Back to Journals » Clinical Ophthalmology » Volume 13
Salzmann nodular degeneration: prevalence, impact, and management strategies
Authors Paranjpe V , Galor A , Monsalve P, Dubovy SR, Karp CL
Received 2 March 2019
Accepted for publication 27 June 2019
Published 25 July 2019 Volume 2019:13 Pages 1305—1314
DOI https://doi.org/10.2147/OPTH.S166280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Vikram Paranjpe,1 Anat Galor,1,2 Pedro Monsalve,1 Sander R Dubovy,1 Carol L Karp1
1Department of Ophthalmology, University of Miami Miller School of Medicine, Miami, FL 33136, USA; 2Department of Ophthalmology, Miami Veterans Administration Medical Center, Miami, FL 33125, USA
Purpose: This review will summarize the clinical and histological presentation of Salzmann nodular degeneration (SND), its prevalence and risk factors, potential underlying mechanisms, diagnostic tools, management options, and impact on cataract surgery and co-morbid ocular surface diseases.
Method: PubMed review of 44 articles published between 1976 and 2018.
Results: SND is a corneal disease characterized by whitish gray or bluish nodules on the peripheral or central cornea. The clinical presentation of SND is variable and the nodules can be asymptomatic or cause foreign body sensation and/or blurred vision. Histologically, SND appears as subepithelial nodules with thin overlying epithelium, disrupted or absent Bowman’s layer, and activated fibroblasts within the nodule. SND pathogenesis is not fully understood but is thought to involve poor epithelial protection and disruption of the epithelial–stromal interface, allowing for penetration of epithelially derived growth factors into the stroma and subsequent activation of stromal fibroblasts, eventually leading to sub-epithelial deposition of disorganized extracellular membrane components. SND most commonly occurs in Caucasian females in a bimodal distribution, occurring in the fifth or eighth and ninth decades of life. Risk factors for SND include ocular surface diseases and surgery. Surgical intervention is recommended in individuals with symptomatic nodules – primarily superficial keratectomy performed with or without intraoperative mitomycin C, photokeratectomy, and/or amniotic membrane transplantation. These procedures have been successful in removing the lesion and reducing corneal irregularity, but have variable recurrence rates (0–31%).
Conclusion: The pathogenesis of SND is complex and multifactorial. Advances in diagnostic and treatment modalities have allowed for earlier and more accurate diagnosis and effective treatment of SND.
Keywords: Salzmann nodular degeneration, cornea, diagnosis, imaging, treatment
Introduction
Salzmann nodular degeneration (SND) was first described in 1925 as a bluish or whitish gray corneal opacity occurring in isolation or in multiples in any location on the cornea.1 Since then, numerous studies have added to the understanding of the epidemiology, clinical presentation, associated risk factors, and underlying pathogenesis of SND.2–6 Furthermore, advances in imaging modalities and surgical techniques have transformed the diagnosis and management of SND. This review will summarize the clinical and histological presentation of SND, its epidemiology and risk factors, diagnostic tools and management strategies, and finally the impact of SND on cataract surgery and co-existing ocular surface disease.
Clinical presentation
SND can present asymptomatically (16%, n=19, in one study), with foreign body sensation (68%, n=19) or decreased vision (5%, n=19).5 In two studies, decreased vision was the most common complaint, found in 85.5% (n=93) and 31% (n=108) of individuals, respectively.2,3
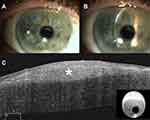
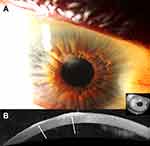
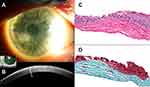
Clinically, the bluish or whitish gray corneal opacity/ies occur more often peripherally than centrally (Figure 1A-B, 2A, 3A, and 4A).6 In a retrospective review of 30 eyes, 28% of the nodules were found in the superior-nasal quadrant, 23% in the superior-temporal quadrant, 20.5% in the inferior-nasal quadrant, 18% in the inferior-temporal quadrant, and 10% of the nodules were located centrally.5 While the opacities are typically discrete, cases of ring-like peripheral SND have been described.7
Imaging
Corneal opacities are conventionally visualized by slit-lamp examination, but with the advent of high-frequency ultrasound biomicroscopy (UBM), confocal microscopy, high-resolution OCT (HR-OCT), and corneal topography and tomography technologies, there has been an interest in utilizing these technologies to better visualize corneal pathology.6,8,9
In a case of SND occurring after laser-assisted in-situ keratomileusis (LASIK), the nodules were visualized using in vivo high-frequency UBM.9 Nodules appeared as hyper-reflective surfaces covered by an abnormally thin epithelium.9 High-frequency UBM was able to identify the lamellar flap interface, the hyperechogenic nodule, and an abrupt end to Bowman’s layer, indicating localization of the nodule within the LASIK flap margin and destruction of Bowman’s layer.9
Multiple studies have reported the use of confocal microscopy to visualize SND nodules.10–13 Two studies also compared in vivo confocal microscopy with histological findings.10,13 On confocal microscopy, the SND nodule appeared as an unstructured area with increased reflectivity extending from the epithelium to the anterior stroma. The nodule contained an irregular network of highly reflective structures thought to represent activated keratocytes, the epithelium consisted of irregular, elongated, superficial cells, and Descemet’s membrane and the endothelium appeared normal.10 Confocal microscopy of the central cornea, away from the nodule, showed normal epithelium but decreased corneal nerve density in the sub-basal nerve plexus with increased nerve thickness and absent branching compared to historical images.13 Stromal nerves in the central cornea were also abnormally thick and tortuous.13 Overall, findings of in vivo confocal microscopy correlated with histologic findings.10
Ultra-high-resolution OCT (UHR-OCT) has been used to non-invasively characterize SND nodules.14 SND lesions appear as bright, white, sub-epithelial deposits in the cornea that are wedged between Bowman’s layer and the epithelium. The epithelium above the SND is thinned. (Figure 1C, 2B, and 4B).14 These characteristic features on UHR-OCT can help diagnose SND in cases that are clinically ambiguous (Figure 4).7 UHR-OCT findings of SND have also been found to correlate with histological characteristics.14 Our experience has been that OCT is a useful tool to diagnose SND without the need for a tissue sample.
Corneal topography and tomography can assess the degree of irregularity and astigmatism caused by SND and evaluate improvements in regularity and astigmatism after surgical intervention. (Figure 3B-C).6,10,15,16
Histology
SND typically consists of a sub-epithelial nodule anterior to Bowman’s layer, thinning of the corneal epithelium overlying the nodule, and disruption of Bowman’s layer, with variable duplication of the epithelial basement membrane.4,5,10 Other described findings include decreased cell density and deposition of extracellular matrix material in the nodule, a variable degree of anterior stromal scarring, an irregularly shaped epithelial–stromal interface, and activated fibroblasts.10,17 A variable amount of inflammation, including B and T cell lymphocytes and major histocompatibility complex class II antigens, has been described in a surgically removed SND specimen, suggesting that inflammation may play a role in the pathophysiology of the disease.18 Overall, there are no pathognomonic histological features of SND, and described findings are not dissimilar from those seen with a degenerative pannus or old corneal scar following inflammation or trauma.18 Therefore, the diagnosis of SND is best made with correlation of both the clinical and histopathologic features.4,5,10
Pathophysiology
The exact mechanism underlying the development of SND is unknown, but most likely, the corneal epithelium, basement membrane, and stroma are all involved. Structurally and functionally, the central corneal epithelium differs from the limbal epithelium. Central corneal epithelium is terminally differentiated and does not contain stem cells, whereas the limbal epithelium is undifferentiated and contains both stem cells and transient amplifying cells (TAC) or cells with a high capacity for proliferation that are not true stem cells.19 One study analyzed the expression of specific markers in the epithelium overlying the central cornea, the limbus, and SND nodules.19 The limbal epithelium had high levels of expression of ABCG2, a stem cell marker, high levels of CK19, a marker of TACs, medium levels of enolase-alpha, an enzyme expressed in mitotically active cells, and low levels of CK3/12, a marker of terminally differentiated epithelium. The central corneal epithelium highly expressed CD3/12 and had low levels of expression of CK19 and ABCG2. The epithelium overlying SND nodules was found to have the highest level of expression of CK19 and enolase-alpha and the lowest level of expression of CD3/12.19,20 Taken together, this suggests that corneal epithelium overlying SND nodules is undifferentiated, highly metabolically active, and consists of TACs but not true limbal stem cells.
Normal basal epithelium also expresses matrix-metalloproteinases (MMPs), transforming growth factor-beta 1 (TGF-β1), and platelet-derived growth factor (PDGF), which may all play a role in SND. Physiologically, the basement membrane sits between the basal epithelial cells and the corneal stroma, acting as a barrier to the penetration of growth factors expressed by the epithelium into the stroma.21 Interestingly, the epithelium overlying SND nodules was found to have increased expression of MMP-2, an enzyme known to dissolve type IV collagen, the primary component of the epithelial basement membrane.4 An increase in epithelial MMP-2 expression could disrupt the integrity of the basement membrane and allow for stromal penetration of TGF-β1 and PDGF at levels sufficient to promote differentiation of quiescent stromal keratocytes into activated fibroblasts and myofibroblasts, which can migrate anteriorly and deposit disorganized extracellular matrix components to form the nodule.4,21 Myofibroblasts have been shown to be responsible for persistent corneal haze after corneal surgery, injury, or infection because of deposition of disorganized extracellular matrix material and decreased transparency of the cells themselves.21 It is unclear why the deposition of extracellular matrix material occurs in a localized nodule in SND compared to larger areas of post-surgical corneal haze.
To summarize, changes in epithelial cells, disruption of basement membrane and Bowman’s layer, differentiation and anterior migration of stromal fibroblasts, and deposition of subepithelial extracellular matrix material are thought to lead to the formation of nodules. However, it is not fully understood what precipitates these changes. Mechanical disruption of the epithelial-stromal barrier from corneal trauma or surgery and chronic corneal irritation from ocular surface disease like aqueous deficient dry eye may lead to prolonged epithelial wound repair and remodeling and create the necessary conditions for deposition of the extracellular material.5,17 However, SND is often seen in individuals without these risk factors, suggesting that other unidentified factors are also involved in precipitating the process.
Prevalence
SND occurs more commonly in women and Caucasians and is a bilateral process more than 50% of the time.5,18 In the largest case series to date consisting of 180 eyes of 108 individuals, 72% of the individuals were female, 76% were white, and 66% had bilateral disease.2 SND seems to have a bimodal age distribution, with a majority of individuals presenting in either the fifth or the eighth and ninth decades of life. Similar results have been found in a case series of 93 individuals (152 eyes) with SND.3
Risk factors
SND may have a genetic component as a number of cases reported SND occurring in multiple generations.22–24 A report of three cases of SND in three consecutive generations of a family highlighted the possibility of an autosomal dominant inheritance pattern.23 This was corroborated by another report of four cases of SND in four consecutive generations.24 However, since no unique genes associated with SND have been identified to date, there has been an unresolved debate on whether these cases represent the presence of a unique genetic link in SND or whether these individuals possessed the same missense mutation in the TGFβI gene that is known to be the genetic basis for many other corneal dystrophies.25,26
SND has been associated with several ocular pathologies including meibomian gland dysfunction, chronic blepharitis, trichiasis, trachoma, previous ocular trauma, Thygeson’s punctate keratitis, vernal keratitis, filamentary keratitis, chronic uveitis, phlyctenular keratitis, and epithelial basement membrane dystrophy.5,14 Previous ocular trauma and previous surgery including cataract extraction, pterygium excision, and laser-assisted in situ keratomileusis (LASIK), and long-term use of contact lenses, especially soft lenses, have also been associated with SND.2,3,5,7 The common factor among all of the conditions associated with SND is poor epithelial protection, due to chronic irritation or mechanical disruption of the epithelial-stromal barrier during trauma or surgery, which is thought to be important in the development of SND.5 However, it is important to remember that conditions such as meibomian gland dysfunction and contact lens use are prevalent in the population and their co-existence with SND does not necessarily imply causation.
Development of SND after LASIK has been reported multiple times.9,27–29 Given the presentations of SND both proximal and remote from the LASIK history, the trigger for post-LASIK SND may be surgical trauma, anatomical disruption in the area of the flap, and/or post-operative dry eye disease.9,27–29 In one case, an individual presented eight months after LASIK complaining of blurry vision and a small growth on the cornea was noted. Dry eye was diagnosed at this visit but was not further defined. The individual was treated with lubrication and presented 5 years later with worsening blurry vision and photosensitivity. At that time, she was found to have three non-inflamed, raised nodules, two in the right eye and one in the left eye, all occurring in the mid-peripheral cornea, overlying the LASIK flap.9 Due to the location of the nodules, SND was treated medically with loteprednol, cyclosporine 0.05% drops, artificial tears, and lower-lid punctal plugs in both eyes. Six months after her initial presentation, her symptoms had resolved, and uncorrected visual acuity improved from 20/50 to 20/20 in the right eye and 20/30−1 to 20/30+1 correctable to 20/20 in the left eye.9 While post-LASIK SND is commonly treated medically, superficial keratectomy has been used to treat concomitant peripheral epithelial ingrowth and overlying SND in the setting of LASIK.29
SND has also been described in the setting of systemic disease. In one case, an individual developed bilateral nodules that were removed with alcohol-assisted superficial keratectomy. At the time of initial presentation, he had no history of systemic diseases. Three years later, nodules recurred in both eyes in the same locations concurrently with a new onset of gastrointestinal manifestations later diagnosed as Crohn’s disease.30
Management
The choice of treatment in SND is dictated by presenting symptoms and extent of disease. As the most commonly reported symptom is foreign body sensation, conservative management with ocular lubrication, warm compresses, and lid hygiene can be used in most individuals, and those with underlying inflammation may also benefit from a short course of topical corticosteroids and/or oral doxycycline.5 Raised nodules, nodules in the central visual axis causing reduced vision, or failure of conservative management are common indications for surgical intervention.5 However, surgery is required in the minority of individuals ranging from 13% to 32% in 3 studies.2,3,5
Surgical options include superficial keratectomy, excimer laser phototherapeutic keratectomy (PTK), and lamellar keratoplasty3,6 These interventions can be coupled with alcohol-assisted delamination,15 amniotic membrane transplantation,18,31 or intraoperative mitomycin C (MMC).16,32 (Table 1).
 |
Table 1 Percentage of eyes with recurrent disease following surgical intervention |
Superficial keratectomy with or without alcohol
Superficial keratectomy with manual excision of nodules is often the procedure of choice for SND. The epithelium overlying the nodules is removed and the nodular edge is separated from the corneal surface with forceps or a flat blade, leaving Bowman’s layer almost untouched.6 Superficial keratectomy and removal of nodules can restore the original corneal contour and reverse the hyperopic shift and astigmatism caused by SND.2,5,33,34 However, in one study, superficial keratectomy did not improve best-corrected visual acuity (BCVA) in individuals with peripheral nodules.2 Recurrences of SND can occur after superficial keratectomy, as was reported in 9 out of 41 cases (22%) over a mean follow-up time of 20.2 months.2
Alcohol can be used to loosen the epithelium prior to its removal.15 In one study, a 25% ethanol solution was applied to the cornea for 25 s. The epithelium and SND were then removed. In that case, pre-operative uncorrected visual acuity (UCVA) was 0.2 and 0.4 in the right and left eye, respectively. Immediately after the procedure, the UCVA improved to 0.8 in both eyes and after 7 days improved to 1.0 and remained stable for 1 year with reduction in astigmatism. Specifically, the cylinder decreased from 3.59 D to 0.32 D in the right eye and 5.92 D to 0.01 D in the left eye and remained stable for 1 year. In this study, there were no recurrences of nodules during the 1-year post-operative period. In our practice, we generally use 20% dehydrated alcohol applied for 40 s with a sponge prior to epithelium removal.
Superficial keratectomy with amniotic membrane transplantation
Amniotic membrane transplantation (AMT) has been used as an adjunct after superficial keratectomy in two studies.18,31 In one, superficial keratectomy was followed by AMT secured by interrupted 10–0 nylon sutures, in one eye with SND; however, data regarding visual outcome and recurrence in this eye are not available.31 In a second study, superficial keratectomy was followed by AMT (technique unspecified) in one eye. The nodules recurred after 1 month, so 7 months later, the individual again underwent superficial keratectomy with amniotic membrane transplantation, but again had a recurrence 1 month later.18
Superficial keratectomy with phototherapeutic keratectomy
If significant corneal haze remains after superficial keratectomy, PTK can be performed.6 A 193-nm ArF excimer laser has been used to ablate the corneal surface keeping the treatment zone in the 6 mm of the central cornea. Ablation depth can range from 25 to 75 μm based on the depth of haze.35,36 In one study, visual acuity improved 1.6±2.9 lines in 41 eyes with SND, 3 months after PTK.37 In another report of PTK following superficial keratectomy in 22 eyes of 14 individuals, mean visual acuity preoperatively was 0.4±0.2 and improved to 0.7±0.3 postoperatively.38 Overall, vision improved in 86% of the cases and recurrences occurred in 31% of the eyes.
Superficial keratectomy with intraoperative mitomycin-C (MMC)
MMC can be applied intraoperatively after superficial keratectomy to target fibroblasts within the stroma.32 In this manner, it is believed to decrease the frequency of recurrence. However, no head-to-head studies exist comparing superficial keratectomy with or without MMC. In one study of superficial keratectomy with intraoperative MMC in 30 eyes of 25 individuals, MMC was applied for 10 s followed by irrigation with a balanced salt solution, with each eye undergoing two applications of MMC. There were no recurrences of SND over a mean follow-up time of 28±15 months.32
Superficial keratectomy with phototherapeutic keratectomy and intraoperative mitomycin-C
The effectiveness of utilizing both PTK and intraoperative MMC following superficial keratectomy was reported in a retrospective study of 8 eyes (5 individuals). All eyes underwent manual superficial keratectomy followed by PTK with 193-nm excimer laser and finally application of mitomycin C for 30 s.16 Individuals were followed for 12–24 months and were found to have a significant improvement in BCVA, a reduction in hyperopia, stable refraction, and no recurrences throughout the follow-up period.16
Lamellar keratoplasty
Keratoplasty is rarely required for treatment of SND. Keratoplasty is only indicated for lesions extending into the mid-stroma, and these lesions occur more often as the result of another underlying disease causing corneal scarring, rather than from SND itself. In these cases, anterior lamellar keratoplasty (ALK) is typically sufficient because most often, the endothelium is intact.6 One study compared the use of automated lamellar keratoplasty (n=21) and PTK (n=28) for the treatment of SND in individuals with peripheral SND and corneal scarring.36 At 6 months, the overall change in corrected distance visual acuity was equivalent between the 2 groups. The frequency of complications was lower in those undergoing PTK compared to automated lamellar keratoplasty.36 Penetrating keratoplasty (PKP) is very rarely required for the treatment of SND, typically only for cases involving postoperative complications after ALK such as presence of infection, graft dehiscence, graft failure, or persistent haze.6
Recurrence of SND after keratoplasty has been reported.5,39 In one report, a 44-year-old male with a history of ALK for SND 22 years prior presented with recurrent nodules in the right eye. He subsequently underwent repeat ALK. In another case, a 24-year-old male with a history of PKP in the left eye for SND 6 years prior presented with recurrent nodules in the left eye and was also treated with ALK.39 A second report describes two cases of recurrent SND occurring months after PKP.5 In these cases, nodules initially appeared at the junction of the host and donor cornea; however, nodules later increased in number and size and extended centrally onto the donor cornea.5
Prosthetic replacement of the ocular surface ecosystem
For individuals who fail conservative management but are not surgical candidates (eg, multiple recurrences after superficial keratectomy), the Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) has been used. This is a custom fitted scleral lens that is fluid filled and fitted to vault the entire cornea and limbus.40 Individuals with SND who used the PROSE scleral lens demonstrated significant improvements in both visual acuity and visual function.40
SND and cataract surgery
Removal of SND should be considered prior to cataract surgery as even peripheral nodules can cause central corneal flattening and astigmatism.41,42 In one case, an older individual presented with extensive bilateral SND and moderate bilateral cataracts with an uncorrected distance visual acuity (UDVA) of 20/50 in both eyes.43 Initially, it was difficult to obtain reliable keratometry measurements for intraocular lens (IOL) calculations because of significant epiphoria provoked by SND. The individual underwent superficial keratectomy in both eyes and had significant improvement in astigmatism 4.9 D to 1.72 D and 7.6 D to 1.94 D in the right and left eye, respectively, and an increase in central corneal power from 39.6D to 44.8D and 34.3D to 45.2D in the right and left eye, respectively. Following superficial keratectomy, reliable keratometry and IOL power calculations were obtained and the individual successfully underwent phacoemulsification cataract surgery in both eyes and improved to an UDVA of 20/20 and 20/25 in the right and left eye, respectively, highlighting the importance of addressing the refractive sequelae of SND prior to cataract surgery.43
SND and co-morbid ocular surface diseases
SND, along with other ocular surface diseases, can mask typical symptoms and slit-lamp findings of ocular surface squamous neoplasia (OSSN), thus making it more difficult to diagnose this condition.8 There have been a number of reports in the literature of OSSN discovered incidentally on biopsy, including in suspected SND.8,44 In one study, HR-OCT identified concomitant SND and OSSN in an individual prior to surgical removal. Histology confirmed the co-existence of the two pathologies.8
Conclusion
Salzmann nodular degeneration is a multifactorial condition resulting in raised nodules typically occurring on the peripheral cornea. Histologically, nodules appear as subepithelial deposits of extracellular matrix material with thinned overlying epithelium and disrupted or absent Bowman’s layer beneath the nodule. The exact pathophysiological mechanism leading to SND is unclear but is thought to involve poor epithelial protection secondary to chronic corneal irritation, trauma or surgery, breakdown of Bowman’s layer, activation and anterior migration of fibroblasts, and deposition of disorganized extracellular matrix material in a subepithelial nodule. SND is associated with various ocular pathologies, ocular trauma, and ocular surgery. Clinically, SND can present with ocular surface irritation, dryness, or decreased vision in the case of central nodules. Advances in UBM, OCT, and confocal microscopy have greatly enhanced the ability to evaluate in vivo characteristics of nodules and diagnose SND. Treatment involves conservative management with ocular surface lubrication or surgical intervention with superficial keratectomy, PTK, or rarely, keratoplasty. Finally, SND can impact the management of cataract, OSSN, and LASIK. There remain many questions regarding the exact pathogenesis of SND as well as the optimal management strategies to maximize post-operative visual outcomes and minimize recurrence of nodules. Future studies should focus on both fully understanding the pathogenesis of SND and on carrying out robust studies comparing the different surgical interventions to understand how to best manage this condition.
Role of sponsors
The sponsors below provided financial support to cover the researchers time but were not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. We would like to note that the contents of this study do not represent the views of the Department of Veterans Affairs or the United States Government.
Acknowlegments
This study was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research EPID-006-15S (Dr. Galor), R01EY026174 (Dr. Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant. This study was also supported by the Dr. Ronald and Alicia Lepke Grant, the Lee and Claire Hager Grant, the H. Scott Huizenga Grant, the Grant and Diana Stanton-Thornbrough, the Robert Baer Family Grant, the Emilyn Page and Mark Feldberg Grant, the Jose Ferreira de Melo Grant, the Richard and Kathy Lesser Grant, the Ted and Michele Kaplan Grant and the Richard Azar Family Grant (institutional grants).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Salzmann MI. über eine Abart der knötchenförmigen Hornhaut-dystrophie. Ophthalmologica. 1925;57(1–6):92–99. doi:10.1159/000295975
2. Graue-Hernandez EO, Mannis MJ, Eliasieh K, et al. Salzmann nodular degeneration. Cornea. 2010;29(3):283–289. doi:10.1097/ICO.0b013e3181b7658d
3. Farjo AA, Halperin GI, Syed N, Sutphin JE, Wagoner MD. Salzmann’s nodular corneal degeneration clinical characteristics and surgical outcomes. Cornea. 2006;25(1):11–15. doi:10.1097/01.ico.0000167879.88815.6b
4. Stone DU, Astley RA, Shaver RP, Chodosh J. Histopathology of Salzmann nodular corneal degeneration. Cornea. 2008;27(2):148–151. doi:10.1097/ICO.0b013e31815a50fb
5. Hamada S, Darrad K, McDonnell PJ. Salzmann’s nodular corneal degeneration (SNCD): clinical findings, risk factors, prognosis and the role of previous contact lens wear. Cont Lens Anterior Eye. 2011;34(4):173–178. doi:10.1016/j.clae.2011.02.004
6. Maharana PK, Sharma N, Das S, et al. Salzmann’s Nodular Degeneration. Ocul Surf. 2016;14(1):20–30. doi:10.1016/j.jtos.2015.08.006
7. Hurmeric V, Yoo SH, Galor A, Canto AP, Wang J. Atypical presentation of Salzmann nodular degeneration diagnosed with ultra-high-resolution optical coherence tomography. Ophthalmic Surg Lasers Imaging. 2011;42(Online):e122–125.
8. Atallah M, Joag M, Galor A, et al. Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases. Ocul Surf. 2017;15(4):688–695. doi:10.1016/j.jtos.2017.03.003
9. VanderBeek BL, Silverman RH, Starr CE. Bilateral Salzmann-like nodular corneal degeneration after laser in situ keratomileusis imaged with anterior segment optical coherence tomography and high-frequency ultrasound biomicroscopy. J Cataract Refract Surg. 2009;35(4):785–787. doi:10.1016/j.jcrs.2008.09.033
10. Meltendorf C, Buhren J, Bug R, Ohrloff C, Kohnen T. Correlation between clinical in vivo confocal microscopic and ex vivo histopathologic findings of Salzmann nodular degeneration. Cornea. 2006;25(6):734–738. doi:10.1097/01.ico.0000214215.75496.a5
11. Ku JY, Grupcheva CN, McGhee CN. Microstructural analysis of Salzmann’s nodular degeneration by in vivo confocal microscopy. Clin Exp Ophthalmol. 2002;30(5):367–368. doi:10.1046/j.1442-9071.2002.00558.x
12. Werner LP, Issid K, Werner LP, Pouliquen Y, Legeais JM, Renard G. Salzmann’s corneal degeneration associated with epithelial basement membrane dystrophy. Cornea. 2000;19(1):121–123. doi:10.1097/00003226-200001000-00024
13. Roszkowska AM, Aragona P, Spinella R, Pisani A, Puzzolo D, Micali A. Morphologic and confocal investigation on Salzmann nodular degeneration of the cornea. Invest Ophthalmol Vis Sci. 2011;52(8):5910–5919. doi:10.1167/iovs.11-7789
14. Hurmeric V, Yoo SH, Karp CL, et al. In vivo morphologic characteristics of Salzmann nodular degeneration with ultra-high-resolution optical coherence tomography. Am J Ophthalmol. 2011;151(2):248 e242–256 e242.
15. Roszkowska AM, Colosi P, De Grazia L, Mirabelli E, Romeo G. One year outcome of manual alcohol-assisted removal of Salzmann’s nodular degeneration. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1431–1434. doi:10.1007/s00417-009-1154-y
16. Khaireddin R, Katz T, Baile RB, Richard G, Linke SJ. Superficial keratectomy, PTK, and mitomycin C as a combined treatment option for Salzmann’s nodular degeneration: a follow-up of eight eyes. Graefes Arch Clin Exp Ophthalmol. 2011;249(8):1211–1215. doi:10.1007/s00417-011-1641-9
17. Qiu J, Cai R, Zhang C. Association between poor wound healing and the formation of Salzmann nodules. J Cataract Refract Surg. 2016;42(10):1527–1530. doi:10.1016/j.jcrs.2016.07.019
18. Yoon KC, Park YG. Recurrent Salzmann’s nodular degeneration. Jpn J Ophthalmol. 2003;47(4):401–404.
19. Eberwein P, Hiss S, Auw-Haedrich C, et al. Epithelial marker expression in Salzmann nodular degeneration shows characteristics of limbal transient amplifying cells and alludes to an involvement of the epithelium in its pathogenesis. Acta Ophthalmol. 2010;88(5):e184–189. doi:10.1111/j.1755-3768.2010.01887.x
20. Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47(11):4780–4786. doi:10.1167/iovs.06-0574
21. Torricelli AA, Singh V, Santhiago MR, Wilson SE. The corneal epithelial basement membrane: structure, function, and disease. Invest Ophthalmol Vis Sci. 2013;54(9):6390–6400. doi:10.1167/iovs.13-12547
22. Auw-Haedrich C, Agostini H, Clausen I, et al. A corneal dystrophy associated with transforming growth factor beta-induced Gly623Asp mutation an amyloidogenic phenotype. Ophthalmology. 2009;116(1):46–51. doi:10.1016/j.ophtha.2008.08.050
23. Singer AR, Pahl S, Lang HM, Ruprecht KW. [A familial anterior corneal degeneration: clinical aspects, histopathology and differential diagnosis]. Klin Monbl Augenheilkd. 1998;213(2):104–107. doi:10.1055/s-2008-1034955
24. Papanikolaou T, Goel S, Jayamanne DG, Mudhar H, Desai SP. Familial pattern of Salzmann-type nodular corneal degeneration–a four generation series. Br J Ophthalmol. 2010;94(11):1543. doi:10.1136/bjo.2009.177055
25. Khan K, Ali M, Inglehearn C. Familial pattern of Salzmann-type nodular corneal degeneration - a four generation series. Reply to Papanikolaou et al. Br J Ophthalmol. 2011;95(6):890. author reply 890
26. Papanikolaou T, Goel S, Jayamanne DGR, Mudhar H, Desai SP. Authors’ response. Br J Opthomol. 2011;95(6):890. doi:10.1136/bjo.2010.199752
27. Lim MC, Chan WK. Salzmann nodular degeneration after laser in situ keratomileusis. Cornea. 2009;28(5):577–578. doi:10.1097/ICO.0b013e3181a2ad81
28. Moshirfar M, Chang JC, Mamalis N. Salzmann nodular degeneration after laser in situ keratomileusis. Cornea. 2010;29(7):840–841. doi:10.1097/ICO.0b013e3181c378df
29. Stem MS, Hood CT. BMJ Case Rep Published online: [2 Feb 2019] doi:10.1136/bcr-2014-207776
30. Roszkowska AM, Spinella R, Aragona P. Recurrence of Salzmann nodular degeneration of the cornea in a Crohn’s disease patient. Int Ophthalmol. 2013;33(2):185–187. doi:10.1007/s10792-012-9648-8
31. Rao A, Sridhar U, Gupta AK. Amniotic membrane transplant with superficial keratectomy in superficial corneal degenerations: efficacy in a rural population of north India. Indian J Ophthalmol. 2008;56(4):297–302. doi:10.4103/0301-4738.39664
32. Bowers PJ
33. Oster JG, Steinert RF, Hogan RN. Reduction of hyperopia associated with manual excision of Salzmann’s nodular degeneration. J Refract Surg. 2001;17(4):466–469.
34. Das S, Link B, Seitz B. Salzmann’s nodular degeneration of the cornea: a review and case series. Cornea. 2005;24(7):772–777. doi:10.1097/01.ico.0000153100.74033.ef
35. Sharma N, Prakash G, Sinha R, Tandon R, Titiyal JS, Vajpayee RB. Indications and outcomes of phototherapeutic keratectomy in the developing world. Cornea. 2008;27(1):44–49. doi:10.1097/ICO.0b013e318157a111
36. Sharma N, Prakash G, Titiyal JS, Vajpayee RB. Comparison of automated lamellar keratoplasty and phototherapeutic keratectomy for Salzmann nodular degeneration. Eye Contact Lens. 2012;38(2):109–111. doi:10.1097/ICL.0b013e31823fdb2a
37. Maloney RK, Thompson V, Ghiselli G, Durrie D, Waring GO
38. Das S, Langenbucher A, Pogorelov P, Link B, Seitz B. Long-term outcome of excimer laser phototherapeutic keratectomy for treatment of Salzmann’s nodular degeneration. J Cataract Refract Surg. 2005;31(7):1386–1391. doi:10.1016/j.jcrs.2004.12.037
39. Sinha R, Chhabra MS, Vajpayee RB, Kashyap S, Tandon R. Recurrent Salzmann’s nodular degeneration: report of two cases and review of literature. Indian J Ophthalmol. 2006;54(3):201–202. doi:10.4103/0301-4738.27075
40. Chiu GB, Bach D, Theophanous C, Heur M. Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE) scleral lens for Salzmann’s nodular degeneration. Saudi J Ophthalmol. 2014;28(3):203–206. doi:10.1016/j.sjopt.2014.06.001
41. Koch DD. Impact of Salzmann’s lesions on corneal curvature. J Cataract Refract Surg. 1995;21(2):111–112. doi:10.1016/S0886-3350(13)80490-7
42. Jeng BH, Millstein ME. Reduction of hyperopia and astigmatism after superficial keratectomy of peripheral hypertrophic subepithelial corneal degeneration. Eye Contact Lens. 2006;32(3):153–156. doi:10.1097/01.icl.0000182875.61214.cf
43. Kim BZ, Wilson PJ, McGhee CN. Annular Salzmann degeneration: avoiding perturbations and pitfalls in phacoemulsification surgery. J Cataract Refract Surg. 2015;41(11):2580–2583. doi:10.1016/j.jcrs.2015.10.037
44. Mendoza PR, Craven CM, Ip MH, Wilson MW, Coroneo MT, Grossniklaus HE. Conjunctival squamous cell carcinoma with corneal stromal invasion in presumed pterygia: a case series. Ocul Oncol Pathol. 2018;4(4):240–249. doi:10.1159/000485425
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.