Back to Journals » Clinical Ophthalmology » Volume 15
Safety and Efficacy of BroadBand Intense Pulsed Light Therapy for Dry Eye Disease with Meibomian Gland Dysfunction
Authors Murtaza F , Toameh D, Al-Habib S, Maini R, Chiu HH , Tam ES , Somani S
Received 27 July 2021
Accepted for publication 31 August 2021
Published 2 October 2021 Volume 2021:15 Pages 3983—3991
DOI https://doi.org/10.2147/OPTH.S331289
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Fahmeeda Murtaza,1 Dana Toameh,2 Saed Al-Habib,3 Raj Maini,2 Hannah H Chiu,2,4,5 Eric S Tam,2,4,5 Sohel Somani2,4,5
1Temerty Faculty of Medicine, University of Toronto, Toronto, Ontario, Canada; 2Uptown Eye Specialists, Brampton, Ontario, Canada; 3U Sculpt Plastic Surgery, Vaughan, Ontario, Canada; 4Department of Ophthalmology & Vision Sciences, University of Toronto, Toronto, Ontario, Canada; 5William Osler Health System, Brampton, Ontario, Canada
Correspondence: Sohel Somani
Uptown Eye Specialists, 401-7900 Hurontario Street, Brampton, Ontario, L6Y 0P6, Canada
Tel +1 416 292-0330
Fax +1 416 292-0331
Email [email protected]
Background: BroadBand light intense pulsed light (BBL-IPL) therapy has shown to reduce hordeolum and blepharitis frequency. This study aims to evaluate the efficacy and safety of BBL-IPL therapy in patients with dry eye disease (DED) from meibomian gland dysfunction (MGD).
Methods: This is a retrospective, consecutive case series of 48 patients with DED from MGD who underwent BBL-IPL therapy from October 2016 to January 2019 at a single, outpatient clinic in Ontario, Canada. Clinical outcomes included first and average non-invasive keratograph tear break-up times (NIKBUT), bulbar redness (BR) scores, tear meniscus heights (TMH), visual acuity (VA) and meibograph grades. Patient-reported outcomes included the Canadian dry eye assessment (CDEA) questionnaire and patient subjective assessment (PSA) scores. Outcomes were measured at baseline and after completion of 4 monthly BBL-IPL sessions.
Results: The mean severity of dry eye symptoms as measured by the CDEA and PSA decreased significantly from 19.78 ± 9.62 to 12.08 ± 7.40 (p< 0.001) and from 7.65 ± 1.74 to 4.77 ± 2.03 (p< 0.001), respectively. Twenty-five percent of patients reported no dry eye symptoms after treatment. The meibograph grade improved significantly in both eyes (p< 0.001). Approximately 71.0% and 80.1% of patients had an improved meibograph grade in the right and left eye, respectively. Near-significant improvements were observed for BR scores and VA. There was also a trend towards improved first/average NIKBUT and TMH scores. No adverse events were noted.
Conclusion: BBL-IPL appears to be an effective and safe treatment modality in improving dry eye symptoms and meibomian gland function in patients with DED from MGD.
Keywords: BroadBand light, intense pulsed light, meibomian gland dysfunction, dry eye disease
Introduction
Dry eye disease (DED) is a multifactorial disease characterized by the loss of homeostasis of the tear film, resulting in tear film instability, hyperosmolarity and inflammation of the ocular surface.1 Patients with DED experience ocular discomfort, including foreign body sensation, irritation, burning, redness, and visual disturbances. DED prevalence varies between 5% and 50% across certain populations, making it one of the most common diseases encountered in ophthalmic practice.2,3
The vast majority of DED patients have evaporative dry eye caused by meibomian gland dysfunction (MGD).4 MGD is a chronic condition characterized by terminal duct obstruction and/or changes in glandular secretion affecting the stability of the tear film lipid layer.4–6 Currently, treatment options for DED with MGD include artificial tears, warm compresses, eyelid scrubs, punctal plugs, cyclosporine drops, steroid drops, omega-3 fatty acid supplements and oral tetracycline and azithromycin.4–7 However, many patients do not achieve complete or long-term relief with these treatments.
In the last few years, intense pulsed light (IPL) therapy has emerged as a possible treatment option for DED with MGD. IPL is widely used in treating dermatological conditions, including facial rosacea, acne, dyspigmentation and dermal vascular lesions.2,8–13 In 2002, Toyos et al14 reported a significant improvement in dry eye symptoms in patients treated for facial rosacea with IPL. Although the exact mechanism of action remains unknown, many studies since then have reported significant improvements in dry eye symptoms, tear break-up times, lipid layer grade and thickness, and/or meibomian gland function, with limited adverse events.2,15–23 These studies have used a variety of IPL devices, including M22™ (Lumenis, Israel),17,20,22 Quadra Q4 (DermaMed Solutions, USA),2,14 E>Eye (E-SWIN, France),15,16,18,21 and customized treatment protocols, ranging in energy levels, treatment frequencies, number of treatment sessions and treatment durations.
BroadBand Light (BBL™) is a high-quality, enhanced IPL modality that uses polychromatic, non-coherent, continuous light waves from the visible (420 nm) to the infrared (1400 nm) spectrum.23 BBL-IPL is known for its relative safety, high skin coverage rate, and minimal recovery time.20 As compared to IPL alone, BBL offers larger spot sizes, a wider range of filters and a continuous pulsing mode, which enhance the efficacy and safety of treatments.24 Recently, Zhang-Nunes et al23 reported an improvement in dry eye symptoms and blepharitis and hordeolum frequency with some temporary adverse effects after BBL-IPL treatment in patients with DED from MGD. To our knowledge, no other studies have evaluated the use of Sciton® BBL-IPL in treating DED with MGD. We carried out a single-center, retrospective study to evaluate the safety and efficacy of BBL-IPL treatment in patients diagnosed with DED with MGD.
Methods
Patient Selection
This retrospective, consecutive case-series was approved by the William Osler Health System Research Ethics Board and adhered to the Declaration of Helsinki. Medical records of patients treated for DED with MGD using BBL-IPL at a single outpatient clinic between October 2017 and January 2019 were extracted.
Inclusion criteria consisted of patients over the age of 18 diagnosed with DED from MGD by their ophthalmologist (R.M./E.S.T./H.H.C.). The diagnosis of MGD was based on anatomical features of terminal duct obstruction, meibomian gland (MG) dropout, and changes in meibum quality and outflow.3,4,25 MGD was classified from stage 1 to stage 4, where stage 1 represents minimal MGD and stage 4 represents severe, marked MGD.25 Only patients with stages 2 to 4 MGD were included. The Fitzpatrick skin type was determined based on sun sensitivity and appearance,26 where I represents fair skin, and VI represents deeply pigmented skin. Patients with skin types I, II, III and IV were included.
Treatment Procedure
Patients received 4 sessions of IPL laser therapy using the BroadBand Light™ (Sciton® Palo Alto, CA, USA) IPL device. All sessions were administered by a plastic surgeon (S.A.). Before treatment, the skin of the upper and lower eyelids was prepped with povidone 5% and anesthetized with clear lidocaine hydrochloride ophthalmic gel 3.5%. Protective metal shields were placed over the patient’s sclera and cornea and ultrasonic gel was applied to the skin of both eyelids. A 7-mm circular adaptor was placed on top of a rectangular sapphire crystal and treatment was directed to both eyelids with two passes and 20% overlap. Treatment settings were customized to the patient’s skin type (Table 1). Each patient received approximately 30 pulses per session: 5 pulses in 6 treatment areas from the nasal to the temporal side of each eyelid. Treatment sessions were repeated approximately every month for a total of 4 sessions. Patients were advised to avoid direct exposure to sunlight during the treatment course. Patients on any active treatments for DED during the BBL-IPL course were advised to continue them as usual. Patients were advised to withhold additional treatments until after the final follow-up visit.
 |
Table 1 Customized BBL-IPL Treatment Settings Based on Patient Skin Type |
Patient-Reported and Clinical Outcomes
Patient-reported outcomes include the Canadian Dry Eye Assessment (CDEA) questionnaire and patient subjective assessment (PSA). The 12-item CDEA questionnaire is a modification of the validated Ocular Surface Disease Index (OSDI) used to determine the severity of dry eye symptoms.27,28 Total scores range from 0 to 48 and symptoms are interpreted as normal (<5), mild (5–20), moderate (21–30), or severe (31–48).28 The question “How much do your eyes bother you?” within the CDEA was evaluated independently and named the patient subjective assessment (PSA). PSA scores range from 1 to 10, with a higher score indicating increased severity of symptoms.
Clinical outcomes included the first and average non-invasive keratograph tear break-up times (NIKBUT), bulbar redness (BR) scores, tear meniscus heights (TMH), visual acuity (VA) and meibograph grades for both eyes. NIKBUT, BR, TMH were measured using the Keratograph® 5M (OCULUS, GmbH, Wetzlar, Germany) by a trained ophthalmic technician blinded to the treatment status.
For NIKBUT measurements, patients were instructed to keep their eyes open as long as possible. First NIKBUT was measured as the time between the last complete blink and the first perturbation of a grid projected onto the surface of the cornea, which the device detects automatically.29 Average NIKBUT was calculated from the average of all break-up events. A break-up time of ≤5 seconds suggests dry eyes.30
The TMH is the distance between the darker edge of the lower eyelid and the tear strip. The final TMH was calculated from the average of the TMH measurements at the left, right and center of each eyelid. A TMH of <0.25 mm is suggestive of DED.31
The BR score was determined by the area percentage ratio of vessels to the bulbar conjunctiva under illumination. The maximum ratio is 40%, therefore, BR scores range from 0.0 to 4.0. A higher score represents increased BR.32
Snellen visual acuity was converted to minimum angle of resolution (logMAR) for analysis based on previous published literature.33
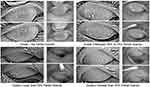
Meibograph pictures of only the upper eyelids were used to grade MG dropout on a 4-point gestalt scale by an independent ophthalmologist grader (S.S.) blinded to the patient’s ocular or treatment status. MG dropout was classified from grade 1 to 4 depending on severity (Figure 1).34
Pre-treatment outcomes were measured approximately 30 days before the first session, while post-treatment outcomes were measured approximately 30 days after the final session.
Statistical Analysis
Statistical analyses were performed using SPSS Statistics Version 27.0 (IBM, Armonk, NY). Continuous variables were described using proportions, means ± standard deviations (SD), and medians with interquartile ranges (IQR). Categorical variables were described using proportions. Data normality was assessed using histograms and the Shapiro–Wilk normality test. A paired-sample t-test or Wilcoxon signed ranks test for non-normal data was used for comparisons of outcomes before and after treatment. All tests were two-tailed, and Holm-Bonferroni correction was used to confer statistical significance. Regression analyses were conducted to determine associations between gender, treatment interval and skin type and the change in outcomes. A p-value of <0.05 was used to confer statistical significance.
Results
Baseline Features
Of 80 patients treated with BBL-IPL, 48 patients met the inclusion criteria. The median age was 64 years (range 25–97), and the sample was 64.6% (31/48) female (Tables 2 and 3). The median Fitzpatrick skin type was 3 (IQR 2–4) and the median treatment interval was 136 days (IQR 116.5–204) (Table 2). All patients continued their DED medications or therapies throughout the BBL-IPL treatment course, which included lubricant drops, cyclosporine drops, antibiotic drops, steroid drops, Omega-3 supplements, oral doxycycline, as well as warm compresses and lid scrubs (Table 3). Most patients were taking at least two topical or oral medications to manage their DED symptoms (Table 3). Approximately 25.0% (12/48) patients had prior refractive surgery, 2.1% (1/48) had Sjogren’s disease and 14.6% (7/48) had diabetes, of which 71.4% (5/7) were being followed for diabetic retinopathy.
 |
Table 2 Patient Demographics |
 |
Table 3 Baseline Patient and Clinical Demographics |
Clinical and Patient-Reported Outcomes
The mean CDEA score decreased significantly from 19.78 ± 9.62 to 12.08 ± 7.40 (p<0.001; Table 4). Approximately 86.1% (31/36) of patients reported an improved CDEA score, with 25.0% (9/36) experiencing no dry eye symptoms after treatment (Figure 2). The mean PSA score decreased significantly from 7.65 ± 1.74 to 4.77 ± 2.03 (p<0.001; Table 4). Approximately 93.5% (29/31) of patients reported an improved PSA score.
 |
Table 4 Clinical and Patient-Reported Outcomes Before and After BBL-IPL Therapy |
The first NIKBUT increased from 4.01 ± 2.71s to 4.60 ± 2.99s in the right eye and from 4.11 ± 3.15s to 5.00 ± 3.64s in the left eye (OD: p=0.350, OS: p=0.555; Table 4). Average NIKBUT changed from 7.35 ± 3.14s to 8.50 ± 4.61s in the right eye and from 8.00 ± 4.57s to 8.04 ± 4.62s in the left eye (OD: p=0.178, OS: p=0.600; Table 4).
The mean TMH increased from 0.28 ± 0.12mm to 0.31 ± 0.12mm in the right eye and from 0.29 ± 0.1mm to 0.32 ± 0.16mm in the left eye (OD: p=0.206, OS: p=0.197; Table 4).
The mean BR decreased from 1.55 ± 0.48 to 1.33 ± 0.47 in the right eye and from 1.59 ± 0.51 to 1.39 ± 0.44 in the left eye (OD: p=0.008, OS: p=0.013; Table 4).
The mean VA improved from 0.34 ± 0.39 (Snellen 20/44) to 0.30 ± 0.41 (Snellen 20/40) in the right eye and from 0.20 ± 0.20 (Snellen 20/32) to 0.14 ± 0.18 (Snellen 20/28) in the left eye (OD: p=0.043, OS: p=0.003; Table 4).
The median meibograph grade decreased significantly from 4 (IQR 3–4) to 3 (IQR 2–3) in both eyes (p<0.001; Table 4). Approximately 71.0% (22/31) and 80.1% (25/31) of patients had an improved meibograph grade in the right and left eye, respectively (Figure 3). No patients experienced worsening in meibograph grade after treatment.
No significant correlations between gender, treatment interval and skin type and patient-reported or clinical outcomes were found (data not reported).
Adverse Events
No temporary or permanent adverse events were noted.
Discussion
With the recent US Food and Drug Administration approval of the M22™ Lumenis IPL device for treatment of DED, the use of IPL therapy is expected to rise in routine ophthalmic practice. Our study demonstrates that BB-IPL, an enhanced IPL modality, can improve dry eye symptoms and meibomian gland function in patients with DED due to MGD.
After four monthly sessions of BBL-IPL, there was a significant improvement in CDEA and PSA scores. Over 85% of patients reported an improvement in dry eye symptoms, which concurs with improvements seen with IPL therapy alone.2,14–21 The use of patient-reported outcomes in dry eye research is an area of ongoing development. Although the reliability and validity of the CDEA are yet to be established, some studies have found no correlations between symptoms reported in dry eye questionnaires and clinical tests for dry eye, such as tear break-up time (TBUT), TMH and MG structure.27,35 However, we observed improvements in both patient-reported and clinical outcomes.
We observed a significant improvement in the meibograph grades in both eyes. Nearly two-thirds of our patients had severe MG dropout at baseline, yet over 70% experienced an improvement after treatment. In contrast to IPL, BBL-IPL uses continuous pulsed light in the red and infrared spectrum to provide more evenly distributed heat to the skin and subcutaneous tissues.24 We believe the heat generated from continuous pulsed light has a two-fold effect in improving MG structure and function. Firstly, as the melting point of meibum is higher in patients with MGD,4,39 BBL-IPL can stimulate MGs making meibum less viscous, and thereby promoting outflow.15,16,21,22 Secondly, heat from BBL-IPL may reduce harmful bacteria and Demodex mite infection in the eyelids and ocular surface, and limit inflammation and obstruction of MG orifices.2,15,16,18,23
We also found a trend towards improved NIKBUT and TMH in both eyes, which is consistent with findings in literature on IPL therapy alone.2,14–21 A low NIKBUT contributes to blurred vision, therefore the improvements in first and average NIKBUT values may explain the improvements in VA in both eyes.15,16 Despite significant improvements in meibomian gland structure, four sessions of BBL-IPL therapy may not have produced significant functional improvements in tear quality and volume. We may see further improvements in tear quality and volume with additional sessions.
We found a near-significant decrease in BR, which can explain the significant improvements reported in dry eye symptoms. Through selective photo thermolysis, IPL induces thrombosis of abnormal vascularization in the eyelid margin and conjunctiva.2,15,22 The reduction in redness and inflammation is attributed to the limited access of inflammatory precursor molecules causing tear film instability at the ocular surface.6,23,38 Studies have reported a significant reduction in pro-inflammatory cytokines, including IL-4, IL-6, IL-10, IL-17A, PGE2, and TNF-alpha in tears of patients treated with IPL therapy.2,12,17,20 Improvements in patient-reported visual symptoms may also be explained by the effects of IPL in the 600–950 nm spectrum in relieving chronic inflammatory pain and neurogenic sensitivity.36,37
Safety
Zhang-Nunes et al23 reported reduced blepharitis and hordeolum frequency after one to four sessions of BBL-IPL treatment. However, four of their patients experienced temporary adverse effects including corneal abrasion, hyperpigmentation, and eyelash thinning.23 Despite two patients using oral doxycycline, a known skin-sensitizing agent, we observed no temporary or permanent adverse events. We also did not observe worsening in VA after treatment. Some studies suggest limiting IPL exposure to only the lower eyelids and Fitzpatrick skin types I–IV.2,14 Even though BBL-IPL therapy delivers more evenly distributed heat than IPL alone, our findings support the safety of BBL-IPL in Fitzpatrick skin types I–IV and both eyelids in the context of adequate eye protection for improving MG function.
Overall, BBL-IPL therapy offers similar functional benefits as IPL therapy alone with respect to dry eye symptoms, tear film quality and quantity, conjunctival injection, visual acuity, and meibomian gland health. However, we believe that in routine practice, BBL-IPL is superior to IPL because continuous pulsed light delivers more evenly distributed energy and heat, without compromising safety. Furthermore, BBL-IPL has a wider range of spot sizes and filters allowing for targeted treatment and a better safety profile for darker skin types.24 Interestingly, some recent photobiomodulation modalities, such as low-level light therapy (LLT), which involves athermal photoactivation, have also been shown to improve dry eye symptoms, tear breakup times, and meibomian gland structure with minimal side effects.41,42 Future research can compare the effects of BBL-IPL with other athermal treatment modalities like LLT on the improvement of DED from MGD.
Strengths
Unlike previous studies that used customized treatment protocols, a major strength of our study is that we used a standardized treatment protocol that can be easily implemented into routine clinical practice and give reproducible results. We also used objective DED metrics as measured by the Keratograph 5M, which have been found to correlate with dry eye symptoms.32,40 The meibograph grade was assessed by an ophthalmologist (S.S.) blinded to treatment status, thereby improving the internal validity of findings, and limiting investigator bias. Furthermore, some studies measured post-treatment results on the same day or shortly after an IPL session,15,16 while our post-treatment results were measured at least 30 days after the last session. Therefore, our study findings demonstrate the sustained effects of BBL-IPL treatment. Lastly, we used PSA as an additional patient-reported measure to better ascertain the effects of BBL-IPL therapy on the severity of dry eye symptoms.
Limitations
The retrospective design and a small sample size with no comparison group weaken the validity of study findings. Given the significant improvement in meibograph grade but non-significant improvements in NIKBUT and TMH scores, other variables of tear film quality and stability, such as lipid-layer grade and thickness, tear evaporation rate, tear film osmolarity, and matrix metalloproteinases levels, could also be investigated. To better isolate the effects of BBL-IPL treatment on the tear film, lid margin debridement could have been performed before sessions.16 Generally, studies with larger patient samples, variable treatment sessions and longer follow-up periods are needed to better evaluate the efficacy and safety of BBL-IPL therapy for DED with MGD.
Conclusion
BBL-IPL appears to be a safe and effective treatment modality for dry eye disease with meibomian gland dysfunction. Patients experienced an improvement in dry eye symptoms, tear break-up times, bulbar redness, visual acuity and meibomian gland function after 4 monthly sessions of BBL-IPL therapy, without any temporary or permanent adverse effects.
Ethical Corrections
Patient consent to review medical records was not required by theWilliam Osler Health System Research Ethics Board as this was a retrospective case series. All efforts were made to protect patient confidentiality. This research project adhered to the Declaration of Helsinki.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Shimazaki J. Definition and diagnostic criteria of dry eye disease: historical overview and future directions. Invest Ophthalmol Vis Sci. 2018;59(14):DES7–DES12. doi:10.1167/iovs.17-23475
2. Gupta PK, Vora GK, Matossian C, Kim M, Stinnett S. Outcomes of intense pulsed light therapy for treatment of evaporative dry eye disease. Can J Ophthalmol. 2016;51(4):249–253. doi:10.1016/j.jcjo.2016.01.005
3. Stapleton F, Alves M, Bunya VY, et al. Tfos dews ii epidemiology report. Ocul Surf. 2017;15(3):334–365.
4. Nichols KK. The international workshop on meibomian gland dysfunction: introduction. Invest Ophthalmol Vis Sci. 2011;52(4):1917–1921. doi:10.1167/iovs.10-6997
5. Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797.
6. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472–478. doi:10.1097/ICO.0b013e318225415a
7. Kashkouli MB, Fazel AJ, Kiavash V, Nojomi M, Ghiasian L. Oral azithromycin versus doxycycline in meibomian gland dysfunction: a randomised double-masked open-label clinical trial. Br J Ophthalmol. 2015;99(2):199–204. doi:10.1136/bjophthalmol-2014-305410
8. Raulin C, Weiss RA, Schönermark MP. Treatment of essential telangiectasias with an intense pulsed light source (PhotoDerm VL). Dermatol Surg. 1997;23(10):941–945. doi:10.1111/j.1524-4725.1997.tb00755.x
9. McGill DJ, MacLaren W, Mackay IR. A direct comparison of pulsed dye, alexandrite, KTP and Nd: YAG lasers and IPL in patients with previously treated capillary malformations. Lasers Surg Med. 2008;40(6):390–398. doi:10.1002/lsm.20638
10. Park JM, Tsao H, Tsao S. Combined use of intense pulsed light and Q‐switched ruby laser for complex dyspigmentation among Asian patients. Lasers Surg Med. 2008;40(2):128–133. doi:10.1002/lsm.20603
11. Neuhaus IM, Zane LT, Tope WD. Comparative efficacy of nonpurpuragenic pulsed dye laser and intense pulsed light for erythematotelangiectatic rosacea. Dermatol Surg. 2009;35(6):920–928. doi:10.1111/j.1524-4725.2009.01156.x
12. Mariwalla K, Rohrer TE. Use of lasers and light‐based therapies for treatment of acne vulgaris. Lasers Surg Med. 2005;37(5):333–342. doi:10.1002/lsm.20276
13. Piccolo D, Di Marcantonio D, Crisman G, et al. Unconventional use of intense pulsed light. Biomed Res Int. 2014;2014(10):1–10. doi:10.1155/2014/618206
14. Toyos R, McGill W, Briscoe D. Intense pulsed light treatment for dry eye disease due to meibomian gland dysfunction; a 3-year retrospective study. Photomed Laser Surg. 2015;33(1):41–46. doi:10.1089/pho.2014.3819
15. Jiang X, Lv H, Song H, et al. Evaluation of the safety and effectiveness of intense pulsed light in the treatment of meibomian gland dysfunction. J Ophthalmol. 2016;2016(1):1–8.
16. Craig JP, Chen YH, Turnbull PR. Prospective trial of intense pulsed light for the treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci. 2015;56(3):1965–1970. doi:10.1167/iovs.14-15764
17. Liu R, Rong B, Tu P, et al. Analysis of cytokine levels in tears and clinical correlations after intense pulsed light treating meibomian gland dysfunction. Am J Ophthalmol. 2017;183:81–90. doi:10.1016/j.ajo.2017.08.021
18. Xue AL, Wang MT, Ormonde SE, Craig JP. Randomised double-masked placebo-controlled trial of the cumulative treatment efficacy profile of intense pulsed light therapy for meibomian gland dysfunction. Ocul Surf. 2020;18(2):286–297. doi:10.1016/j.jtos.2020.01.003
19. Ocak SY, Karakus S, Ocak OB, et al. Intense pulse light therapy treatment for refractory dry eye disease due to meibomian gland dysfunction. Int Ophthalmol. 2020;40(5):1–7.
20. Choi M, Han SJ, Ji YW, et al. Meibum expressibility improvement as a therapeutic target of intense pulsed light treatment in meibomian gland dysfunction and its association with tear inflammatory cytokines. Sci Rep. 2019;9(1):1–8.
21. Piyacomn Y, Kasetsuwan N, Reinprayoon U, Satitpitakul V, Tesapirat L. Efficacy and safety of intense pulsed light in patients with meibomian gland dysfunction—a randomized, double-masked, sham-controlled clinical trial. Cornea. 2020;39(3):325–332. doi:10.1097/ICO.0000000000002204
22. Arita R, Fukuoka S, Morishige N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul Surf. 2019;17(1):104–110. doi:10.1016/j.jtos.2018.11.004
23. Zhang-Nunes S, Guo S, Lee D, Chang J, Nguyen A. Safety and efficacy of an augmented intense pulse light protocol for dry eye syndrome and blepharitis. Photobiomodul Photomed Laser Surg. 2020;39(3):178–184.
24. Bitter P. Intense pulsed light: where we are now? Dermatol Rev. 2021;2(2):60–68. doi:10.1002/der2.63
25. Tomlinson A, Bron AJ, Korb DR, et al. The international workshop on meibomian gland dysfunction: report of the diagnosis subcommittee. Invest Ophthalmol Vis Sci. 2011;52(4):2006–2049. doi:10.1167/iovs.10-6997f
26. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–871. doi:10.1001/archderm.1988.01670060015008
27. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Reliability and validity of the ocular surface disease index. Arch Ophthalmol. 2000;118(5):615–621. doi:10.1001/archopht.118.5.615
28. Jackson WB. Management of dysfunctional tear syndrome: a Canadian consensus. Can J Ophthalmol. 2009;44(4):385–394. doi:10.3129/i09-015
29. Hong J, Sun X, Wei A, et al. Assessment of tear film stability in dry eye with a newly developed keratograph. Cornea. 2013;32(5):716–721. doi:10.1097/ICO.0b013e3182714425
30. Alfaro-Juárez A, Caro-Magdaleno M, Montero-Iruzubieta J, et al. Keratograph 5M as a useful and objective tool for evaluating the ocular surface in limbal stem cell deficiency. Clin Ophthalmol. 2019;13:2025. doi:10.2147/OPTH.S218313
31. Yokoi N, Komuro A. Non-invasive methods of assessing the tear film. Exp Eye Res. 2004;78(3):399–407. doi:10.1016/j.exer.2003.09.020
32. Wu S, Hong J, Tian L, Cui X, Sun X, Xu J. Assessment of bulbar redness with a newly developed keratograph. Optom Vis Sci. 2015;92(8):892–899. doi:10.1097/OPX.0000000000000643
33. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–1240. doi:10.1167/iovs.05-0981
34. Nichols JJ, Berntsen DA, Mitchell GL, Nichols KK. An assessment of grading scales for meibography images. Cornea. 2005;24(4):382–388. doi:10.1097/01.ico.0000148291.38076.59
35. Nichols KK, Nichols JJ, Mitchell GL. The lack of association between signs and symptoms in patients with dry eye disease. Cornea. 2004;23(8):762–770. doi:10.1097/01.ico.0000133997.07144.9e
36. De Godoy CHL, da Costa Silva PF, De Araujo DS, et al. Evaluation of effect of low-level laser therapy on adolescents with temporomandibular disorder: study protocol for a randomized controlled trial. Trials. 2013;14(1):1–6.
37. Irvine J, Chong SL, Amirjani N, Chan KM. Double‐blind randomized controlled trial of low‐level laser therapy in carpal tunnel syndrome. Muscle Nerve. 2004;30(2):182–187. doi:10.1002/mus.20095
38. Mathers WD, Shields WJ, Sachdev MS, Petroll WM, Jester JV. Meibomian gland dysfunction in chronic blepharitis. Cornea. 1991;10(4):277–285. doi:10.1097/00003226-199107000-00001
39. Nagymihályi A, Dikstein S, Tiffany JM. The influence of eyelid temperature on the delivery of meibomian oil. Exp Eye Res. 2004;78(3):367–370. doi:10.1016/S0014-4835(03)00197-0
40. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27(10):1142–1147. doi:10.1097/ICO.0b013e3181814cff
41. Stonecipher K, Abell TG, Chotiner B, Chotiner E, Potvin R. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin Ophthalmol. 2019;13:993–999. doi:10.2147/OPTH.S213664
42. Stonecipher K, Komm C, Potvin R, et al. Low level light therapy as an adjunct treatment for meibomian gland dysfunction. Acta Sci Ophthalmol. 2020;3(11):13–18. doi:10.31080/ASOP.2020.03.0177
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.