Back to Journals » OncoTargets and Therapy » Volume 11
Safety and efficacy of transarterial chemoembolization with degradable starch microspheres (DSM-TACE) in the treatment of secondary liver malignancies
Authors Schicho A , Pereira PL , Michalik K , Beyer LP , Stroszczynski C, Wiggermann P
Received 31 July 2017
Accepted for publication 6 November 2017
Published 12 January 2018 Volume 2018:11 Pages 345—350
DOI https://doi.org/10.2147/OTT.S147852
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Andreas Schicho,1 Philippe L Pereira,2 Katharina Michalik,1 Lukas P Beyer,1 Christian Stroszczynski,1 Philipp Wiggermann1
1Department of Radiology, University Hospital Regensburg, Regensburg, 2Department of Radiology, Minimal-invasive Therapies and Nuclear Medicine, SLK Kliniken Heilbronn, Heilbronn, Germany
Purpose: To evaluate the safety and efficacy of degradable starch microspheres (DSM) as embolic agents in transarterial chemoembolization (TACE) in the treatment of secondary liver metastases.
Methods: This was a national, multicenter observational study. Primary endpoints were safety and treatment response according to Modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria.
Results: A total of 77 DSM-TACE procedures were performed in 20 patients. Minor immediate adverse events (AEs) were epigastric pain with an incidence of 45.5% (35/77), and nausea and vomiting at an incidence of 23.4% (18/77). Delayed minor AEs were epigastric pain in 13/77 (16.9%) treatments and nausea and vomiting in 10 (13.0%) treatments. No severe AEs were documented. Therapeutic efficacy of DSM-TACE procedures according to mRECIST was as follows: complete response 0/77, partial response 17/77, stable disease 33/77 and progressive disease 6/77, no data was available for 21/77 treatments. Overall, objective response was achieved in 8 of 20 patients (40.0%).
Conclusion: DSM as embolic agent for TACE is safe in the treatment of liver metastases. An objective response in 40.0% of patients and disease control in 64.9% of procedures was achieved, and this should lead to further evaluation of DSM-TACE as treatment option for nonresectable liver metastases.
Keywords: TACE, DSM, safety, efficacy, metastases, secondary malignancies
Introduction
A large percentage of hepatic malignancies are secondary metastases. With hepatic metastatic seeding, solid organ cancers mostly disqualify for curative treatment approaches. Evidence for curability of hepatic metastases is emerging, limited to patients with isolated liver metastases, usually further restricted to non-bilobar disease;1,2 thus, less than 1/5 of patients with secondary liver malignancy qualify for curative treatment at the time of initial diagnosis.3 Accordingly, the majority of patients face sparse options for targeted therapy of hepatic metastases; within the cluster of interventional transarterial therapies, degradable starch microspheres – transarterial chemoembolization (DSM-TACE) is one option in palliative treatment besides, eg, radioembolization using Yttrium90 or hepatic arterial infusion of chemotherapeutic agents. The injection of a chemotherapeutic agent in TACE, mixed with an embolic material such as Lipiodol, DSM, or drug-eluting beads, aims at reaching a steep drug concentration gradient within the tumor and low systemic concentrations.4 Thus, systemic side effects are limited, while higher drug dosages favor the local antitumor efficacy. As for all transarterial therapies, data on DSM-TACE is sparse since treatment regimes for hepatic metastasis of advanced-stage solid tumors vary widely. Furthermore, with TACE being one treatment option, it is not standardized. TACE-caused complications can be severe, eg, ascites, acute liver and/or renal failure, hepatic encephalopathy, and gastrointestinal bleedings. Minor adverse events (AEs) after TACE are summarized as postembolization syndrome, it comprises upper right quadrant abdominal pain, fever, and nausea and vomiting within 24 hours post TACE. Despite the palliative treatment situation, treatment regimes are required which reduce side effects and improve tumor response rates.
This study reports results of a national multicenter observational study on safety and efficacy of DSM-TACE in secondary liver malignancies as an interventional, locoregional treatment option.
Methods
Patients
All patients received standard of care, and treatment regimes were defined in an interdisciplinary tumor conference. Patients aged 18 years and older with a secondary liver malignancy of any stage not suitable for resection or any other curative treatment strategy were eligible for inclusion, independent of type, stage, and pretreatment of the primary malignancy. Both nodular and infiltrative growth, as well as single-lobar and bilobar disease were suitable for inclusion. Excluded from study participation were patients with prior chemoembolization, enrollment in any other clinical trial, and/or having other contraindications against DSM-TACE such as lacking a safe arterial access to the intrahepatic malignancy.
Approval by the local ethics committee (University of Regensburg) was waived due to the observational nature of the study, and it was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization Guideline on Good Clinical Practice, and national laws and regulations where applicable. Written informed consent for DSM-TACE was obtained at least 24 hours prior to embolization.
Study design
This was a national, multicenter observational study on safety and efficacy of DSM-TACE in palliative treatment for secondary liver malignancies. Recruitment period for the study was from January 2010 to June 2014.
Safety and toxicity
The key point of the observational study was to evaluate DSM-TACE treatment-emergent adverse events (TEAEs). Separately registered were side effects within 24 hours of a DSM-TACE procedure and between two treatments (6 weeks apart), all according to the CTCAE V4.0 classification of acute and subacute toxic side effects listing both treatment-emergent serious AEs and TEAEs.
Efficacy
DSM-TACE efficacy in secondary liver malignancy treatment was rated according to mRECIST response criteria for the assessment of tumor necrosis in locoregional therapies 6 weeks after TACE.5,6 Using mRECIST first hand,7 complete remission is defined as no malignancy left, partial remission as tumor decline >30%, stable disease as a tumor neither of decline >30% nor progress >20%. Tumor progress (progressive disease) is defined by a growth of >20%. Objective response rate (ORR) is complete remission + partial remission, and disease control is ORR + stable disease, as reported before.8
DSM-TACE
After staging (magnetic resonance imaging [MRI] and/or computed tomography [CT]), patients received DSM-TACE as outlined below. Six weeks later, in a restaging including MRI and/or CT, a decision was made if another TACE was suitable.
Femoral artery was punctured in all DSM-TACE procedures; selective (A. hepatica propria, A. hepatica sin., A. hepatica dex.) or superselective catheterization was used, at the interventionalist’s discretion with respect to tumor burden, hepatic vascular status, and patient characteristics.
EmboCept S DSM 35/50 (PharmaCept GmbH, Berlin, Germany) is a short-term embolizate made of degradable starch microspheres with an average diameter of 50 micrometers. They have a half-life of about 35–50 minutes, both in vivo and in vitro. A specific side effect of all vascular occlusion strategies such as TACE is a backflow of the embolizate to nontarget regions, causing ischemia and severe pain. DSM lowers the risk of severe organ damage due to its self-limiting degradation; partial resumption of the blood flow will be evident after about 10–15 minutes.4
Preparation and dosage of DSM was chosen per the manufacturer’s recommendation; choice of chemotherapeutic agent or a combination of up to three drugs were up to the individual patient and case characteristics. DSM allows the use of a variety of coadministered contrast agents.
Imaging
Within this observational study, imaging modality for staging of intrahepatic lesions and assessment of therapeutic efficacy was chosen at the interventionalist’s discretion. Before and after DSM-TACE, all patients underwent CT and/or MRI imaging using a multiphase liver imaging protocol. The same imaging modalities were used throughout each patient’s participation in the study to enable a reliable rating according to mRECIST as outlined before.
Statistical analysis
For statistical analysis, GraphPad Prism ver. 5.00a for Mac (GraphPad Software, San Diego, CA, USA) was used. Arising from the observational characteristic of the reported study, descriptive statistics were used.
Results
In our national, multicenter observational study on safety and efficacy of DSM-TACE in the treatment of secondary liver malignancies, we included 20 patients between January 2010 and October 2014. They received a total of 77 DSM-TACE procedures. Data on baseline characteristics of patients, pretreatment, and disease extent is summarized in Table 1. Data on primary malignancies is provided in Figure 1.
  | Table 1 Baseline characteristics of patients, pretreatment, and disease extent |
  | Figure 1 Primary malignancies with hepatic metastases. |
DSM-TACE procedure characteristics
DSM-TACE was used in a palliative treatment setting in all cases. Details on TACE procedure and chemotherapeutics used are shown in Tables 2 and 3, respectively.
  | Table 3 Chemotherapy regimes used in DSM-TACE |
Safety
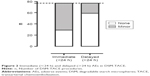
Immediate minor AEs were as follows: nausea and vomiting occurred in 18 out of 77 treatments (23.4%). In 35 treatments (45.5%), moderate, transient epigastric pain was registered. After 4 DSM-TACE procedures (5.2%), body temperature was elevated; after 2 procedures (2.6%), sweating occurred; and after 1 procedure (1.3%), the feeling of abdominal pressure was registered. Delayed minor AEs were as follows: 13 (16.9%) treatments were followed by epigastric pain, and in between 10 treatments (13.0%) nausea and vomiting was reported. There were also 2 reports (2.6%) of diarrhea, 6 (7.8%) of fever, 5 (6.5%) of shivering, and 1 (1.3%) of an ulcer in between treatments. No immediate or delayed severe AEs were recorded. Overall, minor immediate AE occurred in 48/77 (62.3%) treatments and minor delayed AE in 18/77 (23.4%) treatments (Figure 2).
Efficacy and tumor response
In a total of 77 DSM-TACE procedures with clinical and imaging follow-up, progress was registered after 6 procedures (7.8%), 33 (42.9%) showed no change according to mRECIST, and partial response was achieved in 17 procedures (22.1%). No case of complete response was reported. No data was available for 21 procedures (27.3%). Thus, the ORR for TACE procedures was 22.1%; disease control was achieved in 64.9% of TACE procedures. All cases of objective response were attributable to 8 of 20 (40.0%) patients included.
Discussion
Minimally invasive locoregional approaches are of special interest in the treatment of secondary liver malignancies, since 80% of patients disqualify for surgical and potentially curative treatment options due to disease extent at the time of diagnosis.9 DSM-TACE is one option for the palliative treatment of secondary liver malignancies.
TACE yields high local chemotherapeutic concentrations with a steep gradient to healthy liver parenchyma due to the specific architecture of hepatic blood supply.10 Low levels of free circulating chemotherapeutics allow for detaining and minimizing systemic side effects. Thus, the aim of this national multicenter observational study regarding DSM-TACE for the treatment of secondary liver malignancies was to assess safety and efficacy of this treatment regime.
While reports on TACE regimes using nondegradable microspheres (eg, drug-eluting beads) as embolic agent are increasing, studies on DSM are limited. Studies to be found are mostly centered around hepatic metastases of colorectal carcinoma, since up to 80% of secondary liver malignancies are of gastrointestinal origin due to its portal venous drainage.11 Nishiofuku et al12 reported a response rate for DSM-TACE with cisplatin (80 mg/m2 BSA) of 61.1% (11/18 patients) after failure of systemic folinic acid, fluoruracil, oxaliplatin regime in hepatic metastases of colorectal carcinoma. Besides others, nausea, vomiting, and pain were reported in 45.8%, 50.0%, and 45.8% of patients, respectively. As severe AEs, thrombocytopenia, cholecystitis, and high-grade aspartate transaminase elevation were found in 12.5%, 4.2%, and 33.3% of patients, respectively. A comparable response rate of 59% was reported by Tsuchiya et al,13 in 2007, likewise in TACE of hepatic metastases of colorectal carcinoma. Further comparable results of response rates after DSM-TACE are summarized in Table 4.14
  | Table 4 ORRs in DSM-TACE in current literature |
The results of our study are in line with previously published data. With regard to safety, we report transient epigastric pain to be the dominant TEAE with an incidence of 45.5%. Nausea and vomiting followed with a rate of 23.4%. Both symptoms are known to occur with high incidence in TACE procedures. Yet, standards for treatment are missing. A profound antiemetic and analgesic therapy should be able to ameliorate a significant proportion of these TEAEs.15,16 No severe TEAEs were documented. From 20 patients followed until drop-out, none discontinued TACE treatment due to primary treatment-emergent reasons, emphasizing the high tolerance of DSM-TACE.
With DSM-TACE, an objective response was achieved in 40.0% of cases, with partial response in 22.1% of procedures and no further progress after 42.9% of procedures, summing up to 64.9% of procedures achieving disease control. These results should be valued as promising, keeping in mind that DSM-TACE is a minimally invasive, palliative treatment option in a patient cohort showing a high disease burden with associated comorbidities.17
The significance of the presented results must be evaluated considering the study’s limitations. The small sample size and the heterogeneous nature of the primary malignancy are evident as is the nonstandardized TACE treatment protocol including diagnostic pathways for follow-up imaging. Further clinical studies are needed to compare the efficacy including long-term survival and/or progression-free survival in comparison to the standard TACE with Lipiodol.
Conclusion
In summary, TACE with DSM as embolic agent is safe to use in the treatment of secondary liver malignancies not suitable for surgery. Efficacy is promising, with disease control in 64.9% of procedures and an objective response in 40.0% of patients. Further reduction of AEs and increase in efficacy should be achievable by standardizing both the DSM-TACE treatment regime as well as the accompanying side medications prescribed (eg, analgesics, antiemetics) according to well-established standards.
Main points
- DSM can be used as embolic agent in TACE of hepatic malignancies
- DSM-TACE is safe in the treatment of liver metastases taking into consideration adverse and severe AEs
- DSM-TACE shows promising efficacy in the treatment of liver malignancies in a variety of primary tumors.
Disclosure
Philippe L Pereira in the last 3 years has received speaker’s honoraria and grants, has helped organize symposia, and is on the advisory board or acting as a consultant for: Bayer Global and Bayer Germany, Biocompatibles and BTG, Celonova (Boston Scientific), Cook Medical, Pharmacept, SIRTEX, Terumo.
Christian Stroszczynski and Philipp Wiggermann received financial support from PharmaCept GmbH for the present study. The authors report no other conflicts of interest in this work.
References
Doci R, Gennari L, Bignami P, et al. Morbidity and mortality after hepatic resection of metastases from colorectal cancer. Br J Surg. 1995;82:377–381. | ||
Barnett I, van Sluijs EMF, Ogilvie D. Physical activity and transitioning to retirement. Am J Prev Med. 2012;43:329–336. | ||
Nordlinger B, Van Cutsem E, Gruenberger T, et al. Combination of surgery and chemotherapy and the role of targeted agents in the treatment of patients with colorectal liver metastases: recommendations from an expert panel. Ann Oncol. 2009;20:985–992. | ||
Håkansson L, Håkansson A, Morales O, Thorelius L, Warfving T. Spherex (degradable starch microspheres) chemo-occlusion – enhancement of tumor drug concentration and therapeutic efficacy: an overview. Semin Oncol. 1997;24:S6-100–S6-109. | ||
Minocha J, Lewandowski RJ. Assessing imaging response to therapy. Radiol Clin North Am. 2015;53:1077–1088. | ||
Kim MN, Kim BK, Han KH, Kim SU. Evolution from WHO to EASL and mRECIST for hepatocellular carcinoma: considerations for tumor response assessment. Expert Rev Gastroenterol Hepatol. 2015;9:335–348. | ||
Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. | ||
Vogl TJ, Zangos S, Eichler K, et al. Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol. 2007;17:1025–1034. | ||
Germer CT, Buhr HJ, Isbert C. Möglichkeiten und Grenzen der Ablationsverfahren zur Behandlung von Lebermetastasen unter kurativer Intention. [Nonoperative ablation for liver metastases. Possibilities and limitations as a curative treatment]. Chir Z Alle Geb Oper Medizen. 2005;76:552–554, 556–563. German. | ||
Breedis C, Young G. The blood supply of neoplasms in the liver. Am J Pathol. 1954;30:969–977. | ||
Alberts SR. Update on the optimal management of patients with colorectal liver metastases. Crit Rev Oncol Hematol. 2012;84:59–70. | ||
Nishiofuku H, Tanaka T, Matsuoka M, et al. Transcatheter arterial chemoembolization using cisplatin powder mixed with degradable starch microspheres for colorectal liver metastases after FOLFOX failure: results of a phase I/II study. J Vasc Interv Radiol. 2013;24:56–65. | ||
Tsuchiya M, Watanabe M, Otsuka Y, et al. [Transarterial chemoembolization with irinotecan (CPT-11) and degradable starch microspheres (DSM) in patients with liver metastases from colorectal cancer]. Gan To Kagaku Ryoho. 2007;34:2038–2040. Japanese. | ||
Wasser K, Giebel F, Fischbach R, et al. Transarterielle Chemoembolisation von Lebermetastasen kolorektaler Karzinome mit abbaubaren Stärkepartikeln (Spherex®). [Transarterial chemoembolization of liver metastases of colorectal carcinoma using degradable starch microspheres (Spherex®): personal investigations and review of the literature]. Radiol. 2005;45:633–643. | ||
Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358:2482–2494. | ||
Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;374:1356–1367. | ||
Weitz J, Koch M, Debus J, et al. Colorectal cancer. Lancet Lond Engl. 2005;365:153–165. | ||
Wollner IS, Walker-Andrews SC, Smith JE, Ensminger WD. Phase II study of hepatic arterial degradable starch microspheres and mitomycin. Cancer Drug Deliv. 1986;3:279–284. | ||
Lorenz M, Herrmann G, Kirkowa-Reimann M, et al. Temporary chemoembolization of colorectal liver metastases with degradable starch microspheres. Eur J Surg Oncol. 1989;15:453–462. | ||
Taguchi T, Ogawa N, Bunke B, et al. The use of degradable starch microspheres (Spherex) with intra-arterial chemotherapy for the treatment of primary and secondary liver tumours – results of a phase III clinical trial. Reg Cancer Treat. 1992;4:161–165. | ||
Fujimoto S, Koike S, Takahashi M, et al. Intra-arterial infusion chemotherapy with degradable starch microspheres and mitomycin C for unresectable hepatic metastasis from gastrointestinal cancer. Reg Cancer Treat. 1993;1:7–11. | ||
Civalleri D, Pector JC, Håkansson L, Arnaud JP, Duez N, Buyse M. Treatment of patients with irresectable liver metastases from colorectal cancer by chemo-occlusion with degradable starch microspheres. Br J Surg. 1994;81:1338–1341. | ||
Voigt W, Behrmann C, Schlueter A, et al. A new chemoembolization protocol in refractory liver metastasis of colorectal cancer – a feasibility study. Onkologie. 2002;25:158–164. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.