Back to Journals » Clinical Ophthalmology » Volume 15
Short-Term Therapeutic Effects of Topical Corticosteroids on Refractory Dry Eye Disease: Clinical Usefulness of Matrix Metalloproteinase 9 Testing as a Response Prediction Marker
Authors Ryu KJ, Kim S, Kim MK, Paik HJ, Kim DH
Received 1 January 2021
Accepted for publication 5 February 2021
Published 22 February 2021 Volume 2021:15 Pages 759—767
DOI https://doi.org/10.2147/OPTH.S300047
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Kyung Jae Ryu,1 Seunghoon Kim,2 Mee Kum Kim,3 Hae Jung Paik,1 Dong Hyun Kim1
1Department of Ophthalmology, Gil Medical Center, Gachon University College of Medicine, Incheon, Korea; 2RudaCure Co., LTD, Incheon, Korea; 3Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea
Correspondence: Dong Hyun Kim
Department of Ophthalmology, Gachon University College of Medicine, Gil Medical Center, 1198, Guwol-dong, Namdong-Gu, Incheon, 21565, Korea
Email [email protected]
Purpose: To compare the short-term therapeutic effects of topical corticosteroids in patients with refractory dry eye disease (DED) according to the tear matrix metalloproteinase 9 (MMP-9) point-of-care positivity.
Methods: This study was conducted on 137 patients who were referred from other eye clinics, complaining of irresponsive DED or acute DED flares despite routine treatment with topical cyclosporin or diquafosol. The patients received treatment with topical corticosteroids for 1 month. DED was evaluated by SANDE (Symptom Assessment in Dry Eye) questionnaire, tear film breakup time, ocular surface staining score, and meibomian gland dysfunction stage. The InflammaDry MMP-9 immunoassay was conducted in more symptomatic eyes of all patients. The changes in the subjective symptoms were additionally surveyed as symptom improvement score.
Results: The mean age of the patients was 57.8± 13.4 years, and the tear MMP-9 positivity was 73.0%. Topical corticosteroids treatment showed significant improvement of symptoms and signs in the patients with refractory DED irrespective of the positivity of MMP-9 (each p< 0.001). The changes in SANDE score and OSS, and symptom improvement score were higher in the MMP-9 positive group than in the MMP-9 negative group (p=0.002/0.010/0.011). The overall rates of subjective symptoms improvement and SANDE reduction were 73.0% and 90.6% after topical corticosteroids treatment, respectively.
Conclusion: Short-term topical corticosteroids had excellent therapeutic effects in patients with refractory DED or acute DED flares, irrespective of the tear MMP-9 level. Tear MMP-9 positivity may serve as a reliable response predictor of topical corticosteroids treatment in DED.
Keywords: dry eye disease, ocular surface inflammation, tear MMP-9 positivity, corticosteroid
Introduction
Dry eye disease (DED) is a multifactorial disease of the ocular surface, characterized by a loss of homeostasis of the tear film that is accompanied by ocular symptoms.1 DED is frequently associated with the instability and hyperosmolarity of tear films, inflammation of the ocular surface, and neurosensory abnormalities.1,2 DED is one of the most prevalent ocular diseases worldwide, and significantly reduces the quality of life.
The inflammation of the ocular surface is a key component of the pathophysiology of DED. Owing to the role of inflammation in DED, numerous novel therapeutic agents have been investigated for inhibiting various inflammatory cascades.3–5 To date, the most well-known anti-inflammatory agents approved by the US Food and Drug Administration (FDA) for the management of DED include cyclosporin A (CsA), lifitegrast (LG), corticosteroid.6 corticosteroids are widely used for the treatment of both acute and chronic inflammation, and topical corticosteroids are generally indicated for the treatment of ocular inflammatory diseases.7,8 corticosteroids inhibit pro-inflammatory cytokines and chemokines, stabilize macrophages and neutrophils, and repress the key enzymes involved in the initiation or maintenance of the inflammatory response.8 Therefore, corticosteroids are generally thought to be more potent than other therapeutic agents for DED, including CsA, LG, and diquafosol, owing to acting on the multiple mechanisms by which corticosteroids inhibit inflammation. Topical corticosteroids are also effective in controlling the signs and symptoms of chronic, severe DED associated with the Sjogren syndrome, and in the treatment of moderate and severe meibomian gland dysfunction (MGD).9,10 Additionally, short-term induction therapy before long-term treatment with CsA, or combined therapy with corticosteroids and CsA, shows greater symptomatic relief and faster recovery from the signs of DED, compared to monotherapy with CsA.11,12
Matrix metalloproteinase 9 (MMP-9) participates in the degradation of the extracellular matrix and the loss of epithelial cells at the tight junctions. MMP-9 also triggers the release of inflammatory cytokines and the mitogen-activated protein kinase (MAPK)/Nuclear Factor-κB (NF-κB) signaling pathways, in addition to inducing the maturation of antigen-presenting cells (APCs).13 Desiccating stress and tear hyperosmolarity are known to be associated with increased levels of MMP-9 in the ocular surface, as observed in an experimental model of DED, and the increased levels of MMP-9 induce a vicious cycle of inflammation in the ocular surface in DED.14,15 InflammaDry (Rapid Pathogen Screening, Inc., Sarasota, FL) is a disposable, single-use, non-invasive, in-office assay that allows the measurement of the tear levels of MMP-9.16 Several studies demonstrated that the InflammaDry assay is helpful and provides consistent results for the diagnosis of DED, but other studies presented that tear MMP-9 positivity was not related with DED parameters.17–19 Recently, ASCRS Cornea Clinical Committee recommended the measurement of the tear levels of MMP-9 as a method for screening patients with ocular surface disease.20 Considering the relationship between the tear levels of MMP-9 and DED, it is possible to assume that the tear levels of MMP-9 may serve as a predictive biologic marker about the response to anti-inflammatory treatment in DED.
To the best of our knowledge, the therapeutic effects of topical corticosteroids on DED have not been previously compared according to the tear MMP-9 positivity. In this study, we compared the short-term therapeutic effects of topical corticosteroids according to tear MMP-9 level in patients with DED who showed no improvement despite previous treatments with artificial tears, topical CsA, and DQA.
Methods
Study Design and Subjects
A retrospective chart review was conducted at the cornea specialty clinic of Gachon University Gil Medical Center. The study protocol was approved by the institutional review board of Gachon University Gil Medical Center, and a waiver for collecting the informed consent along with authorization for using research information from the patients was also obtained from the review board (IRB number: GCIRB2019-388). Privacy data of all patients were not included in the analysis. The protocol complied with the tenets of the Declaration of Helsinki. The subjects who participated in this study enrolled between August 1, 2018, and August 12, 2020. Patients with DED who were aged ≥19 years were enrolled in this study in accordance with the diagnostic guidelines of the Korean Corneal Disease Study Group and TFOS DEWS II.1,2 The enrolled patients had been previously diagnosed with DED and referred from other eye clinics because there had been no improvement or acute exacerbation with topical CsA, DQA, and artificial tears. Only patients who had used CsA or DQA for at least 1 month or longer, but DED symptoms were not improved, or had had poor compliance due to the irritation feeling from those eyedrops, were included in this study. Patients with evidence of any active ocular infection, eyelid deformity, corneal opacity, or limbal stem cell deficiency were excluded from the study. The additional exclusion criteria were ocular surgery within 6 months of the study visit, ongoing pregnancy, and punctal plug placement within 3 months of testing. Subjects who used topical corticosteroids within 30 days of the study visit were also excluded. All the patients were administered topical corticosteroids and artificial tears, 4 times a day for 1 month [0.1% fluorometholone without preservative (Fumelon®; Hanlim Pharm. Co., Seoul, South Korea) + 0.15% sodium hyaluronate (New Hyaluni®, Taejoon Pharm Co., Seoul, South Korea) or 0.5% loteprednol etabonate (Lotepro®; Hanlim Pharm. Co., Seoul, South Korea) + 0.15% sodium hyaluronate (New Hyaluni®, Taejoon Pharm Co., Seoul, South Korea)]. The patients with MGD were recommended to perform warm compress and maintain eyelid hygiene.
At each visit, DED parameters of all patients were evaluated by an experienced ophthalmologist (DH Kim). The Symptom Assessment in Dry Eye (SANDE) questionnaire was conducted for evaluating the symptoms related to DED.21 The tear film break-up time (TBUT), tear secretion (Schirmer’s test without anesthesia), and ocular staining score (OSS) were also measured. The OSS was determined using the oxford scheme scale (0–5 points).22 MGD was classified into 5 stages (stages 0–4) according to the guideline prescribed by the International MGD workshop.22 The DED parameters were evaluated in the eye in which the patient felt more discomfort, and the right eye was evaluated if the symptoms were similar in both the eyes. Further surveys were conducted for simplifying the improvement in the subjective symptoms before and after topical corticosteroids treatment. Scores of 0, 1, and 2 indicated no improvement, partial improvement (better than before, but a little discomfort left), and complete improvement (no discomfort due to a greater improvement than before) after treatment, respectively. At each visit, the IOP was measured for all the patients with a pneumatic applanation tonometer (KOWA KT-800, Shizuoka, Japan). The patients visited the hospital after 1 month of treatment for a follow-up examination. The question whether warm compression and lid hygiene were performed after 1 month.
MMP-9 Point-of-Care Immunoassay
The tear levels of MMP-9 were detected using InflammaDry (Rapid Pathogen Screening, Inc., Sarasota, FL).23 For collecting the tear samples, a fleece was gently dabbed at 6–8 locations along the palpebral conjunctiva until a sufficient tear sample was obtained. The sample collector was firmly pressed to snap the test cassette body. An absorbent tip was immersed in a buffer vial for 20 seconds. The cap was replaced and the test cassette was laid flat on a horizontal surface for 10 minutes. After 10 minutes, the result window was evaluated for obtaining the levels of MMP-9 in the tear film. A result window without a blue line was considered to be invalid. A result window with a blue line was considered to be negative, while a red line indicated positive results. When a result window without a red line was obtained, the test cassettes were allowed to incubate for an additional 5 min before confirming the results and was considered negative when the red line did not appear. For the samples that showed positive results, the red line was compared with the grading index for classifying the result as trace-positive (grade 1), weakly positive (grade 2), positive (grade 3), and strong positive (grade 4), according to the manufacturer’s instructions. The grading index of InflammaDry was interpreted by an experienced ophthalmologist (DH Kim).
Statistical Analyses
The data are presented as the mean ± standard deviation (SD). Chi-square tests or independent t-tests were performed for comparing the SANDE score, TBUT, OSS, and MGD stage between the MMP-9 positive and MMP-9 negative groups. Paired t-tests were used for analyzing the differences within the groups. The Mann–Whitney U-test was performed in the additional subgroup analyses. All the statistical analyses were performed using SPSS Complex Samples procedures (PASW Statistics for Windows, version 21.0, SPSS, IMB Corp., Armonk, NY, USA). Differences with P<0.05 were considered to be statistically significant.
Results
There were 137 patients enrolled in this study. The baseline characteristics of the enrolled patients at the first visit are summarized in Table 1. The study population comprised 34 men and 103 women with a mean age of 57.8±13.4 years. The number of MMP-9 positive and negative patients were 100 and 37 and the rate of MMP-9 positivity was 73.0%. The InflammaDry MMP-9 immunoassay was conducted in more symptomatic eyes (137 eyes) of all patients. The age, sex, SANDE score, TBUT, OSS, tear secretion, MGD stage at the first visit did not differ significantly between 2 groups. (p>0.05) Mean SANDE score in all patients were 81.9±15.4. In 49 MMP-9 positive patients, 21 were trace-positive (grade 1), 56 were weakly positive (grade 2), 18 were positive (grade 3), and 5 individuals were strong positive (grade 4). The numbers of patients who treated with 0.5% loteprednol etabonate and 0.1% fluorometholone, were 23 and 77 (loteprednol/fluorometholone) in MMP-9 positive group and 7 and 30 in MMP-9 negative group, respectively. The patients with DED were previously treated with CsA+AT or DQA+AT or only AT. The proportion of previously used drug in the MMP-9 positive and negative groups did not differ significantly (p>0.05, Table 1). A correlation analysis between the grade of MMP-9 and the parameters of DED revealed that the OSS at the initial visit was related to the grade of MMP-9 (R=0.355, p<0.001), but SANDE, TBUT, and MGD stage were not related to the grade of MMP-9 (for each, p>0.05, Supplementary Table 1). Lid hygiene compliance rates were 52.0% and 51.3% in MMP-9 positive and negative groups, respectively (p=0.946).
 |
Table 1 Baseline Characteristics of Patients with DED at First Visit |
Table 2 demonstrates the therapeutic effects of treatment with topical corticosteroids for 1 month in all the enrolled subjects. Treatment with topical corticosteroids significantly improved the SANDE score, OSS, and MGD stage, and increased the TBUT in all patients (each p<0.001). Even when the patients were divided into the MMP-9 positive and MMP-9 negative groups, all the parameters for DED and MGD stage also improved significantly regardless of MMP-9 positivity in the tear [MMP-9 positive: SANDE: 80.7±14.3 to 54.3±20.2, TBUT: 3.2±1.4 to 4.6±1.1, OSS: 0.7±0.7 to 0.3±0.5, and MGD stage: 1.5±0.7 to 0.9±0.6; for each test, P<0.001; MMP-9 negative: SANDE: 75.1±24.8 to 53.7±27.4, TBUT: 2.8±1.3 to 4.4±1.2, OSS: 0.4±0.5 to 0.2±0.5, and MGD stage: 1.7±0.8 to 1.0±0.6; for each test, p<0.05]. The IOP at the first and follow-up visits were 10.8±2.7 and 10.9±2.5 mmHg, respectively (P=0.137). There was no patient whose IOP elevated more than 21mmHg during treatment with topical corticosteroids.
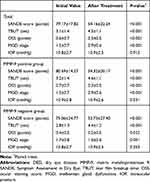 |
Table 2 Comparison of the Parameters of DED in Patients Between Pre-Treatment and Post-Treatment with Topical corticosteroids |
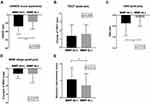
Figure 1 depicts the changes in SANDE score, TBUT, OSS, MGD stage, and subjective symptom change score between the MMP-9 positive and negative groups. The changes of SANDE score (MMP-9 positive: −27.44±19.14; MMP-9 negative: −16.99±17.14; P=0.002) and OSS change (MMP-9 positive: −0.4±0.6; MMP-9 negative: −0.6±1.0; P=0.010) in the MMP-9 positive group were significantly greater than in MMP-9 negative group [(MMP-9 positive/negative) – SANDE: −27.44±19.14/-16.95±17.13 (p=0.002), OSS: −0.4±0.6/-0.1±0.4 (p=0.010)]. However, the TBUT and MGD stage was significantly different between 2 groups (each p>0.05), although all the parameters for DED significantly improved in both the MMP-9 positive and MMP-9 negative groups (Table 2). The subjective symptom improvement score was significantly higher in the MMP-9 positive group compared to that of the MMP-9 negative group (MMP-9 positive: 1.1±0.7; MMP-9 negative: 0.7±0.7; P=0.011). The overall subjective symptoms improvement rate (symptom improvement score≥1) was 74.5% after topical corticosteroids treatment in overall refractory DED patients (Figure 2A). The improvement rate was 79.0% and the complete improvement rate was 27.0% in the MMP-9 positive group. In contrast, the overall improvement rate was 62.1% and the complete improvement rate was 16.2% in the MMP-9 negative group. (p=0.041, Figure 2A, Supplementary Table 2) The overall reduction rate in SANDE score was 90.5% after corticosteroids treatment. The reduction rate was 94.0% and 81.1% in the MMP-9 positive and negative groups, respectively. (p=0.022, Figure 2B, Supplementary Table 2)
Table 3 compares the therapeutic effects of 0.5% loteprednol etabonate and 0.1% fluorometholone. The changes in the TBUT, OSS, and MGD stages did not differ significantly between the groups (p>0.05). The SANDE score in the loteprednol group had much reduced compared to the fluorometholone group (loteprednol: −31.15±21.13; fluorometholone: −24.0±19.5, p=0.078). Similarly, the symptom improvement score was significantly higher in the loteprednol groups (loteprednol: 1.3±0.8; fluorometholone: 0.9±0.7, p=0.021). In the MMP-9 positive group, the improvement in the OSS (loteprednol: −0.2±0.4; fluorometholone: −0.4±0.6; P=0.042) and symptom improvement score (loteprednol: 1.3±0.8; fluorometholone: 1.0±0.7; p=0.042) were higher in the loteprednol group. However, the other parameters were not different between the 2 groups (for each group, p>0.05). In the MMP-9 negative subjects, the TBUT in the loteprednol group was higher than in the fluorometholone group. (loteprednol: 2.5±1.2; fluorometholone: 1.4±0.9; p=0.031)
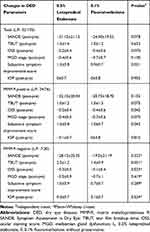 |
Table 3 Comparison of the Therapeutic Effects of Topical Corticosteroids Between 0.5% Loteprednol Etabonate and 0.1% Fluorometholone |
Supplementary Table 3 compares the therapeutic effects of topical corticosteroids based on the grade of MMP-9 (grades 1 and 2 versus grades 3 and 4). The all parameters of DED did not differ significantly between 2 groups. (each p>0.05)
Discussion
This retrospective clinical study demonstrated that short-term treatment with topical corticosteroids shows excellent therapeutic effects in patients with refractory DED who had previously failed on regular treatment with CsA, DQA, and AT, irrespective of the tear MMP-9 positivity. The improvement rate in the symptoms of DED was much higher in the MMP-9 positive group (79.0%) than in the MMP-9 negative group (62.1%), but the OSS improved better in the MMP-9 positive group. The therapeutic effects of 0.5% loteprednol were slightly higher than those of 0.1% fluorometholone especially in symptom improvement. There was no significant elevation of intraocular pressure by usage of topical corticosteroids for 1 month.
The tear levels of MMP-9 have been recently recognized as a potential inflammatory biomarker for DED, and the InflammaDry test is widely used for evaluating the tear levels of MMP-9 in patients with DED.20,24,25 There are numerous studies on the clinical results of inflammatory tests and their correlation with DED. Sambursky et al reported that the tear levels of MMP-9 are significantly higher in the patients with DED than in control subjects.17 Aragona et al also reported that there is a significant correlation between the levels of MMP-9 and several parameters of DED in patients with MGD or the Sjogren syndrome.26 However, Lanza et al reported that there were no significant differences in the signs and symptoms of DED between MMP-9 positive (39%) and MMP-9 negative groups (61%).19 Previous studies have demonstrated that MMP-9 positivity of InflammaDry test were 39, 40% and 77.5% in Europe, U.S, and South Korea (study from other teams), respectively.17,19,27 A MMP-9 positivity rate in this study was 73.0%. This higher MMP-9 positivity appears to be attributed to the higher prevalence of DED in Asians. Additionally, the positive results obtained in the InflammaDry test may vary according to the loading tear volumes.28 The baseline characteristics and DED parameters were similar between the MMP-9 positive and MMP-9 negative groups in this study, and those were similar with the results reported by Lanza et al.19 In our study, the grade of MMP-9 was only correlated with OSS, while the other studies demonstrated a significant correlation between the levels of MMP-9 and the overall DED parameters, including DED symptoms, TBUT, and OSS.26,27 OSS are known to be related with ocular surface inflammation in DED and our results are matched with this.29 Interestingly, topical corticosteroids, which is a potent anti-inflammatory agent used in treating DED, showed a greater improvement in the symptoms of DED in the MMP-9 positive group, compared to that of the MMP-9 negative group in this study. Our study also demonstrated that treatment with topical corticosteroids improved the symptoms of DED even in the MMP-9 negative group, although the rate of improvement was lower than that of the MMP-9 positive group. This could imply that the inflammation of the ocular surface is somewhat etiological even in MMP-9 negative patients. Although there are several factors for adjusting and verifying the InflammaDry test, we believe that the tear MMP-9 positivity may serve as a reliable biomarker in therapeutic response with topical corticosteroids in patients with DED.
corticosteroids are potent immunosuppressive agents. The therapeutic effects of corticosteroids are mediated by the transactivation and transrepression of gene transcription via the glucocorticoid receptor, and most immune cells are affected by corticosteroids.30,31 corticosteroids attenuates the innate immune response by inhibiting the activation and differentiation of APCs and reducing the secretion of pro-inflammatory cytokines. Additionally, corticosteroids reduce the activation of neutrophil and B cell, and modulate T cell polarization and apoptosis.30,31 These anti-inflammatory activities of corticosteroids are mediated via its interactions with numerous molecules involved in intracellular signaling transduction, including JNK, STAT, MAPK, NF-kB, and guanine nucleotide-binding proteins (G proteins).30,31 The exposure of epithelial cells in the ocular surface to desiccating stress also activates several signal transduction molecules, and elevates the levels of proinflammatory cytokines, chemokines, and MMPs.15,32 These mediators promote the maturation of APCs, and the APCs trigger the expansion of the helper T cell subtypes 1 and 17 (afferent arm activation).15,32 The activated T cells infiltrate the ocular surface, where additional proinflammatory cytokines cause further epitheliopathy of the ocular surface, and stimulate a vicious inflammatory cycle (efferent arm activation).15,32 This is the pathophysiology of DED as we know to date. As topical corticosteroids can efficiently inhibit both the afferent and efferent arms in patients with DED, it appears to have better therapeutic effects compared to the other drugs, including CsA, LG, DQA, and AT. Sheppard et al demonstrated that induction therapy with topical corticosteroids for 2 weeks before treatment with CsA provides a more rapid relief from the signs and symptoms of DED compared to treatments with CsA and AT.12 Lee et al also demonstrated the efficacy of topical corticosteroids for moderate and severe MGD.10 A recent study also demonstrated that combination therapy with topical corticosteroids and CsA is more effective than monotherapy with topical CsA in patients with moderate DED.11 Our study also demonstrated the excellent therapeutic benefits from corticosteroids in patients with DED who showed no improvement despite routine treatments. During the topical corticosteroids treatment for 1 month, no patient showed significant intraocular pressure elevation. Therefore, topical treatment with corticosteroids can be useful as a short-term rescue therapy in acute DED flares or refractory DED, although the long-term usage of topical corticosteroids is clinically restricted owing to several complications. Additionally, safer and more potent anti-inflammatory agents are necessary for substituting the topical corticosteroids for a more effective treatment of DED. We recently reported that topical 8-oxo-2ʹ-deoxyguanosine promotes corneal epithelial healing in a dose-dependent manner and shows excellent anti-inflammatory effects equivalent to that of 1% prednisolone acetate in ethanol injury and alkali burn models, respectively.33,34 Therefore, 8-oxo-2ʹ-deoxyguanosine is expected to be a promising therapeutic candidate for DED.
This study has a few limitations. First, the study design was retrospective. Second, the sample size was small. Third, the other clinical factors related to DED were not adjusted during analysis. Fourth, we did not perform a molecular assay for measuring the inflammation of the ocular surface. Fifth, the patients that used LG for the treatment of DED could not be included in this study because LG is not yet approved in Korea. Sixth, tear MMP-9 and secretion were not measured after topical corticosteroids treatment. Seventh, tear MMP-9 tests were not performed in both eyes. Despite these limitations, this study is significant due in the fact that it is the first study to compare the therapeutic effects of topical corticosteroids according to the tear MMP-9 positivity. Additionally, this study also demonstrated that topical corticosteroids significantly improved the symptoms and signs of DED in only patients who were refractory to other treatment or patients in acute DED flares.
In conclusion, topical corticosteroids showed excellent short-term therapeutic effects in patients with refractory DED in both the MMP-9 positive and negative groups. The subjective symptom improvement rate was much higher in the MMP-9 positive group than in the MMP-9 negative group. Tear MMP-9 positivity may serve as a potential response predictor about topical corticosteroids treatment in DED, and topical corticosteroids may serve as a useful as a short-term rescue therapy for irresponsive DED or acute DED flares. Further investigations are necessary for the development of safe and potent anti-inflammatory agents for substituting the topical corticosteroids.
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT (Grant number: NRF-2020R1C1C1007372).
Disclosure
None of the authors have any proprietary or conflicting interests in any of the methods or materials described in this article. Seunghoon Kim is an employee of RudaCure Co., LTD. The authors report no other potential conflicts of interest for this work.
References
1. Hyon JY, Kim HM, Lee D, et al. Korean guidelines for the diagnosis and management of dry eye: development and validation of clinical efficacy. Korean J Ophthalmol. 2014;28(3):197–206. doi:10.3341/kjo.2014.28.3.197
2. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283.
3. Perez VL, Pflugfelder SC, Zhang S, Shojaei A, Haque R. Lifitegrast, a novel integrin antagonist for treatment of dry eye disease. Ocul Surf. 2016;14(2):207–215. doi:10.1016/j.jtos.2016.01.001
4. McCabe E, Narayanan S. Advancements in anti-inflammatory therapy for dry eye syndrome. Optometry. 2009;80(10):555–566. doi:10.1016/j.optm.2009.02.010
5. Calonge M, Enríquez-de-salamanca A, Diebold Y, et al. Dry eye disease as an inflammatory disorder. Ocul Immunol Inflamm. 2010;18(4):244–253. doi:10.3109/09273941003721926
6. Yang Y, Huang C, Lin X, et al. 0.005% preservative-free latanoprost induces dry eye-like ocular surface damage via promotion of inflammation in mice. Invest Ophthalmol Vis Sci. 2018;59(8):3375–3384. doi:10.1167/iovs.18-24013
7. Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicenter comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138(3):444–457. doi:10.1016/j.ajo.2004.04.052
8. Coutinho AE, Chapman KE. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol. 2011;335(1):2–13. doi:10.1016/j.mce.2010.04.005
9. Jung HH, Ji YS, Sung MS, Kim KK, Yoon KC. Long-term outcome of treatment with topical corticosteroids for severe dry eye associated with Sjogren’s syndrome. Chonnam Med J. 2015;51(1):26–32. doi:10.4068/cmj.2015.51.1.26
10. Lee H, Chung B, Kim KS, Seo KY, Choi BJ, Kim TI. Effects of topical loteprednol etabonate on tear cytokines and clinical outcomes in moderate and severe meibomian gland dysfunction: randomized clinical trial. Am J Ophthalmol. 2014;158(6):1172–1183.e1171. doi:10.1016/j.ajo.2014.08.015
11. Singla S, Sarkar L, Joshi M. Comparison of topical cyclosporine alone and topical loteprednol with cyclosporine in moderate dry eye in Indian population: a prospective study. Taiwan J Ophthalmol. 2019;9(3):173–178. doi:10.4103/tjo.tjo_15_18
12. Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40(5):289–296. doi:10.1097/ICL.0000000000000049
13. Tseng HC, Lee IT, Lin CC, et al. IL-1β promotes corneal epithelial cell migration by increasing MMP-9 expression through NF-κB- and AP-1-dependent pathways. PLoS One. 2013;8(3):e57955. doi:10.1371/journal.pone.0057955
14. Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006;47(8):3293–3302. doi:10.1167/iovs.05-1382
15. Stern ME, Pflugfelder SC. Inflammation in dry eye. Ocul Surf. 2004;2(2):124–130. doi:10.1016/S1542-0124(12)70148-9
16. Messmer EM, von Lindenfels V, Garbe A, Kampik A. Matrix metalloproteinase 9 testing in dry eye disease using a commercially available point-of-care immunoassay. Ophthalmology. 2016;123(11):2300–2308. doi:10.1016/j.ophtha.2016.07.028
17. Sambursky R, Davitt WF
18. Sambursky R, Davitt WF
19. Lanza NL, McClellan AL, Batawi H, et al. Dry eye profiles in patients with a positive elevated surface matrix metalloproteinase 9 point-of-care test versus negative patients. Ocul Surf. 2016;14(2):216–223. doi:10.1016/j.jtos.2015.12.007
20. Starr CE, Gupta PK, Farid M, et al. An algorithm for the preoperative diagnosis and treatment of ocular surface disorders. J Cataract Refract Surg. 2019;45(5):669–684. doi:10.1016/j.jcrs.2019.03.023
21. Mathers WD. Ocular evaporation in meibomian gland dysfunction and dry eye. Ophthalmology. 1993;100(3):347–351. doi:10.1016/S0161-6420(93)31643-X
22. Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. doi:10.1097/00003226-200310000-00008
23. Nichols KK, Foulks GN, Bron AJ, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52(4):1922–1929. doi:10.1167/iovs.10-6997a
24. Lanza NL, Valenzuela F, Perez VL, Galor A. The matrix metalloproteinase 9 point-of-care test in dry eye. Ocul Surf. 2016;14(2):189–195. doi:10.1016/j.jtos.2015.10.004
25. Baudouin C, Irkec M, Messmer EM, et al. Clinical impact of inflammation in dry eye disease: proceedings of the ODISSEY group meeting. Acta Ophthalmol. 2018;96(2):111–119. doi:10.1111/aos.13436
26. Aragona P, Aguennouz MH, Rania L, et al. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology. 2015;122(1):62–71. doi:10.1016/j.ophtha.2014.07.048
27. Park JY, Kim BG, Kim JS, Hwang JH. Matrix metalloproteinase 9 point-of-care immunoassay result predicts response to topical cyclosporine treatment in dry eye disease. Transl Vis Sci Technol. 2018;7(5):31. doi:10.1167/tvst.7.5.31
28. Huh J, Choi SY, Eom Y, Kim HM, Song JS. Changes in the matrix metalloproteinase 9 point-of-care test positivity according to MMP-9 concentration and loading volume. Cornea. 2020;39(2):234–236. doi:10.1097/ICO.0000000000002096
29. Yang S, Lee HJ, Kim D, et al. The use of conjunctival staining to measure ocular surface inflammation in patients with dry eye. Cornea. 2020;38(6):698–705. doi:10.1097/ICO.0000000000001916
30. Ramamoorthy S, Cidlowski JA. Corticosteroids: mechanisms of action in health and disease. Rheum Dis Clin North Am. 2016;42(1):15–31. doi:10.1016/j.rdc.2015.08.002
31. Ferrara G, Petrillo MG, Giani T, et al. Clinical use and molecular action of corticosteroids in the pediatric age. Int J Mol Sci. 2019;20(2):444. doi:10.3390/ijms20020444
32. Stevenson W, Chauhan SK, Dana R. Dry eye disease: an immune-mediated ocular surface disorder. Arch Ophthalmol. 2012;130(1):90–100. doi:10.1001/archophthalmol.2011.364
33. Im S-T, Kim HY, Yoon JY, et al. Therapeutic effects of topical 8-Oxo-2ʹ-deoxyguanosine on ethanol-induced ocular chemical injury models. Cornea. 2018;37(10):1311–1317. doi:10.1097/ICO.0000000000001671
34. Kim DH. Comparison of the therapeutic effects between topical 8-oxo-2′-deoxyguanosine and steroid in ocular chemical burn experimental model. J Immunol. 2020;204:235–239.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.