Back to Journals » Open Access Surgery » Volume 14
Short-Term and Mid-Term Outcomes of Video-Assisted Thoracic Surgery in Patients with Early-Stage Non-Small Cell Lung Cancer
Authors Nguyen Van N, Hung PN , Dung LT, Anh LV , Pho DC , Anh BDT , Hai VA
Received 8 May 2021
Accepted for publication 1 July 2021
Published 23 July 2021 Volume 2021:14 Pages 29—36
DOI https://doi.org/10.2147/OAS.S315389
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Luigi Bonavina
Nam Nguyen Van,1 Pham Ngoc Hung,2,3 Le Tien Dung,4 Le Viet Anh,1 Dinh Cong Pho,5 Bui Dang The Anh,2 Vu Anh Hai1
1Department of Thoracic Surgery, Military Hospital 103, Vietnam Military Medical University, Hanoi, Vietnam; 2Department of Epidemiology, Vietnam Military Medical University, Hanoi, Vietnam; 3Department of Training, Vietnam Military Medical University, Hanoi, Vietnam; 4Department of Thoracic Surgery, Phạm Ngọc Thạch Hospital, Ho Chi Minh City, Vietnam; 5Department of Infection Control, Military Hospital 103, Vietnam Military Medical University, Hanoi, Vietnam
Correspondence: Vu Anh Hai
Department of Thoracic Surgery, Military Hospital 103, Vietnam Military Medical University, Ha Dong District, Hanoi, Vietnam
Tel +84986112345
Email [email protected]
Background: Mid-term outcomes of video-assisted thoracoscopic surgery (VATS) lobectomy for early stage non-small cell lung cancer (NSCLC) in Vietnam should be evaluated and discussed.
Methods: This prospective descriptive study was conducted on 94 patients with NSCLC under stages I–IIA who were treated with VATS from November 2011 to July 2014.
Results: The median patient age was 55.5 ± 10.8 years. The rate of successful VATS for NSCLC treatment was 98.9%, and the conversion rate (from VATS to thoracotomy) was 1.1%. The operative time was 143.8 ± 38.9 minutes, the amount of blood loss was 194.8 ± 150.5 mL, and the postoperative complication rate was 10.6%. The ICU length stay was 2.8 ± 1.0 days, and the postoperative hospital length stay was 7.7 ± 2.1 days. Among the 89 patients with successful follow-up, one (1.1%) had a recurrent tumor, and ten (11.8%) had metastasis. The 1- and 2-year relative survival rates were 95.9% (3 deaths) and 80.8% (9 deaths), respectively.
Conclusion: VATS treatment for early stage NSCLC is an effective method with safe outcomes. The mid-term outcomes were acceptable with 1- and 2-year relative survival rates of 95.9% and 80.8%, respectively.
Keywords: video-assisted thoracoscopic surgery, VATS, non-small cell lung cancer, NSCLC, early-stage, short-term outcomes, mid-term outcomes
Introduction
Surgical therapy in the early stage of disease plays an important role in the multimodal therapy of lung cancer treatment. Among which, thoracotomy is an effective method, but its complication rate, extended hospital stay, and postoperative pain are still of foremost concern. Thoracoscopic surgery was developed to overcome these points and showed high safety and feasibility.1,2 However, video-assisted thoracic surgery (VATS) is inferior to thoracotomy as a NSCLC treatment regarding long-term outcomes and recurrence and overall survival rates.3–5 The reason is that in patients with NSCLC, VATS can accomplish difficult oncologic resection with restricted handling of surgical instruments.
Although the short-term outcomes of VATS are advantageous with respect to postoperative complications and hospital length stay, reports on its mid- and long-term outcomes are insufficient,6,7 especially in Vietnam. In this study, VATS as treatment for NSCLC in stages I and IIA was examined. The short- and mid-term results may provide meaningful information for NSCLC surgical treatment.
Materials and Methods
This prospective, descriptive study was conducted on 94 patients with NSCLC who were treated by VATS from November 2011 to July 2014. Inclusion criteria were as follows: patients with NSCLC diagnosed based on histopathological results (transbronchial biopsy, computed tomography [CT]-guided biopsy, VATS biopsy, and histopathological result after surgery) and appropriate information in the medical record and were treated by VATS. Exclusion criteria were as follows: patients treated by VATS who have a different histopathological result from NSCLC; those lacking information in the medical record; and patients who refused to participate in this study. The flowchart of this study is shown in Figure 1.
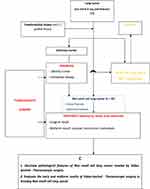 |
Figure 1 Flowchart of study. |
Research Procedure
((1) Clinical examinations and laboratory tests
(2) Indications of VATS8 lobectomy treating NSCLC:
+ NSCLC with the clinical stage is from the clinical stage from I to IIA.
+ Computed tomography image features tumor size < 6 cm without invasion and non-calcified hilar nodes.
(3) Preparing patients for surgery:
Patients are instructed on breathing exercises, nutrition, and personal hygiene prior to surgery. General anesthesia is applied, and selective lung ventilation is performed with double-lumen intubation in the lateral decubitus position. The surgical team consists of one leading surgeon and two assistant surgeons. A video monitor is positioned on the left of the operating table and slightly elevated at 15°–30° on the surgeon’s opposite side.
(4) Technique of VATS
Step 1: Trocar insertion.
Step 2: Evaluate injuries, including pleural space, pleural thickening classification, features of fissures, and tumor position.
Step 3: Lung biopsy, including tumor biopsy and wedge resection.
Step 4: Lobectomy and lymph node dissection.
- Pulmonary lobectomy: Technique of pulmonary lobectomy according to Gottsot D. (2010).9
- Lymph node dissection: Investigate and remove visible lymph nodes according to American Cancer Society guidelines.10
Step 5: Collecting samples, final checks, insert a chest tube (under thoracoscopic guidance), and close the incisions. In the Department of Pathology, specimens were handled following a procedure and identified according to the WHO recommended classification of NSCLC in 2004.11
(5) Postoperative care
- Regular monitoring for all patients. Chest tubes are connected to a drainage system device. Postoperative pain management is initiated after epidural analgesia in the operating room, by administering local anesthesia (Bupivacaine 0.5/20 mL and Sufentanyl 50 mg in NaCl 90/00) maintained using the patient-controlled analgesia device. When the patient is moved to the ward, epidural analgesia is replaced with oral Paracetamol 0.5 * 2 per day. Postoperative complications, such as hemorrhage, prolonged air leak, chylothorax, arrhythmia, atelectasis, and pneumothorax after drain removal, are managed.
After discharge, the follow-up regimen is in accordance with the guidance of ACCP (2007)12 and BTS (2010).13 Patients are checked at the following time points: 1, 3, 6, 12, 18, and 24 months after surgery and at the end of research in the outpatient clinic of Phạm Ngọc Thạch Hospital.
Data Analysis
Information of patients is filled in parameter estimates table of SPSS 16.0 statistical software. We are using statistical formulas. Analytics: (χ2) is used to compare the qualitative parameters in two groups. In case of the shortage of data or 1 in 4 parameters of 2×2 table less than 5, Fisher’s equation is used instead of (χ2). To evaluate a quantitative value: drain duration, length of stay of hospital after surgery. We use T - student, Mann–Whitney, and Kruskal–Wallis equations. Accreditation is significant (either difference or relations) if p < 0.05.
Ethics in Research
Approved by Ethical Committee of Pham Ngoc Thach Hospital.
Results
General Characteristics
The median age was 55.5 ± 10.8 years. The common symptoms were chest pain (59.8%), dry cough (43.6%), hemoptysis (20.2%), and locally decreased lung sounds (22.3%). Approximately 14.9% of patients had no Image in symptoms. CT images showed 100% peripheral tumors with 2.1/1 ratio of the right lung/the left lung. The largest tumor was 5 cm in diameter. Biopsy prior to pulmonary lobectomy by VATS can be classified as transbronchial biopsy (12.8%), trans-thoracic wall under computed tomography biopsy (46.8%), and during VATS (40.4%). Clinical TNM staging was from IA to IIA with stage IA rate of 54.3% and IB rate of 40.4%.
Surgical Characteristics
The rate of VATS lobectomy for NSCLC treatment was 98.9%, and the conversion rate (from VATS to thoracotomy) accounted for 1.1% (one case of severe bleeding). During the operation, the rates of severe complications and blood transfusion were 3.2% and 5.3%, respectively. The operative time was 143.8 ± 38.9 minutes, and the amount of blood loss was 194.8 ± 150.5 mL. In the upper lobe resection, the blood loss during surgery (229.6 ± 176.5 mL, p < 0.05) and the rate of blood transfusion (p < 0.05) were statistically significant. Detailed information is shown in Table 1. The postoperative complication rate was 10.6% (8/9 mild cases accounting for 88.9%). The prolonged air leak was the highest with 5.3% (Table 2).
 |
Table 1 Intra-Operative Result |
 |
Table 2 Postoperative Complications |
Table 3 shows the postoperative characteristics. The amount of pleural fluid drainage decreased gradually from the 1st to the 2nd day after surgery. Drain duration after surgery was identified in 93 patients (one case was converted to thoracotomy). Drain removal in the first 3 days was accomplished in 59.1% of patients. The average duration of using epidural anesthesia for pain relief was 2.6 ± 0.8 days. The mean VAS score on the 5th day after surgery was 2.7 ± 1.0. The ICU length stay was 2.8 ± 1.0 days, and the postoperative hospital length stay was 7.7 ± 2.1 days.
 |
Table 3 Postoperative Characteristics |
Midterm Results
At the end of this study, communication was continued with 89/94 patients (94.7%). Among them, the survival rate was 81.9%, and the death rate was 12.8%.
One case (1.1%) had a recurrent tumor. Ten cases (11.8%) had metastasis, and the mean detection time was 10.7 ± 9.7 months after surgery. The relationship among lesion macroscopically, pathology, and metastasis is shown in Table 4.
 |
Table 4 Relationship Among Lesion Macroscopically, Pathology and Metastasis |
The twelve 1-year relative survival rate was 95.9% (3 deaths), and the 2-year relative survival rate was 80.8% (9 deaths). The median survival time was 30.8 ± 1.2 months (min-max: 26.6–43.5, CI 95% = 28.52–33.09). The factors associated with the survival rate are listed in Table 5. Details on the disease-free survival according to TNM stages after surgery are shown in Table 6.
 |
Table 5 Survival Associated with Several Factors |
 |
Table 6 Disease-Free Survival According to Pathological TNM Stages |
Discussion
A high rate of lung cancer is associated with advanced age,14 with estimates of 0.02% at the age of 40 years and up to 2.0% at the age of 80 years.15 In this study, 84.0% of patients were aged 40–69 years, which is close to the 81.8% of lung cancer occurring in individuals aged 54–84 years.15 Smoking is one of the leading risks of lung cancer.16 In this work, the general rate of smoking was 40.4%. Among which, 79.2% were male, and no female had history of smoking. The male/female ratio for NSCLC was 1/1, indicating that in addition to smoking, many other risk factors contribute to this disease. The status of passive smoking in female patients has not been evaluated in this research. When choosing a surgical method, female patients often agree to endoscopic surgery related to aesthetics and postoperative pain. VAST was considered as suitable treatment for NSCLC in the early stage. Among the 1015 NSCLC cases undergoing VATS, those at Phase I accounted for 87.9%.17 In some cases, VATS was only used in patients at stage I with non-hypertrophic N1 and N2 lymph nodes.18,19
In the present study, the surgical time was 143.8 ± 38.9 minutes, which is similar to the results of Swanson et al20 at 130 minutes, Tomaszek et al at 139 minutes,21 Congregado M. at 153 minutes,18 and Marty–Ane at 152 minutes.22 Other authors showed relatively long surgical time of up to 200,23 and 258.1 minutes.24 The surgical time showed positive trends with blood loss and blood transfusion during surgery. In the present work, the amount of blood loss during surgery was 194.8 ± 150.5 mL, which was lower than previous values, such as 253.2 mL,24 and 672 mL,25 but higher than those in other studies such as 10023 and 150 mL.21 During surgery, the amount of blood loss dramatically depends on the type of surgery, the lesion’s characteristics, the medical equipment, and the surgeon’s experience. No effective hemostatic devices, such as the ultrasonic systems (Hamonic® scalpel) and the electrothermal bipolar-activated devices (LigasureTM) that can be a risk factor for increasing blood loss surgery, were applied in the current work. Another risk factor was pulmonary blood congestion due to the ligation of the pulmonary vein before the artery. Severe bleeding during the operation is a serious complication in pulmonary lobe surgery. Vascular injury is a common etiology.1,26,27 Atrial injury and bronchial artery injury rarely happen but have also been recorded.1,27 In the case of failure to control the bleeding, thoracotomy is the next step in the procedure. The rate of changing to thoracotomy in VATS for NSCLC treatment varies from 0% to 14.7%.2,17,23,24,26 Among the 94 cases in the present study, 3.2% (3 cases) had severe bleeding caused by upper lobal segmental artery injury. In two cases, bleeding was successfully controlled using a clamping clip of blood vessels. One case (1.1%) was transferred to thoracotomy.
The rate of postoperative complications was 10.6%, including prolonged air leak (5.3%), atelectasis (2.1%), chylothorax (1.1%), arrhythmia (1.1%), and pneumothorax after chest tube removal (1.1%). Shortening the hospitalization time is the advantage of VATS. In this study, the hospitalization time was 7.3 ± 2.2 days, which was higher than that in some studies, such as 428 and 6.5 days.29 One of the reasons is that the homes of almost all the patients are located far from our hospital. Hence, they want to extend the hospitalization time to achieve a stable condition prior to discharged and coming home. Postoperative care also remarked with chest tube drainage and postoperative pain. The chest tube duration was 3.7 ± 2.1 days, with 59.1% removed during the first 3 days. The pleural fluid at postoperative day 1 and 2 was 358.7 ± 151.7 and 216.1 ± 110.8 mL, respectively. These results were similar to those in other studies.24,29 Postoperative pain management is another crucial factor affecting recovery and quality of life.30,31 The time of analgesia infusion was 2.6 ± 0.8 days, and the VAS score at postoperative day 5 was 2.7 ± 1.0. These values indicate promising results in pain management.
Mid-Term Results
Recurrence and metastatic evaluation is fundamental in cancer monitoring. Kelsey et al showed that the mean duration of local recurrence and metastasis was 14.1 and 12.5 months, respectively.32 With 36 months of follow-up after surgery, no statistically significant difference in recurrence rate and distant metastasis was observed between thoracoscopy and thoracotomy.7 A long-term study on phase I lung cancer with follow-up time of 91 months showed that recurrence was mainly observed in 60% of patients during the first 2 years after surgery.33 In the present work, one case (1.3%) had local recurrence 3 months after surgery. Ten cases (11.8%) showed metastatic detection time of 11.7 ± 9.7 months after the surgery. These recurrence and metastatic rates were considered low. A large sample size with a long follow-up time is required for paranomical evaluation. The current work also revealed several factors related to metastasis. The tumor with parietal pleural invasion had higher metastatic rate than that without invasiveness (20.0% vs 1.3%, p < 0.05). The (distant) metastasis rate was statistically significant for the stages of lymph node metastasis with N1-metastasis at 62.5%, N2-metastasis at 30%, and with no lymph node metastasis at 3.0%.
Overall survival rate is a key in evaluating the effectiveness of treatment from oncological perspectives.7 In patients with NSCLC stage I, the overall survival after resection did not differ between those receiving thoracotomy and VATS.7,34,35 In general, the 5-year relative survival rate of NSCLC ranges from 58% to 97%.36 A 3-year follow-up study reported that the 1-, 2-, and 3-year relative survival rates for NSCLC were 85%, 82.2%, and 73.5%, respectively.23 In the current work, the 1- and 2-year relative survival rates were 95.9% and 88.0%, respectively. These rates were acceptable and in line with previous results on early stage.37 The survival rate also varied under different disease stages, that is, the 3-year relative survival rate of stage I was 87.2%, and that of stage II–III was 58.4%.7
Conclusion
VATS as an early stage NSCLC treatment is an effective method with safe outcomes. Its mid-term outcomes were acceptable with 1 and 2-year relative survival rates of 95.9% and 80.8%, respectively.
Data Sharing Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The Ethics Committee of Pham Ngoc Thach Hospital approved the study protocol (QD/HVQY) and authorized its conduct and follow-up. The study was in line with the Declaration of Helsinki. Individual patient consent for inclusion in the study was obtained. Before treatment, written informed consent was provided to all participants after a thorough explanation of the purpose of this study. Patients had signed in written informed consent. Patients had the right to discontinue at any time during the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing interests.
References
1. Choi MS, Park JS, Kim HK, et al. Analysis of 1067 cases of video - assisted thoracic surgery lobectomy. Korean J Thorac Cardiovasc Surg. 2011;44:169–177. doi:10.5090/kjtcs.2011.44.2.169
2. Kim RH, Takabe K, Lockhart CG. Outcomes of a hybrid technique for video - assisted thoracoscopic surgery (VATS) pulmonary resection in a community setting. J Thorac Dis. 2010;2:210–214.
3. Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg. 2010;89(6):S2107–S2111. doi:10.1016/j.athoracsur.2010.03.020
4. Boffa DJ, Dhamija A, Kosinski AS, et al. Fewer complications result from a video-assisted approach to anatomic resection of clinical stage I lung cancer. J Thorac Cardiovasc Surg. 2014;148(2):637–643. doi:10.1016/j.jtcvs.2013.12.045
5. Su S, Scott WJ, Allen MS, et al. Patterns of survival and recurrence after surgical treatment of early stage non-small cell lung carcinoma in the ACOSOG Z0030 (ALLIANCE) trial. J Thorac Cardiovasc Surg. 2014;147(2):
6. Yang HX, Woo KM, Sima CS, et al. Long-term survival based on the surgical approach to lobectomy for clinical stage I nonsmall cell lung cancer: comparison of robotic, video-assisted thoracic surgery, and thoracotomy lobectomy. Ann Surg. 2017;265(2):431–437. doi:10.1097/SLA.0000000000001708
7. Lee PC, Nasar A, Port JL, et al. Long-term survival after lobectomy for non-small cell lung cancer by video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg. 2013;96(3):
8. Fraser S, Routledge T, Scarci M. Videoendoscopic resection of solitary peripheral lung nodule. Multimed Man Cardiothorac Surg. 2011:1–6.
9. Gossot D. Atlas of Endoscopic Major Pulmonary Resections. Paris - France: Springer; 2010.
10. Darling DE, Allen MS, Decker PA, Ballman K, Malthaner RA. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non - small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 trial. J Thorac Cardiovasc Surg. 2011;141(3):662–670. doi:10.1016/j.jtcvs.2010.11.008
11. Goldstraw P, Asamura H, Bunn P, Crowley J, Jett J, Rami-Porta R. Staging Manual in Thoracic Oncology. USA: Editorial Rx Press; 2009.
12. Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of non-small cell lung cancer stage I and stage II. Chest. 2007;132(3):234S- 242S. doi:10.1378/chest.07-1378
13. Lim E, Baldwin D, Beckles M, Duffy J, Entwisle J. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65(Suppl 3):iii1–iii27. doi:10.1136/thx.2010.145938
14. Ost D, Fein AM. The Solitary Pulmonary Nodule: A Systematic Approach. Vol. 1 & 2. United States of America: The McGraw - Hill Companies; 2008.
15. Shields TW, LoCicero III J, Reed CE, Feins RH. General Thoracic Surgery.
16. WHO. Latest World Cancer Statistics. Lyon - France; 2013. N° 223.
17. McKenna RJ, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1100 cases. Ann Thorac Surg. 2006;81(2):421–426. doi:10.1016/j.athoracsur.2005.07.078
18. Congregado M, Jimenez - Merchan R, Gallardo G, Ayarra J, Loscertales J. Video - assisted thoracic surgery (VATS) lobectomy: 13 years’experience. Surg Endosc. 2008;22:1852–1857. doi:10.1007/s00464-007-9720-z
19. Yamamoto K, Ohsumi A, Kojima F, et al. Long - term survival after video - assisted thoracic surgery lobectomy for primary lung cancer. Ann Thorac Surg. 2010;89:353–359. doi:10.1016/j.athoracsur.2009.10.034
20. Swanson SJ, Herndon IIJE, D’Amico TA, et al. Video - assisted thoracic surgery lobectomy: report of CALGB 39802 - a Prospective, Multi - Institution Feasibility Study. J Clin Oncol. 2007;25(31):4993–4997. doi:10.1200/JCO.2007.12.6649
21. Tomaszek SC, Cassivi SD, Shen KR, Allen MS, Nichols III FC. Clinical outcomes of video - assisted thoracoscopic lobectomy. Mayo Clin Proc. 2009;84(6):509–513. doi:10.4065/84.6.509
22. Marty - Ané CH, Canaud L, Solovei L, Alric P, Berthet J-P. Video - assisted thoracoscopic lobectomy: an unavoidable trend? A retrospective single-institution series of 410 cases. Interact Cardiovasc Thorac Surg. 2013;17:36–43. doi:10.1093/icvts/ivt146
23. Amer K, Khan AZ, Vohra HA. Video - assisted thoracic surgery of major pulmonary resections for lung cancer: the southampton experience. Eur J Cardiothorac Surg. 2011;39:173–179. doi:10.1016/j.ejcts.2010.05.029
24. Srisomboon C, Koizumi K, Haraguchi S, Mikami I, Iijima Y, Shimizu K. Complete video - assisted thoracoscopic surgery for lung cancer in 400 patients. Asian Cardiovasc Thorac Ann. 2013;21(6):700–707. doi:10.1177/0218492313479038
25. Kasemsarn C, Namthaisong K, Sungkahaphong V. Video - assisted Thoracic Surgery (VATS) lobectomy: chest disease institute experience. Thai J Surg. 2006;27:42–48.
26. Kim K, Kim HK, Park JS, et al. Video - assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg. 2010;89:S2118–S2122. doi:10.1016/j.athoracsur.2010.03.017
27. Wright GM. Video - assisted thoracoscopic pulmonary resections - the Melbourne experience. Ann Cardiothorac Surg. 2012;1(1):11–15.
28. Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity - matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139(2):336–378. doi:10.1016/j.jtcvs.2009.08.026
29. Wang W, Yin W, Shao W, et al. Comparative study of systematic thoracoscopic lymphadenectomy and conventional thoracotomy in resectable non - small cell lung cancer. J Thorac Dis. 2014;6(1):45–51.
30. Pu Q, Ma L, Mei J, et al. Video - assisted thoracoscopic surgery versus posterolateral thoracotomy lobectomy: a more patient - friendly approach on postoperative pain, pulmonary function and shoulder function. Thorac Cancer. 2013;4:84–89. doi:10.1111/j.1759-7714.2012.00153.x
31. Jiang G, Yang F, Li X, et al. Video - assisted thoracoscopic surgery is more favorable than thoracotomy for administration of adjuvant chemotherapy after lobectomy for non - small cell lung cancer. World J Surg Oncol. 2011;9(170):1–6. doi:10.1186/1477-7819-9-170
32. Kelsey CR, Marks LB, Hollis D, et al. Local recurrence after surgery for early stage lung cancer: an 11 - year experience with 975 patients. Cancer. 2009;115(22):5218–5227. doi:10.1002/cncr.24625
33. Martini N, Bains MS, Burt ME, Zakowski MF. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109(1):120–129. doi:10.1016/S0022-5223(95)70427-2
34. Higuchi M, Yaginuma H, Yonechi A, et al. Long - term outcomes after video - assisted thoracic surgery (VATS) lobectomy versus lobectomy via open thoracotomy for clinical stage IA non - small cell lung cancer. J Cardiothorac Surg. 2014;9(88):1–7. doi:10.1186/1749-8090-9-88
35. Paul S, Isaacs AJ, Treasure T, Altorki NK, Sedrakyan A. Long term survival with thoracoscopic versus open lobectomy: propensity matched comparative analysis using SEER - medicare database. BMJ. 2014;349:1–11. doi:10.1136/bmj.g5575
36. Taioli E, Lee DS, Lesser M, Flores R. Long - term survival in video - assisted thoracoscopic lobectomy vs open lobectomy in lung - cancer patients: a meta - analysis. Eur J Cardiothorac Surg. 2013;44:591–597. doi:10.1093/ejcts/ezt051
37. Ohtsuka T, Nomori H, Horio H, Naruke T, Suemasu K. Is major pulmonary resection by video - assisted thoracic surgery an adequate procedure in clinical stage I lung cancer? Chest. 2004;125:1742–1746. doi:10.1378/chest.125.5.1742
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
