Back to Journals » Clinical Ophthalmology » Volume 14
Sight-threatening Diabetic Retinopathy and Associated Risk Factors Among Adult Diabetes Patients at Debre Tabor General Hospital, Northwest Ethiopia
Authors Alemu Mersha G, Tsegaw Woredekal A , Tilahun Tesfaw M
Received 7 October 2020
Accepted for publication 20 November 2020
Published 30 December 2020 Volume 2020:14 Pages 4561—4569
DOI https://doi.org/10.2147/OPTH.S285606
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Getasew Alemu Mersha,1 Asamere Tsegaw Woredekal,2 Matyas Tilahun Tesfaw3
1Department of Optometry, School of Medicine, University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia; 2Department of Ophthalmology, School of Medicine, University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia; 3Department of Ophthalmology, School of Medicine, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Getasew Alemu Mersha
Department of Optometry, School of Medicine, University of Gondar Comprehensive Specialized Hospital, Gondar 196, Ethiopia
Tel +251932823935
Fax +251-58-114 1240
Email [email protected]
Background: People with diabetes have an increased risk of developing sight-threatening conditions. Sight threatening diabetic retinopathy (STDR) is an advanced microvascular of complication of diabetes on the eye. It remains one of the leading causes of preventable blindness among working age adults around the world. There is a paucity of evidence on the prevalence of STDR and its associated factors in Ethiopia, particularly in the study area. Therefore, the aim of our study was to determine the prevalence of STDR and its associated factors among adult diabetes patients at Debre Tabor General Hospital (DTGH), Northwest Ethiopia.
Materials and Methods: An institution-based cross-sectional study was conducted on 306 diabetes patients at Debre Tabor General Hospital with systematic random sampling technique. Semi-structured questionnaire, document review and physical examination were applied to collect the data. Binary and multivariable logistic regression model were used to identify associated factors for STDR.
Results: The majority of the participants 163 (53.3%) were type 1 diabetes (T1DM) and the mean age of T1 and T2 DM participants was 34.5 (12.8) and 58.7 (10.7) years respectively. The prevalence of STDR was 15.3% (95%CI: 9.6%– 20.9%) and 11.9% (6.6– 17.5) in T1DM and T2DM, respectively. Low family monthly income (adjusted odds ratio (AOR)=4.4, 95%CI: 1.05– 18.40) among T2DM, longer duration of diabetes (AOR=10.9, 95%CI: 2.94– 40.4) among T1DM (AOR=3.54, 95CI: 1.06– 11.8) among T2DM and poor glycemic control (AOR=3.93, 95%CI: 1.06– 14.5) and hypertension (AOR=5.86, 95%CI: 1.20– 28.6) among T1DM and BMI (AOR=4.79, 95%CI: 1.35– 17.00) among T2DM were significantly associated with STDR.
Conclusion and Recommendation: The prevalence of STDR was high. Low family monthly income, longer duration of diabetes, poor glycemic control, hypertension and obesity were positively associated with STDR. Early screening of STDR and improving diabetes self management in all diabetes patients were recommended.
Keywords: STDR, prevalence, Ethiopia
Introduction
Diabetic retinopathy (DR) is a common and specific microvascular complication of diabetes, characterized by spectrum of lesions in the retina. Clinically, diabetic retinopathy can be graded as non-sight-threatening diabetic retinopathy (NSTDR) including mild and moderate nonproliferative abnormalities and sight-threatening diabetic retinopathy (STDR) which includes severe nonproliferative abnormalities, proliferative abnormalities and diabetic maculopathy.1,2
In the initial stage of the disease, people do not have visual impairment and may not notice any visual symptoms. However, in its advance form of complication (STDR), the disease progress into proliferative phase which is characterized by formation of new blood vessels and macular edema owing to accumulation of fluid within the retina producing severe and often irreversible vision loss. In addition, the new blood vessels may bleed, adding further complication of preretinal or vitreous hemorrhage. Finally, neovascular glaucoma associated with the new vessels can be a cause of visual loss.1
Globally, the prevalence of DR and STDR among diabetic adults was estimated to be 34.6% and 10.2% respectively.3 The prevalence of DR in Africa was reported to be 31.6%4 while the national prevalence of DR in Ethiopia was 19.48%.5 Out of 139 million visual impaired worldwide 3.7 (1.9%) million were visually impaired due to STDR.6 Visual impairment as a result of STDR has a significant impact on patients’ quality of life, and can compromise their ability to manage their disease successfully, which can in turn have a positive impact on the incidence of other diabetic complications and negative impact on overall life expectancy and productivity.7
The major risk factors for the development STDR in diabetes patients are longer duration of diabetes, hyperglycemia, inflammation, dyslipidemia, obesity, puberty, pregnancy and hypertension.8–10 Complete understanding of the magnitude of STDR in the patient population is crucial to design policies for prevention and timely treatment of the disease.
Preservation of sight in STDR can be achieved through effective screening, timely laser treatment, intraocular injection of steroids and antivascular endothelial growth-factor agents and intraocular surgery.11,12
Despite the magnitude of DR is widely known through the previous studies, limited data is available on the magnitude and underlying risk factors of STDR in Ethiopia, besides no study has been conducted in the study area so far. The purpose of this study is, therefore, to determine the prevalence of STDR and associated factors among adult diabetes patients attending Debre Tabor General Hospital (DTGH), Northwest Ethiopia.
Methods and Materials
Study Design and Period
A hospital-based cross-sectional study was conducted at Debre Tabor General Hospital from June 29, 2020 to August 28, 2020. The hospital is located in Debre Tabor town, the capital city of South Gondar zone of the Amhara National Regional State, and it is located 667 km from Addis Ababa. According to the Debre Tabor Hospital Planning and Information Department, the hospital is providing preventive and curative health care services for about ~2.7 million people in the zone and nearby districts and has a capacity of 250 beds for inpatient services in five disciplines and 12 outpatient departments (OPDs).13 The hospital has specialty chronic illness clinics where patients with specific chronic diseases are referred for follow-up. On average around 22 DM patients are visiting the two diabetic clinics per day during working hours and general practitioners, internists and nurses are involved in the clinical service of diabetes patients. Secondary eye care service is given in the hospital with three optometrists, two ophthalmic nurses, one cataract surgeon and one ophthalmologist.
Inclusion Criteria
All adult diabetes patients of age ≥18 years visiting the diabetic clinic in DTGH during the study period were included and diabetes patients who were previously diagnosed for DR or not were also included in the study.
Exclusion Criteria
Patient with pregnancy induced diabetes (gestational diabetes), patients who were severely ill unable to sit and be examined with slit lamp indirect ophthalmoscope and patients with media opacity that obscured the view of their retina were excluded from the study.
The sample size was determined based on a single population proportion formula by taking 7.3% of the prevalence of STDR from a similar study done in Jimma, Ethiopia,14 95%CI, 3% margin of error and 10% nonresponse rate. Accordingly, the final computed sample size was 319. A systematic random sampling technique was applied to select study participants: there are around 750 diabetes patients who visit the diabetic clinic over two months.
Based on the decision to collect data in two months a sampling interval “k” was determined by dividing the expected number of DM patients with the sample size 319 which was approximately two. Then every other diabetes patient was approached for the study. Ethical clearance was obtained from University of Gondar, College of Medicine and Health Sciences, School of Medicine ethical review committee. Moreover, permission to conduct the study in the hospital was obtained from Chief Executive Officer and Medical Director Offices of DTGH. Written informed consent was obtained from each participant before they were recruited in the study. The written informed consent was approved by the ethical review committee. Generally, the study was conducted in line with the Ethical Principle of the Declaration of Helsinki.
Data Collection Procedures and Quality Control
Semi-structured interviewer-administered questionnaire, document review and ocular examination were used to collect data. The questionnaire consisted of four sections: Sociodemographic and economic variables (six items), Behavioral measurements (14 items), diabetic follow-up and eye checkup (five items) and checklist for clinical data extraction (seven items). Data quality was ensured through pretesting the questioner on 5% of the sample before the actual data collection period and training of the data collectors. Each day during the data collection 5% of the data was cross checked for completeness by the principal investigator.
Three BSc nurses interviewed the participants and traced data on type of DM, hypertension, FBS, and mode of treatment from the patients’ medical folder they also measured height and weight of participants. Retinal examination was carried out with a 90 diopter of Volk lens with slit lamp biomicroscope by a trained senior optometrist after the pupillary dilation was done using 1% tropicamide eye drop on both eyes. Participants with complexity and/or issue of sight-threatening retinopathy were double seen by a senior ophthalmologist and an agreed diagnosis of STDR was taken. An eye with the highest grade of diabetic retinopathy was labeled as having STDR.
Labeling of NSTDR and STDR was done based on the Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales.15,16 Previously undiagnosed diabetic retinopathy was used to refer retinopathy that was not diagnosed through dilated retinal examination before.
Assessment and Definition of Risk Factors
Known diabetes and hypertension were assigned for the participants who had confirmed diagnosis of diabetes or hypertension previously. Newly diagnosed diabetes was assigned for the patients with zero years of diabetes duration. Type of DM was assigned to participants as it was confirmed and written in their medical folder. Glycemic control was defined as poor if a current fasting blood sugar level was >152 mg/dL, or 7% glycated hemoglobin according to American Diabetic Association Standards of Medical Care in Diabetes.17
Physical activity was defined as
- Physical inactivity: anyone who does not to perform any form of physical activity for at least 10 min per day.
- Low physical activity: anyone who performs moderate activities (walking, running, or cycling) less than five days for at least 30 min or vigorous intensity activities (like carrying or lifting heavy loads or digging) less than three days for at least 20 min per week.
- Moderate physical activity: anyone who performs moderate activity for more than five days for at least 30 min per day or vigorous intensity activity more than three days for at least 20 min per day.18
Alcohol consumption was defined based on National Institute on Alcohol Abuse and Alcoholism:19
- Nondrinkers (ie, abstainers, or no alcohol consumption history),
- Moderate drinkers (ie, up to one drink/day for women and up to two drinks/day for men), and
- Heavy drinkers (ie, >1 drink/day for women and >2 drinks/day for men).
Data Processing and Analysis
The data were entered into Epi Info 7 and exported to SPSS version 20 for analysis. The descriptive statistics was summarized and presented using summary statistics such as frequency tables. The model was checked by Hosmer–Lemeshow goodness of fit test. Binary logistic regression was used to identify candidate variables. Variables with p-value <0.2 in binary logistic regression were entered into a multivariable logistic regression model. Variables having p-values <0.05 were considered as statistically significant.
Results
Sociodemographic Characteristics
A total of 306 (type 1 DM=163 and type 2 DM=143) participants completed the study with a response rate of 95.9%. The mean age of type 1 and type 2 DM participants was 34.5 (12.8) and 58.7 (10.7) years respectively. From both types of DM, the majority of the participants were male (57.1%, 51.0%), being currently married (58.3%, 75.5%) and had no formal education (56.4%, 55.2) (Table 1). The median family monthly income of the type 1 and type 2 DM participants was 3700 (3252.9) and 3180 (3493.8) Ethiopian Birr (ETB) respectively.
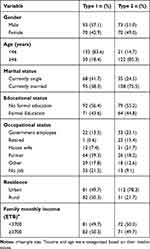 |
Table 1 Sociodemographic and Economic Characteristics of Study Participants at Debre Tabor General Hospital, Northwest Ethiopia, 2020 (n=306) |
Clinical and Behavioral Characteristics of Study Participants
The median duration of T1 and T2DM participants was 5 (6.4) and 4 (5.9) years respectively. The median level of FBS was 140 (105.6) and 159 (65) mg/dL for T1DM and T2DM participants respectively. In both types of DM, majority of the participants had a moderate level of physical activity (65.0%, 45.5%), visited the diabetes clinic every month (73.0%, 76.2%) and not obese (95.7%, 86.7%). Regarding prior retinal evaluation, 76 (46.6%) of T1DM and 104 (72.7%) of T2DM participants had previous dilated retinal examination. Only 47 (28.80) of T1DM participants and 33 (23.1) of T2DM had awareness about diabetic retinopathy (Table 2).
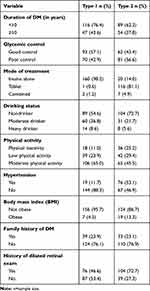 |
Table 2 Clinical and Behavioral Characteristics of Study Participants at Debre Tabor General Hospital Northwest Ethiopia, 2020 (n=306) |
Prevalence of DR and STDR Among Participants
Among the total study participants, the overall prevalence of DR of any severity was found to be 96 (31.4%) among them 34 (27%) did not have prior dilated retinal examination. The prevalence of STDR was 13.7% (95%CI: 9.8–17.6%) among them 13 (10.3%) did not have prior dilated fundus examination (Table 3) and only 3 (3.1%) had prior laser treatment for DR. Based on the type of DM, the prevalence of DR was 51 (31.3%) and 45 (31.5%) in T1DM and T2DM respectively. The prevalence of STDR was 15.3% (95%CI: 9.6–20.9%) among T1DM and it was 11.9% (95%CI: 6.6–17.5%) among T2DM (Table 4).
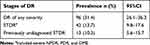 |
Table 3 Prevalence of DR and STDR Among Adult Diabetes at Debre Tabor General Hospital, Northwest Ethiopia, August 2020 (n = 306) |
 |
Table 4 Prevalence of DR and STDR Among Adult Type 1 and Type 2 Diabetes at Debre Tabor General Hospital, Northwest Ethiopia, August 2020 (n = 306) |
Factors Associated with Sight-threatening Diabetic Retinopathy
On applying bivariable logistic regression analysis among T1DM participants: age, family history of diabetes, family monthly income, duration of diabetes, glycemic control, and comorbid hypertension were statistically and significantly associated with STDR. Among T2DM participants: family monthly income, duration of DM and BMI were statistically and significantly associated with STDR. However, in multivariable logistic regression analysis among T1DM duration of diabetes, glycemic control and comorbid hypertension were remained statistically and significantly associated with STDR. Among T2DM family monthly income, duration of DM and BMI were statistically and significantly associated with STDR.
Reference to family monthly income of the T2DM participants, those who had an income of <3180 ETB were 4.4 times (AOR=4.40, 95%CI: 1.05–18.4) more likely to have STDR compared to those who had an income of ≥3180 ETB (Table 6). Regarding the duration of DM, participants who had a duration of 10 years and above among T1DM were nearly 11 times (AOR=10.90, 95%CI: 2.94–40.4) and among T2DM were 3.5 times (AOR=3.54, 95%CI: 1.06–11.8) more likely to develop STDR compared to those who had a duration of less than 10 years (Tables 5 and 6). T1DM participants who had a poor glycemic control had nearly four times (AOR=3.93, 95%CI: 1.06–14.5) an increased risk of having STDR compared to those who had a good glycemic control (Table 5). T1DM Participants who had a comorbid hypertension were nearly six times (AOR=5.86, 95%CI: 1.20–28.6) more at risk to develop STDR (Table 5). Regarding BMI, T2DM participants who were obese were nearly five times (AOR=4.79, 95%CI; 1.35–17.0) more likely to develop STDR compared with participants who were not obese (Table 6).
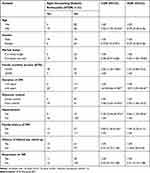 |
Table 5 Bivariable and Multivariable Logistic Regression Analysis of Factors Associated with STDR Among Type I DM at Debre Tabor General Hospital, Northwest Ethiopia, August 2020 (n=306) |
 |
Table 6 Bivariable and Multivariable Logistic Regression Analysis of Factors Associated with STDR Among Type 2 DM at Debre Tabor General Hospital, Northwest Ethiopia, August 2020 (n=306) |
Discussion
In this study the higher proportion of diabetics were T1DM 163 (53.3%), owing to the current pandemic some of T2DM patients were not coming to the clinic during the study period, taking medication through their attendants. The overall prevalence of STDR among all adult diabetes patients attending at Debre Tabor General Hospital, Northwest, Ethiopia was found to be 13.70%. Our finding was in line with the findings of studies conducted in mainland China (12.6%),20 Zimbabwe (11.40%),21 and Uganda (14.6).22
However, our finding was higher than findings reported from population-based studies in Singapore (8.90%)23 and Zambia (5.5%).24 This variation could be accountable for the difference in study population characteristics and labeling of STDR. For instance, labeling of STDR in the Zambian study excluded diabetic maculopathy, however, our study included diabetic maculopathy in labeling of STDR. Similarly our finding was higher than that reported from previous hospital based studies in Alaska, USA (4.2%)25 and Jimma, Ethiopia (7.3%).14 The difference in the labeling of STDR was the possible cause of these variations.
Alternatively, our finding was lower than population-based studies done in Tanzania (23%),26 besides the study setting the difference in the mean duration of diabetes contributed for this discrepancy. Similarly, our finding was also lower than Hangzhou, China (80%),27 Fiji (27%),28 and Malawi (29.4%).29 The discrepancy could be attributed to the difference in study settings and duration of diabetes. The study in China was at a retina clinic where patients with retinopathy were linked for a better treatment and follow-up, while the Malawi study was in a tertiary center where patients with advanced conditions were actually referred for a better evaluation and treatment and therefore, probing STDR in these settings could result in high prevalence.
With reference, the types of DM, the prevalence of STDR among T1DM participants was 15.3%, this was higher than reported from Slovakia (5.76%)30 and Gondar, Ethiopia (6.3%)31 however, it was lower than Norway (26.0%).32 The difference in mean duration of DM and labeling of STDR in these studies contributed to the variation of figures.
The prevalence of STDR among T2DM participants was 11.9% which was similar to Nepal (8.3%),33 India (9.5%),34 and Saudi Arabia (16.3%).35 Nevertheless, this report was lower than Pakistan (17.6%)36 and higher than California, USA (0.5%),37 Pittsburgh, USA (19.0%),38 and Slovakia (3.35).30 These discrepancies might be attributable to the difference in mean duration of diabetes, the nature of study population and definition of STDR.
Our study also found out that, family monthly income, duration of diabetes, glycemic control, hypertension and BMI were important risk factors of STDR. The likelihood of having STDR was high among T2DM participants who had an average monthly income of <3180 ETB. This finding was in accordance with the finding of studies in India39 and Sudan.40 The possible reason for the association could be, participants with low monthly income may have constraints to cover their transportation, investigation and medication related costs all the time and these factors are interrelated and may lead to poor glycemic control and in turn linked to advanced diabetic complications, including STDR.
Our finding demonstrated that, the likelihood of developing STDR was high for both types of DM participants with longer duration of diabetes (≥10 years) and this was consistent with what has been found previously in Norway,32 Hangzhou, China,27 mainland China,20 India,34 Cameroon,41 and Malawi29 that longer duration diabetes was associated with the development of vision-threatening diabetic retinopathy. This might be due the fact that in diabetes there are abnormalities in energy production which are thought to be the major contributor to the development of advanced diabetic complications like STDR, and these abnormalities are considered to occur late in the development of the disease.2
Our study also indicated that T1DM participants who had a poor glycemic control had an increased risk of developing STDR compared to those who had a good glycemic control. In this regard, our finding was similar to the findings previously reported in Norway,32 mainland China,20 and India.34 A poorly controlled high glucose level instigates a cascade of events leading to retinal vascular endothelial dysfunction eventually leading to advanced diabetic retinopathy.11
Our study identified that T1DM participants with comorbid hypertension were more likely to develop STDR compared to those without comorbid hypertension, this finding was in accordance with studies in California, USA,37 mainland China,20 and Malawi,29 that comorbid hypertension was positively associated with advanced diabetic retinopathy. Hypertension exacerbates diabetic retinopathy through increased blood flow and mechanical damage (stretching) of vascular endothelial cells, stimulating release of vascular endothelial growth factor which further increase the severity of the disease.11
Moreover, our study showed that obese T2DM participants had an increased risk of developing STDR compared to those who were not obese. This result agreed with the finding of a Croatian42 study which reported that the progression of diabetic retinopathy among T2DM significantly increased with higher BMI. Obesity could be associated with local inflammation of the retina and increase oxidative stress to the endothelial cells in turn elevate vascular endothelial factor which is responsible for the progression of DR to STDR.
The limitation of our study includes: single center and hospital based study—the patients recruited into our study may not be representative of the overall population with diabetes, and this affects the generalizability of our finding. Finally, we used current fasting glucose level instead of glycated hemoglobin for glycemic status; the latter shows the glycemic status over three months.
Conclusion
The prevalence of sight-threatening diabetic retinopathy was high compared to the global prevalence and previously reported in Ethiopia. Longer duration of diabetes, poor glycemic control, hypertension, obesity and low family monthly income were found to be independently and significantly associated with the presence of sight-threatening diabetic retinopathy.
Acknowledgments
We would like to express our deepest gratitude to Debre Tabor General Hospital for letting the data collection at the hospital and clinical nurse professionals and senior ophthalmic staffs who faced exhaustive data collection process. We wish to thank the study participants for their willingness to participate in the study. This study did not receive funding from any organization.
Disclosure
The authors report no conflict of interest in this work.
References
1. Fong DS, Aiello L, Gardner TW, et al. Retinopathy in diabetes. Diabetes Care. 2004;27(1):84–87. doi:10.2337/diacare.27.2007.S84
2. Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiological Rev. 2013;93(1):137–188. doi:10.1152/physrev.00045.2011
3. Thomas R, Halim S, Gurudas S, et al. IDF Diabetes Atlas: A review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res Clin Pract. 2019;157:107840. doi:10.1016/j.diabres.2019.107840
4. Burgess P, MacCormick I, Harding S, et al. Epidemiology of diabetic retinopathy and maculopathy in Africa: a systematic review. Diabet Med. 2013;30(4):399–412. doi:10.1111/j.1464-5491.2012.03756.x
5. Fite RO, Lake EA, Hanfore LK. Diabetic retinopathy in Ethiopia. Diabetes MetabSyndr. 2019;13(3):1885–1891.
6. Leasher JL, Bourne RR, Flaxman SR, et al. Global estimates on the number of people blind or visually impaired by diabetic retinopathy: a meta-analysis from 1990 to 2010. Diabetes Care. 2016;39(9):1643–1649. doi:10.2337/dc15-2171
7. Elwali ES, Almobarak AO, Hassan MA, et al. Frequency of diabetic retinopathy and associated risk factors in Khartoum, Sudan: population based study. Int J Ophthalmol. 2017;10(6):948.
8. Jonas JB, Sabanayagam C. Epidemiology and risk factors for diabetic retinopathy. Diabetic Retinopathy Cardiovascular Disease. 2019;20–37.
9. Ting DSW, Cheung GCM, Wong TY. Diabetic retinopathy: global prevalence, major risk factors, screening practices and public health challenges: a review. Clin Exp Ophthalmol. 2016;44(4):260–277.
10. Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi:10.2337/dc11-1909
11. Cheung N, Mitchell P, Wong TY. diabetes care. Lancet. 2010;21:22.
12. Njeri LN. Prevalence of Diabetic Retinopathy and Barriers to Uptake of Diabetic Retinopathy Screening at Embu Provincial General Hospital. Central Kenya; East African Journal of Ophthalmology, 2012.
13. Debre Tabor General Hospital. Summary and Statistical Annual Report of the Hospital, 2019: Information and Planning Office. Debre Tabor, Ethiopia: Debre Tabor General Hospital (DTGH); 2020.
14. Sharew G, Ilako D, Kimani K, et al. Prevalence of diabetic retinopathy in Jimma University Hospital, Southwest. Ethiopia Ethiop Med J. 2013;51(2):105–113.
15. Solomon SD, Goldberg MF. ETDRS grading of diabetic retinopathy: still the gold standard. Ophthalmic Res. 2019;62(4):185–190. doi:10.1159/000501372
16. Wilkinson C, Ferris III FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9):1677–1682. doi:10.1016/S0161-6420(03)00475-5
17. Marathe PH, Gao HX, Close KL. American diabetes association standards of medical care in diabetes. J Diabetes. 2017;9(4):320–324. doi:10.1111/1753-0407.12524
18. Teshome DF, Demssie AF, Zeleke BM. Determinants of blood pressure control amongst hypertensive patients in Northwest Ethiopia. PLoS One. 2018;13(5):196–535. doi:10.1371/journal.pone.0196535
19. Li Z, Guo X, Bai Y, et al. The association between alcohol consumption and left ventricular ejection fraction: an observational study on a general population. Medicine. 2016;95(21):3763. doi:10.1097/MD.0000000000003763
20. Zhang G, Chen H, Chen W, et al. Prevalence and risk factors for diabetic retinopathy in China: a multi-hospital-based cross-sectional study. Br J Ophthalmol. 2017;101(12):1591–1595.
21. Machingura PI, Macheka B, Mukona M, et al. Prevalence and risk factors associated with retinopathy in diabetic patients at Parirenyatwa Hospital outpatients’ clinic in Harare, Zimbabwe. Arch Med Biomed Res. 2017;3(2):104–111. doi:10.4314/ambr.v3i2.6
22. Magan T, Pouncey A, Gadhvi K, et al. Prevalence and severity of diabetic retinopathy in patients attending the endocrinology diabetes clinic at Mulago Hospital in Uganda. Diab Res Clin Pract. 2019;152:65–70. doi:10.1016/j.diabres.2019.04.024
23. Huang OS, Tay WT, Ong PG, et al. Prevalence and determinants of undiagnosed diabetic retinopathy and vision-threatening retinopathy in a multiethnic Asian cohort. Br J Ophthalmol. 2015;99(12):1614–1621. doi:10.1136/bjophthalmol-2014-306492
24. Bellemo V, Lim ZW, Lim G, et al. Artificial intelligence using deep learning to screen for referable and vision-threatening diabetic retinopathy in Africa: a clinical validation study. Lancet Digit Health. 2019;1(1):35–44. doi:10.1016/S2589-7500(19)30004-4
25. Bursell S-E, Fonda SJ, Lewis DG, et al. Prevalence of diabetic retinopathy and diabetic macular edema in a primary care-based teleophthalmology program for American Indians and Alaskan Natives. PLoS One. 2018;13(6):198551. doi:10.1371/journal.pone.0198551
26. Cleland CR, Burton MJ, Hall C, et al. Diabetic retinopathy in Tanzania: prevalence and risk factors at entry into a regional screening programme. Trop Med Int Health. 2016;21(3):417–426. doi:10.1111/tmi.12652
27. Sapkota R, Chen Z, Zheng D, et al. The profile of sight-threatening diabetic retinopathy in patients attending a specialist eye clinic in Hangzhou, China. BMJ Open Ophthalmol. 2019;4(1):236. doi:10.1136/bmjophth-2018-000236
28. Damato EM, Murray N, Szetu J, et al. Sight-threatening diabetic retinopathy at presentation to screening services in Fiji. Ophthalmic Epidemiol. 2014;21(5):318–326. doi:10.3109/09286586.2014.949781
29. Burgess P, Allain T, García‐Fiñana M, et al. High prevalence in Malawi of sight‐threatening retinopathy and visual impairment caused by diabetes. Diabet Med. 2014;31(12):1643–1650. doi:10.1111/dme.12492
30. Ondrejkova M, Jackuliak P, Martinka E, et al. Prevalence and epidemiological characteristics of patients with diabetic retinopathy in Slovakia. PLoS One. 2019;14(12):223788. doi:10.1371/journal.pone.0223788
31. Alemu S, Dessie A, Tsegaw A, et al. Retinopathy in type 1 diabetes mellitus: major differences between rural and urban dwellers in northwest Ethiopia. Diabetes Res Clin Pract. 2015;109(1):191–198. doi:10.1016/j.diabres.2015.04.010
32. Jansson RW, Hufthammer KO, Krohn J. Diabetic retinopathy in type 1 diabetes patients in Western Norway. Acta Ophthalmol. 2018;96(5):465–474.
33. Thapa R, Joshi DM, Rizyal A, et al. Prevalence, risk factors and awareness of diabetic retinopathy among admitted diabetic patients at a tertiary level hospital in Kathmandu. Nepal J Ophthalmol. 2014;6(1):24–30. doi:10.3126/nepjoph.v6i1.10760
34. Singh L, Nigam B, Pathak K, et al. Prevalence and pattern of macular edema in diabetes. JMSCR. 2016;4(11):13891–13897. doi:10.18535/jmscr/v4i11.63
35. Al-Rubeaan K, Youssef AM, Subhani SN, et al. Diabetic retinopathy and its risk factors in a society with a type 2 diabetes. PLoS One. 2014;9(2):88956. doi:10.1371/journal.pone.0088956
36. Sultan S, Fawwad A, Siyal NA, et al. Frequency and risk factors of diabetic retinopathy in patients with type 2 diabetes presenting at a tertiary care hospital. Int J Diabetes Dev Ctries. 2020;40(1):87–92. doi:10.1007/s13410-019-00756-9
37. Stram DA, Jiang X, Varma R, et al. Factors associated with prevalent diabetic retinopathy in Chinese Americans: the Chinese American Eye. Study Ophthalmol Retina. 2018;2(2):96–105. doi:10.1016/j.oret.2017.05.014
38. Kovarik JJ, Eller AW, Willard LA, et al. Prevalence of undiagnosed diabetic retinopathy among inpatients with diabetes: the diabetic retinopathy inpatient study (DRIPS). BMJ Open Diabetes Res Care. 2016;4:1. doi:10.1136/bmjdrc-2015-000164
39. Mannam M, Nalluri L, Pingili R, et al. Assessment of drug utilization pattern, prevalence and risk factors for the development of diabetic retinopathy among type 2 diabetic patients in a south indian tertiary care hospital: a cross-sectional observational study. Int J Res Pharm Sci. 2020;11(2):2383–2398. doi:10.26452/ijrps.v11i2.2229
40. Ibrahim MKM. Social risk factors of diabetic retinopathy among sudanese diabetic patients in Khartoum-Sudan: hospital based cross-sectional study. Int J Public Health Epidemiol. 2017;5(9):301–304.I.
41. Jingi AM, Noubiap JJN, Ellong A, et al. Epidemiology and treatment outcomes of diabetic retinopathy in a diabetic population from Cameroon. BMC Ophthalmol. 2014;14(1):1–5. doi:10.1186/1471-2415-14-19
42. Kastelan S, Tomic M, Gverovic Antunica A, et al. Body mass index: a risk factor for retinopathy in type 2 diabetic patients. Mediators Inflamm. 2013;20(13):8.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
