authors report no conflicts of interest in this work.
References
1. Roh MR, Choi YJ, Lee KG. Papular acantholytic dyskeratosis of the vulva. J Dermatol. 2009;36(7):427–429. doi:10.1111/j.1346-8138.2009.00660.x
2. Bell HK, Farrar CW, Curley RK. Papular acantholytic dyskeratosis of the vulva. Clin Exp Dermatol. 2001;26(5):386–388. doi:10.1046/j.1365-2230.2001.00840.x
3. Callander JA, Davies BM, Hill G. Acquired lymphangioma circumscriptum of the vulva secondary to severe herpes simplex infection. Sex Transm Infect. 2020;96(3):233–234. doi:10.1136/sextrans-2019-054224
4. Joshi M, Phansalkar DS. Simple lymphangioma to generalized lymphatic anomaly: role of imaging in disclosure of a rare and morbid disease. Case Rep Radiol. 2015;2015:603859. doi:10.1155/2015/603859.
5. Ha J, Yu YC, Lannigan F. A review of the management of lymphangiomas. Curr Pediatr Rev. 2014;10(3):238–248. doi:10.2174/1573396309666131209210751
6. Best SR, Coelho DH, Ahrens WA, et al. Laser excision of multiple esophageal lymphangiomas: a case report and review of the literature. Auris Nasus Larynx. 2008;35(2):300–303. doi:10.1016/j.anl.2007.07.016
7. Kentley J, Cerio R, Khorshid M, et al. Acantholytic dermatosis of the vagina: the diagnostic challenge of acantholytic disease in the genital region. Clin Exp Dermatol. 2017;42(2):189–191. doi:10.1111/ced.13026
8. Sáenz AM, Cirocco A, Avendaño M, Gonzalez F, Sardi JR. Papular acantholytic dyskeratosis of the vulva. Pediatr Dermatol. 2005;22(3):237–239. doi:10.1111/j.1525-1470.2005.22312.x
9. Belotto R, Santos RE, Tardivo JP, et al. Photodynamic therapy in vulvar lymphangioma: case report. Photodiagnosis Photodyn Ther. 2019;25:84–86. doi:10.1016/j.pdpdt.2018.09.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.