Back to Journals » International Journal of General Medicine » Volume 13
Safety Considerations in Cannabinoid-Based Medicine
Authors Gottschling S, Ayonrinde O, Bhaskar A, Blockman M , D’Agnone O, Schecter D, Suárez Rodríguez LD, Yafai S, Cyr C
Received 1 August 2020
Accepted for publication 5 October 2020
Published 1 December 2020 Volume 2020:13 Pages 1317—1333
DOI https://doi.org/10.2147/IJGM.S275049
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sven Gottschling,1 Oyedeji Ayonrinde,2 Arun Bhaskar,3 Marc Blockman,4 Oscar D’Agnone,5 Danial Schecter,6 Luis David Suárez Rodríguez,7 Sherry Yafai,8 Claude Cyr9
1Universitätsklinikum des Saarlandes, Homburg (Saar), Germany; 2Queen’s University, Kingston, Ontario, Canada; 3Imperial College Healthcare NHS Trust, London, UK; 4University of Cape Town and Groot Schuur Hospital, Cape Town, South Africa; 5The OAD Clinic, London, UK; 6Spectrum Therapeutics, Smiths Falls, Ontario, Canada; 7Centro de Medicina Integrativa Sanar, Playa del Carmen, Mexico; 8The Releaf Institute, Santa Monica, CA, USA; 9McGill University, Montreal, Quebec, Canada
Correspondence: Claude Cyr
McGill University, 3500 DeMaisonneuve Boulevard, Suite 1520, Montreal, Quebec H3Z3C1, Canada
Tel +1-514-264-9657
Email [email protected]
Abstract: Cannabinoids are a diverse class of chemical compounds that are increasingly recognized as potential therapeutic options for a range of conditions. While many studies and reviews of cannabinoids focus on efficacy, safety is much less well reported. Overall assessment of the safety of cannabinoid-based medicines is confounded by confusion with recreational cannabis use as well as different study designs, indications, dosing, and administration methods. However, clinical studies in registered products are increasingly available, and this article aims to discuss and clarify what is known regarding the safety profiles of cannabinoid-based medicines, focusing on the medical and clinical safety evidence and identifying areas for future research. The two most well-studied cannabinoids are Δ9-tetrahydrocannabinol (THC), or its synthetic variants (dronabinol, nabilone), and cannabidiol (CBD). Across diverse indications, dizziness and fatigue are generally the most common adverse events experienced by patients receiving THC or combined THC and CBD. Patients receiving THC may experience adverse cognitive effects and impairment in psychomotor skills, with implications for driving and some occupations, while CBD may help to lower the psychotropic effects of THC when used in combination. Studies on dependency and addiction in a medical context are limited, but have shown inconsistent findings regarding misuse potential. Generally, the recommended route of administration is oral ingestion, as smoking medicinal cannabinoid products potentially releases mutagenic and carcinogenic by-products. There are several potential drug–drug interactions and contraindications for cannabinoid-based medicines, which physicians should account for when making prescribing decisions. The available evidence shows that, as with any other class of pharmaceuticals, cannabinoid-based medicines are associated with safety risks which should be assessed in the context of potential therapeutic benefits. Each patient should be assessed on an individual basis and physicians must rely on informed, evidence-based decision-making when determining whether a cannabinoid-based medicine could be an appropriate treatment option.
Keywords: cannabis, cannabinoid, safety, cannabidiol, Δ9-tetrahydrocannabinol
Plain Language Summary
Cannabinoids are drugs that are either found in the cannabis plant or made in a laboratory. There are many cannabinoids but researchers are mostly interested in CBD (cannabidiol) and THC (Δ9-tetrahydrocannabinol). Cannabinoids can help with several different diseases, but it is important to know how safe they are. Doctors need to know the facts about the pros and cons of cannabinoids so they know which patients they may be able to help.
Medicines with cannabinoids in them (cannabinoid-based medicines) either contain THC or CBD or both. They can be swallowed or inhaled (smoking or vaping). Because there are so many different types, it is hard to find out how safe they are. This article looks at what we know now and what researchers still need to find out.
Many patients taking cannabinoid-based medicines get side effects, but very few get serious side effects. There are some groups of patients who should not take cannabinoids because they are more likely to get serious side effects. The most likely side effects for patients taking THC are feeling dizzy or tired. Patients who take THC might not be able to drive as they can have problems with moving and thinking clearly (cognitive effects). CBD does not cause these effects. Taking CBD and THC together might help reduce the cognitive effects of THC.
Cannabinoids can be helpful for treating some diseases, but we need more information to understand who they can help most and when it is best to use them.
Note on Terminology
Currently, terminology in this therapy area is non-standardized, with a variety of terms used interchangeably. In this article, we have defined and used the terminology below for clarity.
Cannabinoid-based medicine: medical therapy area pertaining to the use of cannabinoids with therapeutic intent.
Cannabinoid-based medicines: standardized products containing known cannabinoid constituents that are used under medical supervision with therapeutic intent.
Medical cannabis: cannabinoid-containing products derived from the cannabis plant used with therapeutic intent (whether clinically diagnosed or perceived), not necessarily a medical product nor used under medical supervision.
Cannabis use: recreational drug use with the aim of inducing euphoria or other cognitive effects (no therapeutic intent).
Introduction
Cannabinoids are a class of medicines that are increasingly recognized by global and national guidelines as potential treatment options for a range of conditions.1–7 Cannabinoids either occur naturally in the human body (endocannabinoids), are derived from the cannabis plant (phytocannabinoids), or are synthesized in the laboratory (synthetic cannabinoids) (Figure 1).8,9 Although approximately 150 cannabinoids have been identified,9,10 the two most well studied are Δ9-tetrahydrocannabinol (THC) (responsible for the euphoric effects associated with cannabis) and cannabidiol (CBD). These have different pharmacologic properties and have shown efficacy in clinical trials, either alone or in combination with each other.11–14
 |
Figure 1 Types of cannabinoids.8,9 |
The approved indications for specific cannabinoid-based medicines in either North American or European countries (among other countries) include: spasticity associated with multiple sclerosis (~1:1 THC:CBD oromucosal spray, known as nabiximols); AIDS/cancer cachexia or chemotherapy-induced nausea and vomiting (dronabinol or nabilone, respectively synthetic THC and a synthetic THC analog); and Lennox-Gastaut and Dravet syndromes (CBD).15–20 In addition to those mentioned above, other quality-controlled products with defined cannabinoid constituents are available in some countries and are prescribed for a range of other conditions, such as chronic pain (including neuropathic pain).
Cannabinoid-based medicines act on the human endocannabinoid system, a network of CB1, CB2, and other receptors distributed throughout the body. CB1 receptors are congregated predominantly in the central and peripheral nervous systems, with a low concentration in the respiratory center in the brainstem, while CB2 receptors are found largely in the immune and hematopoietic systems, as well as the brain, liver, endocrine pancreas, and bone.21–26 First discovered in 1992, endocannabinoids, such as anandamide or 2-arachidonoylglycerol, are produced naturally in the body and act on these receptors to regulate a variety of processes, including pain perception and neuroendocrine-immune pathways.22,27 Although current understanding of the endocannabinoid system is limited, it remains an important focus for research.
The number of clinical studies assessing the potential therapeutic benefits of cannabinoids is increasing, especially as cultural and legal barriers to research and access continue to ease. However, while there are several valuable reviews and commentaries on the efficacy of cannabinoids for a variety of indications,28–30 both short- and long-term safety are less well reported, despite these being critical factors in regulatory and prescribing decisions.
Many guidelines, recommendations, and reviews on cannabinoids do not differentiate data from studies in recreational and medical contexts when discussing safety, and this amalgamation of disparate data has led to confusion around the safety profiles of cannabinoid-based medicines.31–35 Studies of recreational cannabis use are associated with several confounding factors that may not be applicable to cannabinoid-based medicines. Recreational cannabis use may be associated with uncertain product compositions, with potentially a very high THC (or synthesized equivalent) content, and unknown impurities. Moreover, dosing may be unknown and uncontrolled, and usage may be accompanied by concomitant tobacco or other recreational drug use.36 Recreational cannabis users are often aiming to take a large dose in order to induce euphoria and other psychoactive effects. In contrast, cannabinoid-based medicines are quality-controlled products with defined cannabinoid composition and standardized dosing, taken with the aim of achieving symptom relief. These products are administered under the direction of an informed, licensed healthcare professional, with appropriate monitoring procedures for concomitant medications and adverse events (AEs).
Although studies of recreational cannabis use can be of some value when medical literature is sparse, generalizing findings is challenging, as they are often based on subjective, unverifiable reports along with the associated factors described above. However, published data from studies in approved, registered products are increasingly available and can be leveraged to determine a more accurate picture of the safety profile of cannabinoid-based medicines in their intended therapeutic settings and patient populations.
Analyses of the overall safety of cannabinoid-based medicines are further confounded by variation in dosing and administration methods across products, study designs, and indications. While there are standardized doses for CBD in Lennox-Gastaut and Dravet syndromes, and in investigational studies,11,19,37 a commonly used dosing strategy for products containing THC is “start low, go slow, stay low”.38 Patients, under supervision from their physician, start with a low dose of the cannabinoid-based medicine and gradually “titrate” until a balance between satisfactory symptom reduction and minimal adverse effects is achieved (this practice is employed for a variety of approved medications39). While this personalized approach is helpful for patients as individuals, lack of standardization, even within the same indication, represents a challenge for overall analysis of safety, and more studies to establish appropriate standard doses or dose ranges for each indication are needed. Additionally, while many cannabinoid-based medicines are administered orally, inhalation may be deemed appropriate in some cases and these different administration methods are associated with different pharmacokinetic characteristics. Although the general pharmacokinetic and pharmacodynamic properties of THC and CBD are broadly known,40,41 more studies are needed to understand further the effects of specific cannabinoid combinations on the intended patient populations.
The aim of this article is to discuss and clarify what is known regarding the safety profiles of cannabinoid-based medicines, focusing on the medical and clinical safety evidence and target areas for future research. We do not aim to comment on the regulations of individual countries, which vary widely, nor on the safety of recreational cannabis use.
This review is based on assessment and review of literature analyses of clinical trials and real-world studies of cannabinoid-based medicines, including registered products and other products used for medical purposes, together with multidisciplinary expert opinion and discussion. As well as ClinicalTrials.gov searches (Table 1), we qualitatively evaluated the literature based on PubMed searches including terms related to cannabis AND safety in the title or abstract.
 |  |  |
Table 1 Safety Data from Phase 3 Randomized Controlled Trials in Registered, Approved Cannabinoid-Based Medicines |
Adverse-Event Profiles
Overall analysis of traditional measures of drug safety, such as number and type of AEs, is challenging for cannabinoid-based medicines given the substantial differences between cannabinoids, individual product composition, study designs (particularly non-standardized dosing and administration), indications, and populations studied. Strategy and findings from a ClinicalTrials.gov search for randomized, controlled, Phase 3 clinical trials of cannabinoid-based medicines for approved indications are shown in Table 1, and illustrate that these challenges are apparent even across major clinical trials. Interestingly, there were no clinical trials found for dronabinol or nabilone that met the inclusion criteria for this search (Table 1).12,13,42–48
However, many additional safety data from both randomized controlled trials and real-world evidence studies are available. A pragmatic approach is needed to assess these data and draw key safety findings, which are outlined below.
Across clinical trials, the most common AEs reported by patients receiving THC alone (including plant-derived THC, dronabinol, and nabilone) were generally dizziness, drowsiness/somnolence and fatigue, dry mouth, nausea/vomiting, and effects on cognitive function (eg, perception disorders, euphoria, confusion); balance and coordination problems were also commonly reported.14,28,49–57 The most common AEs remained broadly similar across diverse patient populations, including those with cachexia due to AIDS,14 multiple sclerosis,49 chronic pain conditions including neuropathic pain,28,55,57 chemotherapy-induced nausea/vomiting,51 medication overuse headache,52 or sleep in fibromyalgia.53
The AE profile of CBD is different to THC, with common AEs across clinical trials and real-world studies including diarrhea, somnolence, pyrexia, decreased appetite, vomiting, upper respiratory tract infection, and breakthrough epilepsy symptoms, noting that randomized controlled trials and real-world evidence studies of CBD are almost all in patients with rare forms of epilepsy using doses of 5–20 mg/kg daily.11,12,58–61 The most notable serious AE was elevated liver enzymes (ie, alanine transaminase, aspartate aminotransferase, and gamma-glutamyl transferase11,12). However, dosing regimens for CBD in other conditions such as chronic pain have not yet been determined, with preclinical data suggesting a much lower therapeutic dose than that required for treating seizures, accompanied by a more favorable safety profile.62
The most common AEs reported with nabiximols treatment (2.7:2.5 mg THC:CBD oromucosal spray, maximum of 12 sprays per day18) in patients with multiple sclerosis include dizziness, fatigue, somnolence, nausea, and application site discomfort,13 with few patients experiencing cognitive AEs.48 Elevated liver enzymes did not appear to be a key safety signal, perhaps due to the relatively low dose of CBD compared with that used to treat patients with epilepsy. In patients prescribed other cannabis plant extracts containing both THC and CBD for other indications, the AE profile appeared generally similar to that of THC.
Overall, although many patients receiving cannabinoid-based medicines experienced AEs, the incidence of serious AEs was generally not significantly different compared with control individuals, with no serious AEs reported in some studies.28,29,63 AE profiles from real-world studies broadly correlate with those of clinical trials and are generally dose dependent, with dizziness, dry mouth, and somnolence commonly reported.64,65
The “start low, go slow, stay low” dosing titration method may help to mitigate some AEs by finding a patient-specific balance between efficacy and tolerability.38 Individual titration will depend on the cannabinoid combination, indication, concomitant medications, demographic characteristics, and patient’s previous experience with the medication. However, more studies in specific indications are needed to establish the frequency and intensity of AEs, and to optimize dosing for relevant patient populations.
Real-World Medical Use Patterns
Although the majority of clinical trial data are in the approved indications, real-world studies show that patients are using cannabinoid-based medicines to treat a range of other conditions (Figure 2),64,66 with chronic pain accounting for approximately 30–80% of patients.64,66–68 Additionally, and despite limited evidence of efficacy, mental health conditions were found to account for 27% and sleep disorders for 9.7% of cannabinoid-based medicine use in registered Canadian patients.66 Given that almost half (45.5%) of patients with chronic pain conditions also suffer from sleep disorders and that alleviation of pain may lead to improved sleep quality, cannabinoid-based medicines may have a role in treating both these symptoms.35,69
 |
Figure 2 Patient-reported primary indications for cannabinoid-based medicine uptake in Canada. Adapted under the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/) from Baron EP, Lucas P, Eades J, Hogue O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain. 2018;19(1):37.66 |
Although not specifically indicated for chronic pain, some chronic pain guidelines include cannabinoid-based medicines in recommended treatment algorithms,3,4 and the common AEs associated with cannabinoids seem generally comparable to those seen with other established treatments for chronic pain (Table 2).4 Although there are no studies that directly compare the overall safety profiles of cannabinoid-based medicines with other pain therapies, data from non-clinical studies indicate that there are potentially clinically meaningful differences in mortality and dependency (both in favor of cannabinoids), which warrant further investigation.70,71 This constitutes an important area for future research.
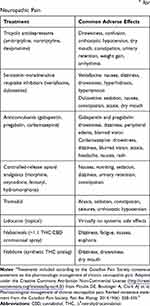 |
Table 2 Common Adverse Effects of Treatment Optionsa for Neuropathic Pain |
These findings represent a clear unmet need for clinical trials to assess the efficacy and safety of standardized cannabinoid-based medicines in the conditions in which they are being used and to compare them with existing standards of care. Meanwhile, existing real-world data could be leveraged to address urgent questions surrounding these therapeutic needs.72
Concomitant Medication Use and Contraindications
There is a theoretical risk of drug–drug interactions between some cannabinoids and some concomitant medications.73,74 However, these have not been well studied in clinical practice and more drug-interaction studies are urgently needed to establish the extent of any interactions, including dose-dependent effects, especially with common medications that patients may be receiving alongside cannabinoids. Caution should be exercised with any concomitant medication that is metabolized by the CYP450 complex, due to pharmacokinetic interactions with THC or CBD;75–77 however, the exact mechanisms of these interactions and their clinical relevance remain unknown. Current recommendations regarding concomitant medications are shown in Table 3.73,77,78
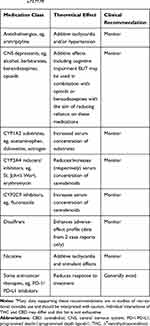 |
Table 3 Current Monitoring Recommendations for Concomitant Medicationsa,73,77,78 |
Products approved and licensed for specific indications by national regulatory bodies (Epidiolex®, Sativex®, Marinol®, Syndros®, and Cesamet®) have clearly defined contraindications,15,16,18–20 and physicians should check the latest prescribing information to ensure compliance. For cannabinoid-based medicines that are not licensed for a specific indication, guidelines recommend contraindications based on currently available evidence (Table 4).73 It should be noted that some of these rely on data from recreational cannabis use studies; therefore, it is not always clear which cannabinoid is relevant for the contraindication. In addition to regulatory compliance, safety should always be considered in the light of the specific cannabinoid and individual risk-benefit evaluation. For example, patients aged <25 years may be at risk of long-term cognitive effects from THC; however, for pediatric patients with refractory chemotherapy-induced nausea and vomiting, the potential benefits of THC-based treatments such as dronabinol or nabilone may outweigh the risks.79 The currently known contraindications do not seem excessive or severely restrictive in the context of other treatments for similar indications.80
 |
Table 4 Recommended Contraindications for Cannabinoid-Based Medicines Based on the Current State of Evidencea,35,73 |
Cannabis-Use Disorder and Cannabis-Withdrawal Syndrome
While dependency is a normal physiologic reaction to many pharmaceutical products, and tolerance and withdrawal symptoms might be expected in patients on long-term medications for chronic conditions, substance-use disorder is generally characterized by continued use despite harm or risky behavior, cravings, and impaired control.81 Diagnostic criteria for cannabis-use disorder and cannabis-withdrawal syndrome are clearly defined elsewhere.81 However, available tools to detect substance-use disorder designed for recreational drug use may not be suitable for assessing problematic medication use in patients.82,83
The majority of data on cannabis-use disorder are in recreational use, with associated confounding factors of concomitant use of other recreational drugs and smoking. Data from recreational cannabis use studies indicate a prevalence of 18–34%.71,84 This is lower than estimated figures for opioid or alcohol users,71 with a much smaller estimated global burden.85 Additionally, recreational cannabis use does not appear to be associated with increased mortality compared with the general population.85 However, these data should be interpreted cautiously and cannot be extrapolated to standardized, quality-controlled products administered (often orally) under medical supervision.
Although studies on cannabis-use disorder and cannabis-withdrawal syndrome in a therapeutic context are limited, available data seem to show that incidence of cannabis-use disorder in patients receiving cannabinoid-based medicines is generally low.65,86 A review of nabilone found no concerns with abuse potential86 and long-term registry data for nabiximols showed no signals of dependence or abuse.65 A recent study showed that nabiximols may be effective in treating existing cannabis dependence,87 while preliminary research indicates a potential role for CBD in treating heroin withdrawal.88
Withdrawal symptoms in patients with neuropathic pain treated with dronabinol (sleep disturbance, excitability, nervousness, and increase of neuropathic pain) were found to be mild and transient.57 Withdrawal symptoms with cannabinoids generally start within 1–2 days and resolve within 1–2 weeks of treatment discontinuation.73 It is important to note that the presence of withdrawal symptoms does not necessarily constitute a diagnosis of cannabis-withdrawal syndrome.81
In addition, patients receiving cannabinoid-based medicines generally do not increase their dose over time, once the therapeutic dose has been achieved.89 This is in contrast to opioid use, where dose escalation and addiction are not uncommon.38,80 In terms of toxicity, one model found that the estimated ratio of toxicologic threshold (based on median lethal dose [LD50] values) to standard daily human intake for THC was much higher than for many other substances, including all opioids studied,90 which may partly explain why cannabis use is not associated with increased mortality. Indeed, official statistics for England and Wales in 2018 showed that opiates were a factor (not necessarily directly attributable) in 2208 deaths registered in 2018, compared with 210 for paracetamol and 22 for cannabis, noting that these values do not differentiate medical from recreational use (Figure 3).70 Based on data in recreational cannabis use (with unknown product composition), acute overdose is associated with agitation, hyperemesis, tachycardia, drowsiness, and psychological disturbances that can include psychosis.91,92
 |
Figure 3 Number of deaths related to selected drugs, where the drug name was listed on the death certificate, England and Wales, 2018. aData from the Office for National Statistics, accessed February 20, 2020: all deaths relating to drug poisoning in England and Wales registered in 2018, where a drug was mentioned on the death certificate. Any opiate includes unspecified opiates and excludes paracetamol compounds. Codeine and dihydrocodeine not from compound formulations (eg, co-codamol). Paracetamol includes paracetamol compounds. Office for National Statistics. Deaths related to drug poisoning, England and Wales. 2019. Available from: (https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsrelatedtodrugpoisoningenglandandwalesreferencetable). Public sector information licensed under the Open Government Licence v3.0 (http://www.nationalarchives.gov.uk/doc/open-government-licence/version/3/).70 |
More high-quality medical research is needed to determine the incidence of cannabis-use disorder in patients treated with cannabinoid-based medicines, including identifying which combinations of cannabinoids, doses, and individual-use patterns cause these effects in which patients. This will decrease reliance on recreational cannabis use data in this area, with its associated confounding factors.
Psychiatric and Cognitive Effects
While both THC and CBD are psychoactive (ie, they act on the central nervous system to alter brain function), CBD does not induce euphoria or measurably impair psychomotor skills.73,93,94 CBD exhibits anxiolytic and neuroprotective properties,93,95 and is approved in some countries to treat children with Lennox-Gastaut or Dravet syndromes.19
Current evidence indicates that THC is anxiolytic at low doses and anxiogenic at higher doses, with unique dose–response curves.35,73,96–98 Interestingly, CBD (and other cannabis plant constituents such as cannabinol, terpenes, and flavonoids) may help to lower the psychotropic effects of THC when used in particular combinations35,99–101 and understanding this important interaction between these two cannabinoids should be a priority for further clinical research.
Studies in recreational cannabis use show that early, frequent, and heavy use of high-potency THC may have long-term effects in the developing brain and is linked with earlier onset of psychosis in those with an individual or familial risk of psychosis or schizophrenia.35,73,96,102 Some synthetic cannabinoids produced illicitly for recreational use that bind to the same receptors as THC are associated with atypical psychosis and long-term cognitive impairment. However, other evidence suggests no association between adolescent cannabis use and structural brain differences in adulthood.103 Currently, products containing THC or its analogs should not be prescribed for patients aged <25 years73 unless there is a strong clinical need that cannot be met by other treatment options and the benefits outweigh the risks.35,79
Due to a short-term decrease in psychomotor skills alongside potential cognitive and motor reflex impairments, patients may not be safe to drive or operate heavy machinery after taking medicines containing THC, with one review recommending abstinence from driving for 8 hours.104,105 Although the extent of impairment may depend on the dose, individual patient, route of consumption, concomitant medications, and rate of metabolism, a study in recreational cannabis use showed that long-term cannabis users were impaired even when they had not consumed cannabis for at least 12 hours.106 In addition to respective national laws, the patient’s occupation and location are, therefore, important considerations when assessing whether a cannabinoid-based medicine is a suitable option. Further studies are needed to clarify the risk of prolonged psychomotor impairment in the therapeutic setting and establish any dose-dependent relationships.
Respiratory and Cardiovascular Safety
Smoking cannabinoid-based medicines is not recommended, as many by-products of pyrolysis are mutagenic and carcinogenic, and may exacerbate existing asthma or chronic obstructive pulmonary disease;35 however, one large pooled analysis found no overall association between cannabis smoking and lung cancer.107
Vaporization carries less respiratory risk, as the product is heated to a lower temperature (without combustion) than smoking and, therefore, does not produce the same level of toxic by-products.73 Oral ingestion carries the least respiratory risk, with no known association between ingestion of cannabinoids and respiratory adverse effects. In contrast to opioids, cannabinoids carry a low risk of respiratory depression due to the lack of endocannabinoid CB1 receptors in the respiratory control centers in the medulla.23,80
The existing literature assessing cardiovascular risk is in recreational cannabis use and shows conflicting findings.108,109 Recreational cannabis use is associated with a variety of cardiovascular effects such as tachycardia and dose-dependent peripheral vasodilation;32,110,111 however, it is unknown whether these effects are experienced by relevant medical populations receiving controlled doses and this is an important area for future research.
Reproductive Safety
Men and women are known to exhibit different endocannabinoid responses.35 Although the majority of available data on the reproductive safety of cannabinoids in humans are in recreational cannabis use, there is ample evidence that cannabinoid-based medicines may impact on both male and female reproductive systems.
In women, while data on sexual behavior and reproductive diseases are limited, use of cannabinoid-based medicines in pregnancy and breastfeeding may be associated with a variety of serious and long-term adverse effects in the offspring.35,73,112,113 Although no currently approved cannabinoid-based medical products have been studied in pregnant women, preclinical studies of CBD, nabilone, and dronabinol found dose-related developmental toxicity, noting that some findings were at much higher exposure levels than the human therapeutic dose.15,16,19,20 Although pregnancy is not specifically listed as a contraindication in the prescribing information for these products,15,16,19,20 guidelines currently contraindicate all cannabinoid-based medicines in pregnant and breastfeeding women.73 However, some real-world studies in recreational cannabis use suggest that it is not an independent risk factor for adverse neonatal outcomes.114 As an estimated 12% of pregnant women in the US use cannabis or cannabinoid-containing products in the first trimester115 many to treat severe nausea and vomiting116,117 informed patient–practitioner conversations are needed to discuss and educate on the risks and benefits of cannabinoid-based medicines compared with other lifestyle and pharmacologic options.
In men, recreational cannabis use may be associated with increased risk of testicular cancer, and has also been linked to reduced sperm count, motility, and libido, and to erectile dysfunction.35,118–120 However, preclinical studies of nabilone in rats showed no effect on fertility or reproductive performance.16
As the majority of data on reproductive safety are in recreational cannabis use, more preclinical and high-quality long-term clinical studies are needed to assess fully the risks, including which cannabinoids are responsible for specific adverse effects.
Potential as Adjunctive Therapy
Cannabinoids and opioids used in combination may augment the analgesic effects of opioids.121 In patients with chronic pain, some open-label and real-world studies have found that initiation of cannabinoid-based medicines may lead to a reduction in opioid use, which could have implications for opioid sparing.64,122–124 In real-world studies, 97% of patients with chronic pain reported that they were able to decrease their opiate dose and 92% found the side effects more tolerable with cannabinoids compared with opiates123 while 14.4% of elderly patients ceased to use opioid analgesics within 6 months.64 Another study found that initiation of cannabinoid-based medicines was associated with 17-fold higher odds of ceasing opioid prescriptions within 21 months.125 Due to the synergy between cannabinoids and opioids, patients receiving both therapies should be closely monitored for increased adverse effects.
There is also evidence that prescribing cannabinoid-based medicines may reduce the use of benzodiazepines and non-steroidal anti-inflammatory drugs.52,64,126
Conclusions and Future Directions
Robust analysis of the safety of cannabinoid-based medicine is challenging due to disparities in formulation, dosing, administration method, indication, and confusion with recreational cannabis use. Further high-quality research is needed to establish the safety profile of each individual cannabinoid formulation for the indications in which it is being used, from which treatment guidelines and algorithms based on credible and validated medical evidence can follow.
However, while the evidence base continues to evolve, the existing safety data can be leveraged to draw some key conclusions.72 As cannabinoid-based medicines are currently used to treat a range of conditions, ongoing pharmacovigilance and real-world studies provide important data sources that complement randomized controlled clinical trials.
The available evidence shows that, as with any other class of pharmaceuticals, cannabinoid-based medicines are associated with risks, which need to be assessed in the context of potential therapeutic benefits. Individual, evidence-based decision-making is required by physicians to determine whether a cannabinoid-based medicine could be an appropriate treatment option for their patients. Healthcare professionals should always bear in mind their local legal framework and access procedures when recommending or prescribing cannabinoid-based medicines.
Abbreviations
AE, adverse event; CBD, cannabidiol; THC, Δ9-tetrahydrocannabinol.
Acknowledgments
Medical writing assistance was provided to the authors by Helena Cant, MChemPhys, of Complete HealthVizion, McCann Health Medical Communications, and funded by Spectrum Therapeutics, Ontario, Canada. The first draft was prepared based on detailed discussion and input from the authors and revised in accordance with their critical feedback.
Author Contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. All authors contributed to the conception and design of the manuscript and data interpretation, and critically revised the manuscript for important intellectual content. The authors take full responsibility for the scope, direction, and content of the manuscript, and have approved the submitted manuscript. They received no compensation related to the development of the manuscript.
Funding
This review was sponsored and funded by Spectrum Therapeutics, Ontario, Canada. The sponsor was involved in the conception and design of the article. Medical writing assistance was funded by Spectrum Therapeutics, Ontario, Canada. Neither honoraria nor payments were made for authorship.
Disclosure
Sven Gottschling has received consultancy fees from Bioevents, Bionorica, Biotest, Boehringer Ingelheim, Cogitando, Experten-Futrue, Grünenthal, Hexal, IQQ Institut, Kyowa Kirin, MedConcept, Novartis, Roche, Sandoz, Spectrum Therapeutics, and Tilray.
Oyedeji Ayonrinde has no disclosures to declare.
Arun Bhaskar has received consultancy fees from Spectrum Therapeutics.
Marc Blockman has received consultancy fees from Spectrum Therapeutics.
Oscar D’Agnone has received consultancy fees from Spectrum Therapeutics.
Danial Schecter is a former employee of Spectrum Therapeutics, a former employee of Canopy Growth Corp from February 2019 to March 2020 and has provided consulting services both prior and after this, was previously Chief Medical Advisor of AusCann, and received honoraria from and provided consulting services for the following: Aleafia Health Inc, Shoppers Drug Mart, Khiron, Tilray, and Organigram, outside the submitted work.
Luis David Suárez Rodríguez has received consultancy fees from Spectrum Therapeutics and funding from the Asociacion Mexicana de Medicina Cannabinoide AC, and reports personal fees and non-financial support from Asociación Mexicana de Medicina Cannabinoide AC, outside the submitted work; and is the current President of the Asociación Mexicana de Medicina Cannabinoide AC.
Sherry Yafai has received consultancy fees from Canopy Growth.
Claude Cyr has received consultancy fees from Aurora, Shoppers/Inventiv, Spectrum Therapeutics, and Tilray.
The authors report no other potential conflicts of interest for this work.
References
1. Allan GM, Ramji J, Perry D, et al. Simplified guideline for prescribing medical cannabinoids in primary care. Can Fam Physician. 2018;64(2):111–120.
2. Cyr C, Arboleda MF, Aggarwal SK, et al. Cannabis in palliative care: current challenges and practical recommendations. Ann Palliat Med. 2018;7(4):463–477. doi:10.21037/apm.2018.06.04
3. Häuser W, Finn DP, Kalso E, et al. European Pain Federation (EFIC) position paper on appropriate use of cannabis-based medicines and medical cannabis for chronic pain management. Eur J Pain. 2018;22(9):1547–1564. doi:10.1002/ejp.1297
4. Moulin DE, Boulanger A, Clark AJ, et al. Pharmacological management of chronic neuropathic pain: Revised consensus statement from the Canadian Pain Society. Pain Res Manag. 2014;19(6):328–335. doi:10.1155/2014/754693
5. Yadav V, Bever C. Jr, Bowen J, et al. Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis. Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2014;82(12):1083–1092. doi:10.1212/WNL.0000000000000250
6. World Health Organization. WHO expert committee on drug dependence. Critical review. Delta-9-tetrahydrocannabinol; 2018. Available from: https://www.who.int/medicines/access/controlled-substances/THCv1.pdf?ua=1.
7. World Health Organization, Cannabidiol (CBD). Critical review report; 2018. Available from: https://www.who.int/medicines/access/controlled-substances/CannabidiolCriticalReview.pdf.
8. ElSohly MA. Chemical constituents of cannabis. Grotenhermen F, Russo E, eds. In: Cannabis and Cannabinoids. Pharmacology, Toxicology, and Therapeutic Potential. Binghamton, NY: The Haworth Press; 2002:27–36.
9. Hanuš LO, Meyer SM, Muñoz E, Taglialatela-Scafati O, Appendino G. Phytocannabinoids: a unified critical inventory. Nat Prod Rep. 2016;33(12):1357–1392. doi:10.1039/c6np00074f
10. Pertwee RG, ed. Handbook of Cannabis. Oxford: Oxford University Press; 2014.
11. Devinsky O, Cross JH, Laux L, et al. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011–2020. doi:10.1056/NEJMoa1611618
12. Thiele EA, Marsh ED, French JA, et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10125):1085–1096. doi:10.1016/S0140-6736(18)30136-3
13. Wade DT, Makela P, Robson P, House H, Bateman C. Do cannabis-based medicinal extracts have general or specific effects on symptoms in multiple sclerosis? A double-blind, randomized, placebo-controlled study on 160 patients. Mult Scler. 2004;10(4):434–441. doi:10.1191/1352458504ms1082oa
14. Beal JE, Olson R, Lefkowitz L, et al. Long-term efficacy and safety of dronabinol for acquired immunodeficiency syndrome-associated anorexia. J Pain Symptom Manage. 1997;14(1):7–14. doi:10.1016/S0885-3924(97)00038-9
15. AbbVie Inc. MARINOL (dronabinol) capsules, for oral use. Prescribing Information; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/018651s029lbl.pdf.
16. Meda Pharmaceuticals Inc. CESAMET - nabilone capsule. Prescribing information; 2015. Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=bb582d64-0f51-11df-8a39-0800200c9a66&audience=consumer.
17. National Institute for Health and Care Excellence. Cannabis-based medicinal products. NICE guideline [NG144]; 2019. Available from: https://www.nice.org.uk/guidance/NG144.
18. Bayer Schering Pharma. Sativex oromucosal spray. Summary of product characteristics; 2019. Available from: https://www.medicinesresources.nhs.uk/upload/documents/News/2010/Sativex_UK_SmPC_FINAL.pdf.
19. Greenwich Biosciences Inc. EPIDIOLEX® (cannabidiol) oral solution, CX. Prescribing information; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf.
20. Insys Therapeutics Inc. SYNDROS (dronabinol) oral solution, CX. Prescribing information; 2016. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/205525s000lbl.pdf.
21. Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nat Rev Drug Discov. 2004;3(9):771–784. doi:10.1038/nrd1495
22. Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58(3):389–462. doi:10.1124/pr.58.3.2
23. Mackie K. Distribution of cannabinoid receptors in the central and peripheral nervous system. Handb Exp Pharmacol. 2005;168:299–325.
24. Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20(Suppl 1):10–14. doi:10.1111/j.1365-2826.2008.01671.x
25. Dai E, Zhang L, Ye L, et al. Hepatic expression of cannabinoid receptors CB1 and CB2 correlate with fibrogenesis in patients with chronic hepatitis B. Int J Infect Dis. 2017;59:124–130. doi:10.1016/j.ijid.2017.03.008
26. Lotersztajn S, Teixeira-Clerc F, Julien B, et al. CB2 receptors as new therapeutic targets for liver diseases. Br J Pharmacol. 2008;153(2):286–289. doi:10.1038/sj.bjp.0707511
27. Devane WA, Hanus L, Breuer A, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. doi:10.1126/science.1470919
28. Lynch ME, Ware MA. Cannabinoids for the treatment of chronic non-cancer pain: an updated systematic review of randomized controlled trials. J Neuroimmune Pharmacol. 2015;10(2):293–301. doi:10.1007/s11481-015-9600-6
29. Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2018;3:CD012182. doi:10.1002/14651858.CD012182.pub2
30. Whiting PF, Wolff RF, Deshpande S, et al. Cannabinoids for medical use. A systematic review and meta-analysis. JAMA. 2015;313(24):2456–2473. doi:10.1001/jama.2015.6358
31. Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medical cannabinoids: a systematic review. CMAJ. 2008;178(13):1669–1678. doi:10.1503/cmaj.071178
32. Ware MA, Tawfik VL. Safety issues concerning the medical use of cannabis and cannabinoids. Pain Res Manag. 2005;10(Suppl A):31A–37A. doi:10.1155/2005/312357
33. Volkow ND, Baler RD, Compton WM, Weiss SR. Adverse health effects of marijuana use. N Engl J Med. 2014;370(23):2219–2227. doi:10.1056/NEJMra1402309
34. The National Academies of Sciences, Engineering, and Medicine. The Health Effects of Cannabis and Cannabinoids. The Current State of Evidence and Recommendations for Research. Washington, DC: The National Academies Press; 2017.
35. Health Canada. Information for Health Care Professionals. Cannabis (marihuana, marijuana) and the cannabinoids. Dried or fresh plant and oil administration by ingestion or other means. Psychoactive agent; 2018. Available from: https://www.canada.ca/content/dam/hc-sc/documents/services/drugs-medication/cannabis/information-medical-practitioners/information-health-care-professionals-cannabis-cannabinoids-eng.pdf.
36. Secades-Villa R, Garcia-Rodriguez O, Jin CJ, Wang S, Blanco C. Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy. 2015;26(2):135–142. doi:10.1016/j.drugpo.2014.07.011
37. Millar SA, Stone NL, Bellman ZD, Yates AS, England TJ, O’Sullivan SE. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85(9):1888–1900. doi:10.1111/bcp.14038
38. MacCallum CA, Russo EB. Practical considerations in medical cannabis administration and dosing. Eur J Intern Med. 2018;49:12–19. doi:10.1016/j.ejim.2018.01.004
39. Schuck RN, Pacanowski M, Kim S, Madabushi R, Zineh I. Use of titration as a therapeutic individualization strategy: An analysis of Food and Drug Administration-approved drugs. Clin Transl Sci. 2019;12(3):236–239. doi:10.1111/cts.12626
40. Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32(11):1053–1067. doi:10.1007/s40263-018-0578-5
41. Huestis MA. Human cannabinoid pharmacokinetics. Chem Biodivers. 2007;4(8):1770–1804. doi:10.1002/cbdv.200790152
42. Collin C, Davies P, Mutiboko IK, Ratcliffe S, for the Sativex Spasticity in MS Study Group. Randomized controlled trial of cannabis-based medicine in spasticity caused by multiple sclerosis. Eur J Neurol. 2007;14(3):290–296. doi:10.1111/j.1468-1331.2006.01639.x
43. Collin C, Ehler E, Waberzinek G, et al. A double-blind, randomized, placebo-controlled, parallel-group study of Sativex, in subjects with symptoms of spasticity due to multiple sclerosis. Neurol Res. 2010;32(5):451–459. doi10.1179/016164109X12590518685660
44. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med. 2018;378(20):1888–1897. doi:10.1056/NEJMoa1714631
45. Notcutt W, Langford R, Davies P, Ratcliffe S, Potts R. A placebo-controlled, parallel-group, randomized withdrawal study of subjects with symptoms of spasticity due to multiple sclerosis who are receiving long-term Sativex® (nabiximols). Mult Scler. 2012;18(2):219–228. doi:10.1177/1352458511419700
46. Novotna A, Mares J, Ratcliffe S, et al. A randomized, double-blind, placebo-controlled, parallel-group, enriched-design study of nabiximols* (Sativex®), as add-on therapy, in subjects with refractory spasticity caused by multiple sclerosis. Eur J Neurol. 2011;18(9):1122–1131. doi:10.1111/j.1468-1331.2010.03328.x
47. Serpell MG, Notcutt W, Collin C. Sativex long-term use: an open-label trial in patients with spasticity due to multiple sclerosis. J Neurol. 2013;260(1):285–295. doi:10.1007/s00415-012-6634-z
48. Wade DT, Makela PM, House H, Bateman C, Robson P. Long-term use of a cannabis-based medicine in the treatment of spasticity and other symptoms in multiple sclerosis. Mult Scler. 2006;12(5):639–645. doi:10.1177/1352458505070618
49. Ball S, Vickery J, Hobart J, et al. The Cannabinoid Use in Progressive Inflammatory brain Disease (CUPID) trial: a randomised double-blind placebo-controlled parallel-group multicentre trial and economic evaluation of cannabinoids to slow progression in multiple sclerosis. Health Technol Assess. 2015;19(12):vii–viii, xxv–xxxi, 1–187. doi:10.3310/hta19120
50. Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain. 2003;106(1–2):169–172. doi:10.1016/s0304-3959(03)00331-2
51. Meiri E, Jhangiani H, Vredenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007;23(3):533–543. doi:10.1185/030079907x167525
52. Pini LA, Guerzoni S, Cainazzo MM, et al. Nabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trial. J Headache Pain. 2012;13(8):677–684. doi:10.1007/s10194-012-0490-1
53. Ware MA, Fitzcharles M-A, Joseph L, Shir Y. The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg. 2010;110(2):604–610. doi:10.1213/ANE.0b013e3181c76f70
54. Frank B, Serpell MG, Hughes J, Matthews JN, Kapur D. Comparison of analgesic effects and patient tolerability of nabilone and dihydrocodeine for chronic neuropathic pain: randomised, crossover, double blind study. BMJ. 2008;336(7637):199–201. doi:10.1136/bmj.39429.619653.80
55. Skrabek RQ, Galimova L, Ethans K, Perry D. Nabilone for the treatment of pain in fibromyalgia. J Pain. 2008;9(2):164–173. doi:10.1016/j.jpain.2007.09.002
56. Fabre LF, McLendon D. The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. J Clin Pharmacol. 1981;21(S1):377S–382S. doi:10.1002/j.1552-4604.1981.tb02617.x
57. Schimrigk S, Marziniak M, Neubauer C, Kugler EM, Werner G, Abramov-Sommariva D. Dronabinol is a safe long-term treatment option for neuropathic pain patients. Eur Neurol. 2017;78(5–6):320–329. doi:10.1159/000481089
58. Devinsky O, Marsh E, Friedman D, et al. Cannabidiol in patients with treatment-resistant epilepsy: an open-label interventional trial. Lancet Neurol. 2016;15(3):270–278. doi:10.1016/S1474-4422(15)00379-8
59. Devinsky O, Nabbout R, Miller I, et al. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia. 2019;60(2):294–302. doi:10.1111/epi.14628
60. Szaflarski JP, Bebin EM, Comi AM, et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia. 2018;59(8):1540–1548. doi:10.1111/epi.14477
61. Thiele E, Marsh E, Mazurkiewicz-Beldzinska M, et al. Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia. 2019;60(3):419–428. doi:10.1111/epi.14670
62. De Gregorio D, McLaughlin RJ, Posa L, et al. Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain. 2019;160(1):136–150. doi:10.1097/j.pain.0000000000001386
63. Ware MA, Wang T, Shapiro S, Collet J-P, for the COMPASS study team. Cannabis for the Management of Pain: Assessment of Safety Study (COMPASS). J Pain. 2015;16(12):1233–1242. doi:10.1016/j.jpain.2015.07.014
64. Abuhasira R, Schleider LB, Mechoulam R, Novack V. Epidemiological characteristics, safety and efficacy of medical cannabis in the elderly. Eur J Intern Med. 2018;49:44–50. doi:10.1016/j.ejim.2018.01.019
65. Etges T, Karolia K, Grint T, et al. An observational postmarketing safety registry of patients in the UK, Germany, and Switzerland who have been prescribed Sativex® (THC:CBD, nabiximols) oromucosal spray. Ther Clin Risk Manag. 2016;12:1667–1675. doi:10.2147/TCRM.S115014
66. Baron EP, Lucas P, Eades J, Hogue O. Patterns of medicinal cannabis use, strain analysis, and substitution effect among patients with migraine, headache, arthritis, and chronic pain in a medicinal cannabis cohort. J Headache Pain. 2018;19(1):37. doi:10.1186/s10194-018-0862-2
67. Park J-Y, Wu L-T. Prevalence, reasons, perceived effects, and correlates of medical marijuana use: A review. Drug Alcohol Depend. 2017;177:1–13. doi:10.1016/j.drugalcdep.2017.03.009
68. Russo EB. Cannabis and pain. Pain Med. 2019;20(11):2083–2085. doi:10.1093/pm/pnz227
69. Jank R, Gallee A, Boeckle M, Fiegl S, Pieh C. Chronic pain and sleep disorders in primary care. Pain Res Treat. 2017;2017:9081802. doi:10.1155/2017/9081802
70. Office for National Statistics. Deaths related to drug poisoning, England and Wales; 2019. Available from: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsrelatedtodrugpoisoningenglandandwalesreferencetable.
71. Marel C, Sunderland M, Mills KL, Slade T, Teesson M, Chapman C. Conditional probabilities of substance use disorders and associated risk factors: Progression from first use to use disorder on alcohol, cannabis, stimulants, sedatives and opioids. Drug Alcohol Depend. 2019;194:136–142. doi:10.1016/j.drugalcdep.2018.10.010
72. Bonn-Miller MO, Pollack CV, Jr., Casarett D, et al. Priority considerations for medicinal cannabis-related research. Cannabis Cannabinoid Res. 2019;4(3):139–157. doi:10.1089/can.2019.0045
73. Canadian Pharmacists Association. CPhA monograph. Cannabis. Compendium of Pharmaceuticals and Specialties; 2018.
74. Antoniou T, Bodkin J, Ho JM. Drug interactions with cannabinoids. CMAJ. 2020;192(9):E206. doi:10.1503/cmaj.191097
75. Zendulka O, Dovrtělová G, Nosková K, et al. Cannabinoids and cytochrome P450 interactions. Curr Drug Metab. 2016;17(3):206–226. doi:10.2174/1389200217666151210142051
76. Brown JD, Winterstein AG. Potential adverse drug events and drug–drug interactions with medical and consumer cannabidiol (CBD) use. J Clin Med. 2019;8(7):989. doi:10.3390/jcm8070989
77. Alsherbiny MA, Li CG. Medicinal cannabis—potential drug interactions. Medicines (Basel). 2018;6(1):3. doi:10.3390/medicines6010003
78. Taha T, Meiri D, Talhamy S, Wollner M, Peer A, Bar-Sela G. Cannabis impacts tumor response rate to nivolumab in patients with advanced malignancies. Oncologist. 2019;24(4):549–554. doi:10.1634/theoncologist.2018-0383
79. Wong SS, Wilens TE. Medical cannabinoids in children and adolescents: A systematic review. Pediatrics. 2017;140(5):e20171818. doi:10.1542/peds.2017-1818
80. Canadian Pharmacists Association. CPhA monograph. Opioids. Compendium of Pharmaceuticals and Specialties. 2018.
81. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. DSM-5™. Arlington, VA: American Psychiatric Publishing; 2013.
82. Adamson SJ, Kay-Lambkin FJ, Baker AL, et al. An improved brief measure of cannabis misuse: the Cannabis Use Disorders Identification Test-Revised (CUDIT-R). Drug Alcohol Depend. 2010;110(1–2):137–143. doi:10.1016/j.drugalcdep.2010.02.017
83. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–371. doi:10.1016/0306-4603(82)90005-3
84. Copeland WE, Hill S, Costello EJ, Shanahan L. Cannabis use and disorder from childhood to adulthood in a longitudinal community sample with American Indians. J Am Acad Child Adolesc Psychiatry. 2017;56(2):124–132.e2. doi:10.1016/j.jaac.2016.11.006
85. Degenhardt L, Ferrari AJ, Calabria B, et al. The global epidemiology and contribution of cannabis use and dependence to the global burden of disease: results from the GBD 2010 study. PLoS One. 2013;8(10):e76635. doi:10.1371/journal.pone.0076635
86. Ware MA, St Arnaud-Trempe E. The abuse potential of the synthetic cannabinoid nabilone. Addiction. 2010;105(3):494–503. doi:10.1111/j.1360-0443.2009.02776.x
87. Lintzeris N, Bhardwaj A, Mills L, et al. Nabiximols for the treatment of cannabis dependence. A randomized clinical trial. JAMA Intern Med. 2019;179(9):1242–1253. doi:10.1001/jamainternmed.2019.1993
88. Wiese B, Wilson-Poe AR. Emerging evidence for cannabis’ role in opioid use disorder. Cannabis Cannabinoid Res. 2018;3(1):179–189. doi:10.1089/can.2018.0022
89. Hoggart B, Ratcliffe S, Ehler E, et al. A multicentre, open-label, follow-on study to assess the long-term maintenance of effect, tolerance and safety of THC/CBD oromucosal spray in the management of neuropathic pain. J Neurol. 2015;262(1):27–40. doi:10.1007/s00415-014-7502-9
90. Lachenmeier DW, Rehm J. Comparative risk assessment of alcohol, tobacco, cannabis and other illicit drugs using the margin of exposure approach. Sci Rep. 2015;5:8126. doi:10.1038/srep08126
91. Li W, Gunja N. Illicit drug overdose. Prevalence and acute management. Aust Fam Physician. 2013;42(7):481–485.
92. Cao D, Srisuma S, Bronstein AC, Hoyte CO. Characterization of edible marijuana product exposures reported to United States poison centers. Clin Toxicol (Phila). 2016;54(9):840–846. doi:10.1080/15563650.2016.1209761
93. Moreira FA, Aguiar DC, Guimarães FS. Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(8):1466–1471. doi:10.1016/j.pnpbp.2006.06.004
94. Pisanti S, Malfitano AM, Ciaglia E, et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol Ther. 2017;175:133–150. doi:10.1016/j.pharmthera.2017.02.041
95. Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3364–3378. doi:10.1098/rstb.2011.0389
96. Micale V, Di Marzo V, Sulcova A, Wotjak CT, Drago F. Endocannabinoid system and mood disorders: priming a target for new therapies. Pharmacol Ther. 2013;138(1):18–37. doi:10.1016/j.pharmthera.2012.12.002
97. Onaivi ES, Green MR, Martin BR. Pharmacological characterization of cannabinoids in the elevated plus maze. J Pharmacol Exp Ther. 1990;253(3):1002–1009.
98. Childs E, Lutz JA, de Wit H. Dose-related effects of delta-9-THC on emotional responses to acute psychosocial stress. Drug Alcohol Depend. 2017;177:136–144. doi:10.1016/j.drugalcdep.2017.03.030
99. Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of ∆9-tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28(1):172–177. doi:10.1016/0014-2999(74)90129-0
100. Englund A, Morrison PD, Nottage J, et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J Psychopharmacol. 2013;27(1):19–27. doi:10.1177/0269881112460109
101. Solowij N, Broyd S, Greenwood LM, et al. A randomised controlled trial of vaporised ∆9-tetrahydrocannabinol and cannabidiol alone and in combination in frequent and infrequent cannabis users: acute intoxication effects. Eur Arch Psychiatry Clin Neurosci. 2019;269(1):17–35. doi:10.1007/s00406-019-00978-2
102. Scott JC, Slomiak ST, Jones JD, Rosen AFG, Moore TM, Gur RC. Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry. 2018;75(6):585–595. doi:10.1001/jamapsychiatry.2018.0335
103. Meier MH, Schriber RA, Beardslee J, Hanson J, Pardini D. Associations between adolescent cannabis use frequency and adult brain structure: A prospective study of boys followed to adulthood. Drug Alcohol Depend. 2019;202:191–199. doi:10.1016/j.drugalcdep.2019.05.012
104. Rogeberg O, Elvik R. The effects of cannabis intoxication on motor vehicle collision revisited and revised. Addiction. 2016;111(8):1348–1359. doi:10.1111/add.13347
105. Neavyn MJ, Blohm E, Babu KM, Bird SB. Medical marijuana and driving: a review. J Med Toxicol. 2014;10(3):269–279. doi:10.1007/s13181-014-0393-4
106. Dahlgren MK, Sagar KA, Smith RT, Lambros AM, Kuppe MK, Gruber SA. Recreational cannabis use impairs driving performance in the absence of acute intoxication. Drug Alcohol Depend. 2020;208:107771. doi:10.1016/j.drugalcdep.2019.107771
107. Zhang LR, Morgenstern H, Greenland S, et al. Cannabis smoking and lung cancer risk: Pooled analysis in the International Lung Cancer Consortium. Int J Cancer. 2015;136(4):894–903. doi:10.1002/ijc.29036
108. Reis JP, Auer R, Bancks MP, et al. Cumulative lifetime marijuana use and incident cardiovascular disease in middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Public Health. 2017;107(4):601–606. doi:10.2105/AJPH.2017.303654
109. Kalla A, Krishnamoorthy P, Gopalakrishnan A, Garg J, Figueredo V. Cannabis use predicts risks of heart failure and cerebrovascular accidents: results from the national inpatient sample. J Am Coll Cardiol. 2017;69(Suppl)(11):1784. doi:10.1016/S0735-1097(17)35173-2
110. Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103(23):2805–2809. doi:10.1161/01.cir.103.23.2805
111. Rezkalla SH, Sharma P, Kloner RA. Coronary no-flow and ventricular tachycardia associated with habitual marijuana use. Ann Emerg Med. 2003;42(3):365–369. doi:10.1016/s0196-0644(03)00426-8
112. Astley SJ, Little RE. Maternal marijuana use during lactation and infant development at one year. Neurotoxicol Teratol. 1990;12(2):161–168. doi:10.1016/0892-0362(90)90129-z
113. Briggs GG, Freeman RK, Towers CV, Forinash AB. Drugs in Pregnancy and Lactation. A Reference Guide to Fetal and Neonatal Risk. Eleventh Edition. Philadelphia, PA: Wolters Kluwer; 2017.
114. Conner SN, Bedell V, Lipsey K, Macones GA, Cahill AG, Tuuli MG. Maternal marijuana use and adverse neonatal outcomes: Asystematic review and meta-analysis. Obstet Gynecol. 2016;128(4):713–723. doi:10.1097/AOG.0000000000001649
115. Volkow ND, Han B, Compton WM, McCance-Katz EF. Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA. 2019;322(2):167–169. doi:10.1001/jama.2019.7982
116. Westfall RE, Janssen PA, Lucas P, Capler R. Survey of medicinal cannabis use among childbearing women: patterns of its use in pregnancy and retroactive self-assessment of its efficacy against ‘morning sickness’. Complement Ther Clin Pract. 2006;12(1):27–33. doi:10.1016/j.ctcp.2005.09.006
117. Badowski S, Smith G. Cannabis use during pregnancy and postpartum. Can Fam Physician. 2020;66(2):98–103.
118. Gurney J, Shaw C, Stanley J, Signal V, Sarfati D. Cannabis exposure and risk of testicular cancer: a systematic review and meta-analysis. BMC Cancer. 2015;15:897. doi:10.1186/s12885-015-1905-6
119. Gorzalka BB, Hill MN, Chang SCH. Male–female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58(1):91–99. doi:10.1016/j.yhbeh.2009.08.009
120. Gundersen TD, Jørgensen N, Andersson A-M, et al. Association between use of marijuana and male reproductive hormones and semen quality: a study among 1,215 healthy young men. Am J Epidemiol. 2015;182(6):473–481. doi:10.1093/aje/kwv135
121. Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL. Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther. 2011;90(6):844–851. doi:10.1038/clpt.2011.188
122. Haroutounian S, Ratz Y, Ginosar Y, et al. The effect of medicinal cannabis on pain and quality-of-life outcomes in chronic pain: a prospective open-label study. Clin J Pain. 2016;32(12):1036–1043. doi:10.1097/AJP.0000000000000364
123. Reiman A, Welty M, Solomon P. Cannabis as a substitute for opioid-based pain medication: patient self-report. Cannabis Cannabinoid Res. 2017;2(1):160–166. doi:10.1089/can.2017.0012
124. Wen H, Hockenberry JM. Association of medical and adult-use marijuana laws with opioid prescribing for Medicaid enrollees. JAMA Intern Med. 2018;178(5):673–679. doi:10.1001/jamainternmed.2018.1007
125. Vigil JM, Stith SS, Adams IM, Reeve AP. Associations between medical cannabis and prescription opioid use in chronic pain patients: A preliminary cohort study. PLoS One. 2017;12(11):e0187795. doi:10.1371/journal.pone.0187795
126. Purcell C, Davis A, Moolman N, Taylor SM. Reduction of benzodiazepine use in patients prescribed medical cannabis. Cannabis Cannabinoid Res. 2019;4(3):214–218. doi:10.1089/can.2018.0020
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
