Back to Journals » Journal of Experimental Pharmacology » Volume 14
SAGE: Novel Therapy to Reduce Inflammation in a Naturally Occurring-Dog Model of Periodontal Disease
Authors Raja V, Gu Y, Lee HM, Deng J , Prestwich G, Ryan M
Received 13 December 2021
Accepted for publication 22 March 2022
Published 30 March 2022 Volume 2022:14 Pages 117—129
DOI https://doi.org/10.2147/JEP.S353757
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Paola Rogliani
Veena Raja,1 Ying Gu,2 Hsi-Ming Lee,1 Jie Deng,1 Glenn Prestwich,3,4 Maria Ryan5
1Department of Oral Biology and Pathology, Stony Brook School of Dental Medicine, Stony Brook, NY, USA; 2Department of General Dentistry, Stony Brook School of Dental Medicine, Stony Brook, NY, USA; 3Department of Medicinal Chemistry, The University of Utah, Salt Lake City, UT, USA; 4Health Sciences Spokane, Washington State University, Spokane, WA, USA; 5Colgate and Palmolive Company, Piscataway, NJ, USA
Correspondence: Veena Raja, Department of Oral Biology and Pathology, School of Dental medicine, Stony Brook University, Stony Brook, NY, 11794-8706, USA, Tel +1 516-813-6250, Fax +1 631 632-9705, Email [email protected]
Objective: To determine the effect of a semi-synthetic-glycosaminoglycan Ether (SAGE) as a universal therapeutic benefit to reduce periodontal inflammation and alveolar bone loss in naturally occurring-beagle-dog model of periodontal disease as a surrogate for human non-risk associated natural periodontitis.
Methods: Six adult female dogs with generalized periodontitis were distributed into two groups: control and SAGE treatment (n=3/group). After a 1-hour full-mouth scaling and root planning (SRP) at baseline, control or SAGE treatment (50mg/mL) bioadhesive gel formulation was locally applied for 12 weeks. Various clinical periodontal measurements (probing depth, CAL) were measured at different time periods (baseline, 4, 8 and 12 weeks), and gingival crevicular fluid (GCF), blood samples and gingival tissue biopsies (12 week) were analyzed for inflammatory mediators, collagenases and cell-signaling molecules. Standardized radiographs were taken at baseline and 12week period.
Results: SAGE treatment significantly reduced gingival inflammation (GCF flow), pocket depth (PD), and clinical attachment loss (CAL) compared to control. SAGE also considerably reduced alveolar bone loss and reduced MMP-9, IL-6, CRP levels in gingival tissue, GCF and plasma. Cell-signaling molecules in the inflammatory cascade system TLR-2, TLR-4, p38 MAPK, ERK1/2 and NF-kB responded to SAGE in a pattern consistent with reductions at the active phase of the inflammatory process and collagenolysis.
Conclusion: In the beagle dog model of periodontitis, local SAGE administration significantly attenuated clinical measures of periodontitis, pro-inflammatory cytokines, MMPs, and signal transduction molecules. All our studies, using in vitro and in vivo models, support the therapeutic potential of SAGE as an innovative adjunct to SRP in the treatment of chronic periodontal disease.
Keywords: periodontitis, bone loss, matrix metalloproteinases, inflammation, semi-synthetic-sulfated polysaccharide
Introduction
Periodontal disease is a chronic inflammatory disease characterized by recurrent bacterial infection followed by immune responses derived from the host, which often advances to connective tissue breakdown and alveolar bone loss.1 In the presence of persistent bacterial challenge, Lipopolysaccharide (LPS) and other virulence factors from bacteria induce the host immune-inflammatory response by binding to Toll-like receptors 2 (TLR-2) and 4 (TLR-4), which in turn trigger Nuclear factor-kappa B (NF-κ B) transcription through p38 Mitogen-activated Protein kinase signal transduction system.2 NF-κ B transcription up-regulates several cellular signaling cascades such as the MAPKs and promotes the expression of pro-inflammatory cytokines such as IL-1β, TNF-α, IL-6 and C-reactive protein (CRP). These critical protein molecules were prevailing to be involved in the pathogenesis of periodontitis, including the activation of extracellular matrix and bone resorption pathways.3–7 In turn, these cytokines stimulate the production of catabolic enzymes such as MMPs, which are primarily responsible for the breakdown of connective tissues in chronic inflammatory diseases8,9 This widely accepted pathologic cascade provides the rationale for a pharmacologic strategy developed by Glycomira Therapeutics Semi-Synthetic-GlycosAminoGlycan Ether(SAGE), a potent inflammation-modulating drug.
This treatment modality evolved from the discovery of novel physicochemical properties of Heparin and Hyaluronic acid (HA). HA, a non-sulfated glycosaminoglycan (GAG) found in the extracellular matrix (ECM) of many soft connective tissues, is one of nature’s most versatile and fascinating macromolecules. HA has various biological functions ranging from cell and matrix organization, tissue repair, wound healing and inflammatory responses to cancer metastasis. It has been documented that high molecular weight HA exhibits anti-inflammatory, whereas low molecular weight HA display pro-inflammatory properties modulating inflammatory cytokines TNF-α, IL-1β and IL-6, RANKL (Receptor Activator of NF-κB Ligand) induced osteoclastogenesis10,39,40 Though HA plays a crucial role in numerous biological processes, and natural HA is not an advantageous biomaterial due to its susceptibility to degradation and inferior mechanical properties.11 On the other hand, heparin is a highly sulfated acidic GAG used clinically as an anticoagulant for decades. Apart from its anticoagulant effects, several studies validate that heparin possesses various anti-inflammatory and broad immunomodulatory activity.12,13 Although heparin has shown assurance in treating various events of inflammatory cascades, it displays drawbacks such as cross-species transfer contamination, excessive bleeding due to its anticoagulant properties and induction of thrombocytopenia resulting in catastrophic platelet aggregation.14–16 The discovery of combined approach of hemocompatible materials operationalized like heparin based on the sulfation and alkylation of the natural glycosaminoglycan hyaluronic acid “impel” the development of a potent inflammation-modulating drug known as SAGE by Glycomira Therapeutics, Inc. SAGE was manufactured from 53 kDa HA and had a final molecular weight of 5.5 kDa.17 (Supplementary Figure 1).
The previous study has demonstrated SAGE was substantial anti-inflammatory activity at a nanomolar concentration by effectively inhibiting PMN proteases, leukocyte adhesion receptor P-selectin and intercommunication of RAGE (Receptor for Advanced Glycation End product) with its disparate ligands such as carboxymethyl lysine-bovine serum albumin CML-BSA, the S100 calgranulin, S100b and the nuclear alarmin HMGB−1.19 Preliminary studies demonstrate SAGE inhibition of pro-inflammatory cytokine release by blocking TLR2 and TLR4, function as a scavenger to reduce the amount of free RANKL-induced osteoclast activation, suppress the growth of keystone pathogens P. gingivalis and A. actinomycetemcomitans commonly associated with periodontitis.18–20 Recently published study showed the potential of SAGE in attenuating LPS induced pro inflammatory mediators and MMP-9 in periodontal relevant cell culture model thereby showing this compound may be effective in preventing tissue damage in patients with periodontal disease.38 All the previous studies showed combined effects of SAGE as a powerful host inflammatory modulatory agent will be valuable as a prophylactic or therapeutic for chronic inflammatory diseases. In this study, a large (dog) animal model with naturally occurring periodontitis was brought into play to further define the multiple beneficial outcomes of SAGE as a host response modulator in reducing inflammation and this animal model is extensively acknowledged as obligatory before advancing to human trials. The current pilot study aims to determine the in vivo effects of SAGE on the clinical and biological processes of periodontitis in the dog and to further examine its cardinal mechanisms on the inflammatory cell-signaling pathway by inhibiting signal transduction molecule expressions in periodontal disease as a curative for periodontitis.
Materials and Methods
Chemical Reagents
SAGE (GM-1111) was provided by GlycoMira Therapeutics, Inc (Salt Lake City, UT) as a part of the grant. Carboxymethylcellulose as control was purchased from Sigma Chemical Co. (St Louis, MO, USA). All chemical reagents were bought from Thermo Fisher Scientific (Waltham, MA, USA).
Animal Studies
Protocols for animal studies were accepted by Stony Brook University’s Institutional Animal Care and Use Committee (IACUC). Six adult female beagle dogs (3–5 years old, 9.5–11.5 kg) with generalized periodontitis were given by Marshall BioResourses (5800 Lake Bluff Rd, North Rose, NY), after an initial screening of 9 similar dogs for significant periodontal diseases in the posterior. The Exclusion criteria of dogs included pregnancy and health or laboratory abnormalities.
Animals were sheltered in the Division of Laboratory Animal Resources (DLAR) at Stony Brook University, with care delivered by the center’s personnel. This facility pursues the Animal Welfare Act, the Public Health Service Act, and NY State law and is an AAALAC International recognized facility. The dogs were housed in standard kennels with the ambient temperature maintained at 18–24°C and all dogs were accustomed to the study environment two weeks before the initiation of the experiment. This research conforms to follow the STROBE guidelines of the case-control study.
Study Design
Data collection to assess efficacy of the drug was evaluated at four-time points as described below (Supplementary Figure 2). During all exams, procedures and sample collections, the dogs were administered general anesthesia. Anesthesia was performed by DLAR veterinarians. At each time point, water and food were removed from cages 12 hours prior to anesthesia and 2 hours after topical application. At the pre-baseline pretreatment, a full-mouth exam was done two weeks preceding baseline data collection. Periodontal measurements including the following clinical parameters probing depth (PD), bleeding-on-probing (BOP), clinical attachment loss (CAL) and tooth mobility were attained from all posterior sites of the maxillary and mandibular teeth using a UNC-15 periodontal probe (Hu-Friedy, Chicago, USA). Based upon the clinical periodontal measurements (PD, CAL, BOP and number of deep pockets), dogs with generalized moderate to severe periodontitis were evenly distributed into control and treatment groups (n=3/group) to guarantee a similar level of disease in both groups. Weight for each dog was documented and blood samples were collected to analyze pro-inflammatory mediators in serum and plasma at each time point.
Baseline
At time-0 clinical photos and standardized radiographs of the jaws were also taken. Gingival crevicular fluid (GCF) samples were collected at the selected pocket sites with probing depth of ≥5mm (8 sites/dog). Then, periodontal disease severity was measured using clinical parameters PD, BOP, CAL and tooth mobility, which were documented for all posterior teeth in both jaws. Gingival index (GI) and Plaque Index (PII) were recorded on a scale of 0 to 3 relied on the scoring criteria listed in the table below (Tables 1 and 2). After sample and data collections, a standard non-surgical periodontal treatment scaling and root planing (SRP) for each dog with an ultrasonic scaler (Parkell Inc, Edgewood, NY, USA) and hand instrumentation for calculus and plaque removal was performed until all deposits were undetectable clinically. After SRP, Local Adjunct Administration (LAA) of SAGE (GM-0111 50mg/mL) in the same vehicle or control (vehicle only: carboxymethylcellulose gel) was locally applied to preselected pocket sited with PD≥5mm through blunt needle injection once at each time point starting from baseline to 12 week time point. In addition, SAGE was applied topically on the gums 1mL gel per quadrant once/day (daily) to each dog for 12 weeks. Food/water was removed from cages 2 hours after systemic application.
 |
Table 1 The Scoring Criteria of Gingival Index (GI) |
 |
Table 2 The Scoring Criteria of Plaque Index (PlI) |
4- and 8-Week Time Periods
GCF collection, clinical measurements and LAA were performed as described in the baseline section and topical application was continued daily.
12-Week Time Periods
In addition to the procedures explained in the previous section, clinical photos and standardized dental radiographs were taken at this final time period. After GCF collection and clinical measurements were taken, two gingival biopsies/dog, approximate 5×10×1 mm3 per piece, were excised with a sterile surgical scalpel from selected sites with deep pockets, then stored at −80°C for extraction, partial purification, and analyses of MMPs by gelatin zymography and cell-signaling molecules by Western blot. At the end of this time period, topical application of SAGE or control was stopped and all dogs were effectively arranged for adoption by Stony Brook University Animal Care Facility. All data acquisition was performed in a blinded fashion.
Clinical Measurements
Clinical measurements (PlI, GI, PD, BOP and CAL=PD-CEJ to GM) (refers to the distance from the cementoenamel junction to the gingival margin) were obtained at all time periods (baseline 4, 8 and 12 weeks) in all posterior sites (three buccal and lingual/palatal sites per tooth).
GCF Extraction
GCF was collected before clinical measurements to prevent irritation of the gingiva at the designated pocket sites with PD≥5mm (6 sites/dog). The sites for GCF collection were isolated with cotton rolls to avoid saliva contamination and dried with sterile gauze sponges. A Periopaper® strip (Oraflow Inc, Hewlett, NY, USA) was introduced to the depth of the selected pocket and left in place for 10s. GCF was instantly measured using a calibrated Periotron® 6000 (Oraflow Inc, Hewlett, NY, USA) according to the manufacturer’s instructions. After collection, the experimental samples were stored at −80°C until further analysis.21,22
The frozen GCF samples were defrosted (4°C) for 10 mins. Then, 400 μL of 50 mM Tris/0.2M NaCl/5 mM CaC12 buffer (pH 7.6), including a proteinase-inhibitor cocktail (which blocks serine, cysteine, and thiol proteinases, but not MMPs), comprising of antipain (1 mg/L), aprotinin (1 mg/L), N-ethylmaleimide (125 mg/L), leupeptin (1 mg/L), and 50 mg/L detergent was added to the GCF samples. The strips containing the GCF were thoroughly mixed and extracted (1hr, 4°C) as described previously, and aliquots were removed for evaluation of inflammatory mediators and MMP’s.21,22
Radiographic Analysis of Alveolar Bone Loss
Standardized intraoral periapical jaw radiographs at selected sites were taken at baseline and 12 weeks. A parallel technique was used to position the X-ray sensor, resulting in minimal geometric distortion.24 Radiographic measurements were analyzed by Image J to assess alveolar bone loss by measuring the distance from a fixed-anatomical landmark, the cementoenamel junction (CEJ), to the alveolar bone crest (ABC). All measurements were measured using a radiopaque probe as a standard internal control.23,24
Gingival Tissue Extraction and Partial Purification
Gingival biopsies from each dog at a 12-week time point were obtained as described above. Gingival tissues were homogenized and the MMPs partially purified as described previously.25,26 Protein content of the extracts was determined by Bio-Rad Protein Assay. The levels of MMPs and inflammatory cytokines in the gingival extracts were analyzed by gelatin zymogram and ELISA and the expressions of cell-signaling molecules were analyzed by Western blot as described below.
Gelatin Zymography
Analyses for MMP-9 (pro-form: 92 kDa in GCF), gingival tissue and plasma from blood samples were described previously.25–28 The gelatin zymography system and SDS-PAGE gels, containing polyacrylamide and gelatin at a final concentration of 1mg/mL were made. After electrophoresis (120V), the gels were washed with 2.5% Triton X-100, incubated at 37∘ C overnight in calcium assay buffer (40mMTris/HCl, 200mMNaCl, 10mM CaCl2, and pH 7.5), and then stained with Coomassie Brilliant Blue R-250. The clear zones of lysis against a blue background specifies gelatinolytic activity and were scanned densitometrically by Image J to assess gelatinase activity.29
ELISA Assay
ELISA kits for cytokines IL-1β (Catalog# DY3747), IL-6 (Catalog# CA6000), and TNF-α (Catalog# CATA00) analysis were bought from R&D Systems, Inc. (Minneapolis, MN, USA). The CRP kit (Catalog# MBS704226) was purchased from MyBioSource, Inc. (San Diego, CA, USA). All assays were performed following the manufacturer’s guidelines and instructions.
Western Blot Analysis
Gingival tissue biopsies were treated with RIPA buffer (pH 7.0) (R0278, Sigma-Aldrich, Inc. USA) containing Halt™ Protease and Phosphatase Inhibitor Cocktail, EDTA-free (1:100, 78,441, Thermo Fisher Scientific, Inc., USA). Prestained Precision Plus Protein™ Kaleidoscope™ (1,610,375, Bio-Rad Laboratories, Inc., USA) were used as molecular weight markers. The samples were electrophoresed on SDS-PAGE gels and then transferred to nitrocellulose membranes. Western blot analysis was performed as described previously.29 Immunoreactivity and expression of cell signaling molecules TLR-2, TLR-4 (Thermo Fischer Scientific, Inc., USA) p38 MAPK, ERK1/2 and NF-kB (Cell Signaling Technology, USA) were visualized as dark bands opposing a clear background. The membranes were carefully scanned with Invitrogen™ iBright™ FL1000 Western Blot Imaging Systems (Thermo Fisher Scientific, Inc., USA) for imaging and documenting chemiluminescent Western blot.
Statistical Analysis
Pro-inflammatory cytokines, MMP’s, protein expressions and alveolar bone loss of both control and SAGE treatment groups were analyzed by Student’s t-test, and also by ANOVA, with p<0.05 taken as statistically significant.
Results
Probing Depth (PD) and Clinical Attachment Loss (CAL)
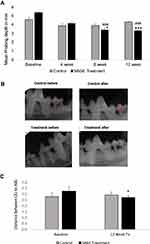
Probing depth measurements and clinical attachment loss in the vehicle-treated control group not only exhibited no improvements over the 12 week protocol (Figure 1A, Supplementary Figure 3A) but also showed a gradual increase in mean probing depth in 12 week time period compared to 4 week and 8 week time period. In contrast, SAGE treatment significantly decreased mean probing depth and clinical attachment loss by about 23% (p<0.01 and p<0.0001) compared to the control group at 8 week and 12 week time period or compared to baseline values (p<0.001) (Figure 1A, Supplementary Figure 3A) at 8 and 12 week time period as well. Furthermore, the total numbers of pockets with PD ≥ 4mm were also significantly reduced by SAGE treatment at the 12 week time period by more than 26% (p<0.05), compared to control; as well as compared to its own baseline at both the 8 and 12 week time periods (p<0.05) (Supplementary Figure 3B).
Alveolar Bone Loss
Consistent with the above-explained clinical parameters, radiographically assessed alveolar bone loss at a 12-week time point showed that control administration produced no significant change in this critical assessment of periodontal destruction. In contrast, SAGE treatment produced a significant (p<0.05) 9% reduction in alveolar bone loss (Figure 1B and C).
Gingival Crevicular Fluid (GCF)
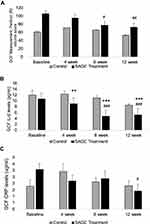
As indicated in Figure 2A, the GCF volume score appeared to be inconsistent, even at baseline measurements. The GCF score was greater in the SAGE treatment group compared to the control group before starting the experimental treatment protocol, but the pattern of change over time for both the groups was similar to that for the clinical measurement of pocket depth (Figure 1A). The scores were significantly decreased in the SAGE group by 27% and 31% at 8 and 12 week time period compared to its own baseline values (p<0.05) (p<0.01) (Figure 2A). However, it is prominent that 12 week control group were not different from its own baseline values, IL- 6 concentrations (pg/mL) showed a statistically significant reduction of about 27%, 57% and 37% (p<0.01) and (p<0.001) in SAGE treated group compared to control group in all 4.8 and 12 week time period, or compared to its own baseline values (p<0.001) in 8 and 12 week time period (Figure 2B). In contrast, the control group did not show any significant change in the 12 week period.
Similar to GCF score CRP levels (µg/mL) and MMP-9 levels in gelatin zymogram appeared to be relatively high in the baseline time period, but there was a significant decrease in CRP levels of about 55%. (p<0.05) in a 12-week time point (Figure 2C) and there was a significant decrease in MMP-9 levels of about 30%, 25% and 37% (p<0.001) and (p<0.01) at 4, 8, and 12 week time point compared to its own baseline (Figure 3A). The vehicle administered control group showed some variability, but there was no significant change over the 12- week time period. (Figures 2B and 3A). Other inflammatory markers IL-1β and TNF-α were undetectable by ELISA measurements.
Blood Samples
Consistent with the above results for GCF, IL-6 concentration (pg/mL) and CRP levels (µg/mL) was significantly reduced over the 12 week time point compared to its own baseline (p<0.05) by 62% (data not shown). Plasma MMP-9 levels assessed by gelatin zymogram showed a significant reduction at 8- and 12-week time point by 46% and 37% respectively compared to the control group or compared to its own baseline at 12 week time point (p<0.05) (Figure 3B). MMP-9 levels in the control group indicated variability in its pattern but did not show much change over 12 week time period. Other inflammatory markers, IL-1β, TNF-α and were undetectable by ELISA measurements.
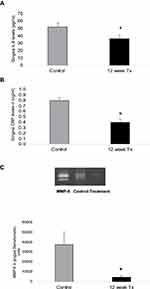
Gingival Extracts
IL-6 concentration (pg/mL) and CRP levels (µg/mL) were significantly reduced compared to control (p<0.05) by 30% and 50% respectively at 12 week time point (Figure 4A and B). Significant changes in levels of MMP-9 and in partially purified extracts of gingival tissues were seen at 12 weeks, the conclusion of the study. As shown in a representative zymogram (Figure 4C), 92 kDa gelatinase or MMP-9 was clearly detectable in the gingival tissues of both groups of dogs; SAGE treatment significantly reduced total forms of this collagenolytic neutral proteinase by 88% (p<0.05) at 12 week time point compared to control group. Other inflammatory markers IL-1 beta and TNF were undetectable by ELISA measurements.
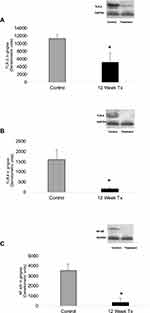
The extracts of gingival tissues were also analyzed for the expression of cell-signaling molecules, TLR-2, TLR-4, p38 MAPK, ERK1/2 and NF-kB (Figure 5A–C, Supplementary Figure 4) by Western blot technique.35 SAGE treatment significantly reduced TLR-2 and 4 (p<0.05) by 54% and 89%, p38 MAPK, ERK1/2 also showed a pattern of reduction of about 80% and 50% but this effect was not statistically significant (Supplementary Figure 4) and also decreased NF-kB (p<0.05) by 89%, at the 12 week period related to control group.
Discussion
Periodontitis is a complex disease that has two primary etiologies, the bacterial biofilm and destructive host response. Aside from the virulence factors usual to most bacteria, their ability to induce the production of pro-inflammatory cytokines and chemokines by macrophages and other inflammatory cells that act to modify the host immune response further amplifies their pathogenicity.30 Experimental studies showed the efficacy of subcutaneous administration of SAGE in a diabetic rat model of periodontitis significantly reduced IL-6, TNF-α, MMP-2 and −9 in gingival tissue extracts and reduced alveolar bone loss by increasing bone density (Results yet to be published).41 This present study was devised to investigate the potential for SAGEs applied locally, the probable route of drug administration in humans to treat naturally occurring periodontitis in beagle dogs.4,17–19,31
Multiple mechanisms of action, exhibited by this novel compound SAGE, proved to be involved in inhibiting LPS induced pro inflammatory cytokines and proteinases contributing to periodontal tissue destruction support SAGE to be investigated as a universal therapeutic benefit for periodontal disease by performing a “clinical trial” in beagle dogs.19,38
In the current pilot study, we investigated the feasibility of SAGE to reduce periodontal inflammation and alveolar bone loss in a naturally occurring beagle dog periodontitis model. Dog models are extensively used for the periodontal study because periodontal inflammation is natural in dogs and its pathological features are related to those of periodontitis in humans.32–34, Based on our findings concerning clinical periodontal measurements as the primary outcome, SAGE was effective at significantly reducing probing depths and alveolar bone loss over compared to the control group, thereby attenuating the active phase of periodontal destruction was seen in the treatment group. There was no significant change in bleeding on probing, gingival index and plaque index within a 12-week time point after scaling and root planing. Regarding Gingival crevicular fluid (GCF), SAGE appeared to prevent post-SRP spikes in systemic inflammation documented in the periodontal literature.35 Western blot results in gingival extracts showed that SAGE was efficacious in attenuating the inflammatory signal cascade system by decreasing the expression levels of TLR-4, TLR-2, p38 MAPK, Erk1/2, and NF-κ B. This initial pilot study findings indicate that the topical administration of a novel semisynthetic sulfated polysaccharide to the Beagle dog model acts as an essential and potent inhibitor of both alveolar bone loss and its inflammatory and collagen-destructive mediators. These in vivo results in a naturally occurring beagle dog model of periodontal disease demonstrated that SAGE treatment efficiently improved clinical parameters of periodontal disease while lessening both local inflammatory cytokines along with signal transduction molecules and local alveolar bone loss.
Conclusion
These in vivo results in a naturally occurring beagle dog model of periodontal disease demonstrated that SAGE treatment efficiently improved on clinical parameters of periodontal disease while reducing both pro-inflammatory mediators and alveolar bone loss. These results and previous studies showed the feasibility of SAGE as universal therapeutic benefit to ameliorate periodontitis and position SAGE as a potential new adjunct to SRP for improved management of the periodontal disease.
Acknowledgments
This study was funded by NIDCR R44DE022216 and Glycomira, Inc. Authors and we would like to thank Rachel Kogen and Peggy Yang (Department of Oral Biology and Pathology, Stony Brook University, New York, USA) for their assistance with the dog study.
Disclosure
GP is CSO and holds equity in GlycoMira Therapeutics. In addition, Dr Glenn Prestwich is an inventor on U.S. Patent 7,855,187 (December 21, 2010) with royalties paid to GlycoMira Therapeutics. Dr Maria Ryan reports funding from a subcontract to Stony Brook based NIDCR SBIR R44DE022216 grant to GlycoMira Therapeutics, during the conduct of the study; is currently an employee of Colgate Palmolive Company. VR, YG, HML, and JD declare that they have no competing interests in this work.
References
1. Ryan ME. Host modulation: conceptualization to clinical trials and integration into clinical practice. J Calif Dent Assoc. 2002;30(4):285–288.
2. Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22(2):240–273. doi:10.1128/CMR.00046-08
3. Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating metabolism with inflammation. Trends Cell Biol. 2012;22(11):557–566.
4. Li Q, Yu H, Zinna R, et al. Silencing mitogen-activated protein kinase-activated protein kinase-2 arrests inflammatory bone loss. J Pharmacol Exp Therap. 2011;336(3):633–642.
5. Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. 2001;107(1):7.
6. Graves D. Cytokines that promote periodontal tissue destruction. J Periodontol. 2008;79(8 Suppl):1585–1591.
7. Giannobile WV. Host-response therapeutics for periodontal diseases. J Periodontol. 2008;79(8S):1592–1600.
8. Robbins JR, Thomas B, Tan L, Choy B, Arbiser JL, Berenbaum F. Immortalized human adult articular chondrocytes maintain specific interleukin phenotype and responses to interleukin‐1β. Arthritis Rheumatol. 2000;43(10):2189–2201.
9. Largo R, Alvarez-Soria MA, Dıez-Ortego I, et al. Glucosamine inhibits IL-1.-induced NFκB activation in human osteoarthritic chondrocytes. Osteoarthr Cartil. 2003;11(4):290–298.
10. Xu X, Jha AK, Harrington DA, Farach-Carson MC, Jia X. Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. Soft Matter. 2012;8(12):3280–3294. doi:10.1039/C2SM06463D
11. Pike DB, Cai S, Pomraning KR, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27(30):5242–5251. doi:10.1016/j.biomaterials.2006.05.018
12. Mousavi S, Moradi M, Khorshidahmad T, Motamedi M. Anti-inflammatory effects of heparin and its derivatives: a systematic review. Adv Pharmacol Sci. 2015;2015. doi:10.1155/2015/507151
13. Rao NV, Argyle B, Xu X, et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol. 2010;299(1):C97–C110. doi:10.1152/ajpcell.00009.2010
14. Wang L, Brown JR, Varki A, Esko JD. Heparin’s anti-inflammatory effects require glucosamine 6-O-sulfation and are mediated by blockade of L-and P- selectins. J Clin Invest. 2002;110(1):127. doi:10.1172/JCI14996
15. Björk I, Lindahl U. Mechanism of the anticoagulant action of heparin. Mol Cell Biochem. 1982;48(3):161–182. doi:10.1007/BF00421226
16. Warkentin TE. Heparin-induced thrombocytopenia. In: Critical Decisions in Thrombosis and Haemostasis. Hamilton. Pagg: BC Decker Inc.; 1998:100–108.
17. Zhang J, Xu X, Rao NV, et al. Novel sulfated polysaccharides disrupt cathelicidins, inhibit RAGE and reduce cutaneous inflammation in a mouse model of rosacea. PLoS One. 2011;6(2):e16658. doi:10.1371/journal.pone.0016658
18. Oottamasathien S, Jia W, McCoard L, et al. A murine model of inflammatory bladder disease: cathelicidin peptide 157 induced bladder inflammation and treatment with sulfated polysaccharides. J Urol. 2011;186(4):1684–1692. doi:10.1016/j.juro.2011.03.099
19. Savage JR, Pulsipher A, Rao NV, et al. A modified glycosaminoglycan, GM-0111, inhibits molecular signalingC involved in periodontitis. PLoS One. 2016;11(6):e0157310. doi:10.1371/journal.pone.0157310
20. Xu L, Yu Z, Lee HM, et al. Characteristics of collagenase-2 from gingival crevicular fluid and peri-implant sulcular fluid in periodontitis and peri-implantitis patients: pilot study. Acta Odontol Scand. 2008;66(4):219–224. doi:10.1080/00016350802183393
21. Golub LM, Lee HM, Stoner JA, et al. Subantimicrobial‐dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol. 2008;79(8):1409–1418. doi:10.1902/jop.2008.070623
22. White SC, Pharoah MJ. Oral Radiology-E-Book: Principles and Interpretation. Elsevier Health Sciences; 2014.
23. Jeffcoat MK, Reddy MS. Advances in measurements of periodontal bone and attachment loss. Monogr Oral Sci. 2000;17:56–72. doi:10.1159/000061636
24. Zaki HA, Hoffmann KR, Hausmann E, Scannapieco FA. Is radiologic assessment of alveolar crest height useful to monitor periodontal disease activity? Dent Clin. 2015;59(4):859–872. doi:10.1016/j.cden.2015.06.009
25. Elburki MS, Moore DD, Terezakis NG, et al. A novel chemically modified curcumin reduces inflammation‐mediated connective tissue breakdown in a rat model of diabetes: periodontal and systemic effects. J Periodontal Res. 2017;52(2):186–200. doi:10.1111/jre.12381
26. Elburki MS. A Novel Chemically Modified Curcumin as a Pleiotropic MMP-Inhibitor: Therapeutic Potential in Locally-and Systemically Induced Periodontal (and other) Connective Tissue Breakdown. [Doctoral dissertation]. Stony Brook, NY: The Graduate School, Stony Brook University; 2015.
27. Lee MH, Golub ML, Cao J, et al. CMT-3, a non-antimicrobial tetracycline (TC), inhibits MT1-MMP activity: relevance to cancer. Curr Med Chem. 2001;8(3):257–260. doi:10.2174/0929867013373660
28. Golub LM, Ramamurthy NS, Llavaneras A, et al. A chemically modified nonantimicrobial tetracycline (CMT‐8) inhibits gingival matrix metalloproteinases, periodontal breakdown, and extra‐oral bone loss in ovariectomized rats. Ann N Y Acad Sci. 1999;878(1):290–310. doi:10.1111/j.1749-6632.1999.tb07691.x
29. Elburki MS, Rossa C, Guimarães-Stabili MR, et al. A chemically modified curcumin (CMC 2.24) inhibits nuclear factor κB activation and inflammatory bone loss in murine models of LPS-induced experimental periodontitis and diabetes-associated natural periodontitis. Inflammation. 2017;40(4):1436–1449.
30. Suzuki N, Yoneda M, Hirofuji T. Mixed red-complex bacterial infection in periodontitis. Int J Dent. 2013;2013:587279.
31. Lee WY, Savage JR, Zhang J, Jia W, Oottamasathien S, Prestwich GD. Prevention of anti-microbial peptide LL-37-induced apoptosis and ATP release in the urinary bladder by a modified glycosaminoglycan. PLoS One. 2013;8(10):e77854.
32. Attström R, Graf‐de Beer M, Schroeder HE. Clinical and histologic characteristics of normal gingiva in dogs. J Periodontal Res. 1975;10(3):115–127.
33. Reddy MS, Weatherford TW, Smith CA, West BD, Jeffcoat MK, Jacks TM. Alendronate treatment of naturally occurring periodontitis in beagle dogs. J Periodontol. 1995;66(3):211–217.
34. Albuquerque C, Morinha F, Requicha J, et al. Canine periodontitis: the dog as an important model for periodontal studies. Vet J. 2012;191(3):299–305.
35. Graziani F, Cei S, Tonetti M, et al. Systemic inflammation following non‐surgical and surgical periodontal therapy. J Clin Periodontol. 2010;37(9):848–854.
36. Yimam M, Brownell L, Do SG, et al. Protective effect of UP446 on ligature-induced periodontitis in beagle dogs. Dent J. 2019;7(2):33.
37. Ramseier CA, Rasperini G, Batia S, Giannobile WV. Advanced reconstructive technologies for periodontal tissue repair. Periodontol 2000. 2012;59(1):185–202.
38. Gu Y, Raja V, Lee HM, Hong H, Prestwich G, Ryan ME. Therapeutic potential of a novel semi-synthetic-sulfated-polysaccharide to suppress inflammatory mediators in P. gingivalis LPS stimulated human monocytes/macrophages. J Inflamm. 2021;18(1):1–11.
39. Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28(3):78–88.
40. Ariyoshi W, Takahashi T, Kanno T, et al. Mechanisms involved in enhancement of osteoclast formation and function by low molecular weight hyaluronic acid. J Biol Chem. 2005;280(19):18967–18972.
41. Raja VS. A Novel Semi-Synthetic-GlycosAminoglycan Ether (SAGE) Attenuates Local and Systemic Inflammation Associated with Periodontitis and Diabetes. [Doctoral dissertation]. Stony Brook: State University of New York; 2018.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.