Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Signet-Ring Cutaneous Metastasis Presenting with Huge Bunches of Grapes
Authors Hao Z , Deng Y, He Y, Xiong X
Received 17 June 2022
Accepted for publication 8 September 2022
Published 21 September 2022 Volume 2022:15 Pages 1997—2001
DOI https://doi.org/10.2147/CCID.S378478
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Zhenyu Hao *, Yongqiong Deng *, Yuanmin He, Xia Xiong
Department of Dermatology STD, The Affiliated Hospital of Southwest Medical University, Luzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongqiong Deng, Department of Dermatology, The Affiliated Hospital of Southwest Medical University, No. 25, Taiping Street, Luzhou, 646000, People’s Republic of China, Tel +8613679670608, Email [email protected]
Abstract: Signet-ring cell (SRC) is a histologic type in which cells show unique features under the microscope. We mainly found signet-ring cells (SRCs) in gastrointestinal and breast tumors. Cutaneous metastasis from internal carcinomas was an uncommon presentation. The cases of signet-ring cell carcinoma (SRCC) metastasis to the skin were rarely reported. Cutaneous metastasis indicated a poor prognosis for a patient. Here, we report a female who had huge grape-like nodules arising from gastrointestinal SRCC in her trunk and thigh.
Keywords: signet-ring cutaneous metastasis, SRCC, signet-ring cell carcinoma, unknown primary tumors, gastrointestinal tumors
Introduction
Signet-ring cell carcinoma (SRCC) is a mucin-secreting adenocarcinoma that contains abundant intracytoplasmic mucin that pushes the nucleus to the periphery. Signet-ring cutaneous metastasis is extremely rare and indicates a poor prognosis, which used to be manifested as papulonodular, erythematous plaque, as well as scar- and erysipelas-like lesions. This case study showed a rare signet-ring cutaneous metastasis presenting with huge bunches of grapes. The condition was solely diagnosed via skin biopsy.
Case Report
A 58-year-old woman was admitted to our hospital in April 2021 for a huge, painful lump in the abdominal wall, coupled with a rapid decline in overall health. An edematous plaque in the abdominal wall inexplicably began about 13 months before admission, when she experienced a stomach ache and dizziness, simultaneously. During the interim, she experienced a gradual weight loss of 5 kg. Her plaque gradually thickened and hardened, forming many grape-like nodules on the surface.
On physical examination (Figure 1), the patient had a huge lump with bunches of grape-like appearance in the abdominal wall. When the grape-like nodules were punctured, a clear yellow fluid came out. The skin at the base of the lump appeared dark red, thickened, and hard, extending to the lower chest, upper thighs, and buttocks. No frank edema, swelling, ulcerations, or hemorrhagic lesions were found. The patient was pale and thin. The dullness of the percussion and decrease in breath sounds were noted in the lower lungs.
 |
Figure 1 A thick, hard, multiple grape-like appearance of nodules in and around the lower chest, abdominal wall, upper thighs, and buttocks. |
The initial diagnostic workup revealed that the hemoglobin levels were 75 g/L. The other findings were as follows: reduced total protein (51.5 g/L) and albumin (29.1 g/L), lactic dehydrogenase (LDH) (247.5 U/L), carcinoembryonic antigen (CEA) (6.37 ng/mL), carbohydrate antigen (CA) 125 (248.15 U/mL), CA 199 (>400 U/mL), CA 50 (392 IU/mL), CA 242 (25.7 IU/mL), CA 724 (116 IU/mL), β-Human chorionic gonadotropin (β-HCG) (8.07m IU/mL), cytokeratin 19 fragment 21-1 (CYFRA 21-1) (10.6 ng/mL), pro-gastrin-releasing peptide (ProGRP) (551 pg/mL). This combination of results suggested tumors that were derived from the digestive system.
A computed tomography scan revealed extensive swelling and effusion on the walls of the chest, abdomen, and basin, in addition to interstitial pulmonary edema in both lungs, lobulated nodules in the upper apical segment of the right lung, as well as a few mucus plugs in the trachea.
Multiple vegetations on the anterior abdominal wall and bilateral thighs were observed through the positron emission tomography/computed tomography (PET/CT) scan, along with intense fluorodeoxyglucose (FDG) uptake. The other FDG-positive pathological lesions were also observed in the superior apical segment of the right lung, multiple enlarged lymph nodes in bilateral neck roots, left upper and lower clavicular fossa (Figure 2A), mediastinum, double hilum, parastinum, sternum, retroperitoneum (Figure 2B), left side of rectum and bilateral iliac vessels, nodules in the upper right posterior lobe of the liver, as well as the left upper femur and nodules of the abdominal wall (Figure 2B). Thoracentesis was done and the result of tumor biomarkers from pleural effusion was consistent with those in serum.
 |
Figure 2 PET/CT: High uptake of 18F-FDG was noted in some left clavicular fossa nodes (A), retroperitoneal lymph nodes, and nodules of the abdominal wall (B) (red arrows). |
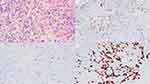
A skin biopsy was performed from the grape-like nodules and dark red patches. There was lymphatic dilation, and dermal mucin deposition in both lesions, but with scattered cells having a signet-ring morphology within the deep reticular dermis and subcutis only in the dark red patch (Figure 3A). Lesion cells stained positive with cytokeratin (CK), Cam 5.2, CK 7, CK 20 and Villin (Figure 3B–D), while negative with S100, caudal type homeobox 2 (CDX 2), thyroid transcription factor −1 (TTF-1), estrogen receptor (ER), and gross cystic disease fluid protein-15 (GCDFP-15).
The elevated tumor biomarkers in both serum and pleural effusion, together with immunophenotypic findings pointed to a metastatic SRCC of gastrointestinal origin. Therefore, esophagogastroduodenoscopy and colonoscopy with biopsy were suggested. Unfortunately, the patient developed atrial fibrillation upon the completion of esophagogastroduodenoscopy and died despite all rescue efforts. The endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) and the left clavicular fossa lymph nodes biopsy could not be completed. Under esophagogastroduodenoscopy, multiple ulcers were found in the gastric antrum and duodenal bulb, but only some inflammatory cell infiltration without tumor cells was found in the stomach tissue, histopathologically.
Discussion
SRC is a morphological description of a cell whose nuclei are pushed to the periphery because of excessive intracytoplasmic mucin. The condition is commonly found in adenocarcinomas, especially in the gastric epithelium.1 According to the classification by WHO, gastric SRCs are a subtype of adenocarcinomas. In addition, signet ring cells can be found in primary cutaneous SRCC of eccrine or apocrine origin, primary cutaneous signet ring basal cell carcinoma, melanomas, and squamous cell carcinoma.2 Signet-ring cutaneous metastasis is extremely rare, and usually occurs in the later stage of the disease, further signifying a poor prognosis. The clinical manifestations of SRCC cutaneous metastases have been reported as papulonodular, erythematous plaque, as well as scar- and erysipelas-like lesions.3,4 This case in this study showed a rare signet-ring cutaneous metastasis presenting with lesions that imitate the shape of huge bunches of grapes, which has not been described throughout the literature, to the best of our knowledge. In our cases speculated the route of cutaneous metastasis of visceral tumors was via lymphatic vessels. The cutaneous metastasis of visceral tumors occurs via hematogenous, lymphatic, direct contiguous tissue invasion, and iatrogenic implantation pathways.5 In our case, some dilated lymphatic vessels in hematoxylin-eosin staining suggested that the patient’s skin lesions were probably due to an accumulation of carcinomatous lymphangitis.
It is difficult to identify the primary cancer of SRCC cutaneous metastasis, but the gross histopathologic evaluation and immunohistochemical stains can be particularly helpful.4,8 Considering that the malignant cells will continue to synthesize tissue-specific proteins that can be identified by targeted immunostaining, these cells have conserved an adequate level of differentiation from the primary tumor. For instance, positive CK and Cam 5.2 may assist particularly distinguish adenocarcinoma. In our case, the presence of SRCs prompted immunostaining for common gastrointestinal malignancies with CK 7, CK 20, and Villin.6 The negative stain of CDX 2 reduced the possibility of considering colorectal origins.7 Additionally, negative S 100, SMA, NSE, TTF-1, ER, and GCDFP-15 helped to exclude melanoma, gynecologic, lung, and breast origins of SRCs, respectively.6–8
Although the incidence of skin metastases from gastrointestinal cancers is low, the involvement of the liver, lung, and bone in this case also provided a distinct clue of the gastrointestinal origin of SRCC.8 Some cases failed to reveal the primary site of origin for SRCC metastases.4,9 About 30–40% of unknown primary tumors are poorly differentiated adenocarcinomas or carcinomas. A hypothesis suggested that primary tumors acquire a special phenotype and/or occurs due to inborn errors, which bring negative impacts for primary growth.10 Moreover, due to the rich intracytoplasmic mucin, lack of membranous glucose transporter-1, and the consequently low FDG uptake, the 18F-FDG PET/CT scan has limited efficacy in diagnosing gastrointestinal SRCC.11
In conclusion, we presented a patient with rare manifestation of lesions that look like huge bunches of grapes in the abdominal wall. This was diagnosed as metastatic SRCC of gastrointestinal origin. It is suggested that the skin biopsy and immunohistochemical staining is not only helpful but essential for the diagnosis of SRCC and determining the primary cancer, which should be taken with no hesitation when a suspicion of cancer arises.
Abbreviations
CA, carbohydrate antigen; CDX 2, caudal type homeobox 2; CEA, carcinoembryonic antigen; CK, cytokeratin; CYFRA 21-1, cytokeratin 19 fragment 21-1; EBUS-TBNA, endobronchial ultrasound-guided transbronchial needle aspiration; ER, estrogen receptor; FDG, fluorodeoxyglucose; GCDFP-15, gross cystic disease fluid protein-15; LDH, lactic dehydrogenase; PET/CT, positron emission tomography/computed tomography; ProGRP, pro-gastrin-releasing peptide; SRC, signet-ring cell; SRCC, signet-ring cell carcinoma; SRCs, signet-ring cells; TTF-1, thyroid transcription factor −1; β-HCG, β-Human chorionic gonadotropin.
Ethics and Consent Statement
All patient samples and clinical data using were approved by the Ethics committee of The Affiliated Hospital of Southwest Medical University. Written informed consent for publication of the case was provided by the patient’s husband for the publication of all patient’s images, samples, and clinical data.
Acknowledgments
We would like to acknowledge the patient and her families. Zhenyu Hao and Yongqiong Deng are co-first authors for this study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Wu SG, Chen XT, Zhang WW, et al. Survival in signet ring cell carcinoma varies based on primary tumor location: a surveillance, epidemiology, and end results database analysis. Expert Rev Gastroenterol Hepatol. 2018;12(2):209–214. doi:10.1080/17474124.2018.1416291
2. Kiyohara T, Kumakiri M, Kouraba S, Tokuriki A, Ansai S. Primary cutaneous signet ring cell carcinoma expressing cytokeratin 20 immunoreactivity. J Am Acad Dermatol. 2006;54(3):532–536. doi:10.1016/j.jaad.2005.04.039
3. Cokgezer S, Samanci NS, Bektas M, Kepil N, Demirelli FH. Cutaneous metastasis of signet cell gastric carcinoma. Indian J Dermatol. 2020;65(2):148–150. doi:10.4103/ijd.IJD_263_18
4. Raval N, Shmuylovich L, Strickley J, Chen TY, Rosman IS, Musiek A. Signet-ring cutaneous metastasis presenting with massive anasarca. JAAD Case Rep. 2021;10:123–125. doi:10.1016/j.jdcr.2021.02.009
5. Hu SC, Chen GS, Wu CS, Chai CY, Chen WT, Lan CC. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60(3):379–387. doi:10.1016/j.jaad.2008.10.007
6. Habermehl G, Ko J. Cutaneous metastases: a review and diagnostic approach to tumors of unknown origin. Arch Pathol Lab Med. 2019;143(8):943–957. doi:10.5858/arpa.2018-0051-RA
7. Alcaraz I, Cerroni L, Rütten A, Kutzner H, Requena L. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34(4):347–393. doi:10.1097/DAD.0b013e31823069cf
8. Gündüz Ö, Emeksiz MC, Atasoy P, Kidir M, Yalçin S, Demirkan S. Cells in the skin: a case of late-onset cutaneous metastasis of gastric carcinoma and a brief review of histological approach. Dermatol Rep. 2017;8(1):6819. doi:10.4081/dr.2016.6819
9. Gregoire C, Muller G, Machiels JP, Goeminne JC. Metastatic signet-ring cell carcinoma of unknown primary origin. Acta Clin Belg. 2014;69(2):135–138. doi:10.1179/0001551213Z.0000000002
10. van de Wouw AJ, Jansen RL, Speel EJ, Hillen HF. The unknown biology of the unknown primary tumour: a literature review. Ann Oncol. 2003;14(2):191–196. doi:10.1093/annonc/mdg068
11. Yamada A, Oguchi K, Fukushima M, Imai Y, Kadoya M. Evaluation of 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography in gastric carcinoma: relation to histological subtypes, depth of tumor invasion, and glucose transporter-1 expression. Ann Nucl Med. 2006;20(9):597–604. doi:10.1007/BF02984657
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.