Back to Journals » Clinical Ophthalmology » Volume 10
Safety and efficacy of a growth factor and cytokine-containing topical product in wound healing and incision scar management after upper eyelid blepharoplasty: a prospective split-face study
Authors Murdock J, Sayed MS, Tavakoli M, Portaliou D, Lee W
Received 30 March 2016
Accepted for publication 19 May 2016
Published 30 June 2016 Volume 2016:10 Pages 1223—1228
DOI https://doi.org/10.2147/OPTH.S109517
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jennifer Murdock,1,* Mohamed S Sayed,1,* Mehdi Tavakoli,1,2 Dimitra M Portaliou,1 Wendy W Lee1
1Bascom Palmer Eye Institute, Department of Ophthalmology, University of Miami Miller School of Medicine, Miami, FL, USA; 2Ophthalmic Research Center, Department of Ophthalmology, Shahid Beheshti University of Medical Sciences, Tehran, Iran
*These authors contributed equally to this work
Purpose: To evaluate the efficacy and safety of a topical product containing a mixture of growth factors and cytokines on the incision scar following upper eyelid blepharoplasty.
Methods: This is a prospective, single-blinded, and split-face study on patients who underwent bilateral upper eyelid blepharoplasty. Two weeks after surgery, one eye of each subject was randomized to receive Lumière Bio-Restorative Eye Cream on one eyelid incision for 12 weeks and no treatment on the other eyelid. Subjects returned at the postoperative weeks 6, 10, and 14. At each visit, patients and the investigator (who was blinded to the treated eyelid) evaluated the scar through specified questionnaires.
Results: A total of 20 subjects with a mean age of 66.3±9.2 years completed the study. Minor side effects were noted in three subjects. At all-time points, all subjects thought eyelids treated with Lumière had a better scar and overall appearance than fellow eyelids (P<0.5); and 60% of patients strongly encouraged others to use the product. The investigator assessment of erythema and pigmentation revealed less erythema and pigmentation in treated eyes at the weeks 6 and 10, although the difference was statistically insignificant. Investigator assessment also revealed a better scar appearance at week 10 in treated eyes (P=0.04). All evaluation parameters were similar in both eyes at the last visit.
Conclusion: Lumière eye cream shows an excellent safety profile and minimal effects on features of the incision scar following upper lid blepharoplasty. It may hasten the wound healing process considering the higher outcomes at the first weeks of application.
Keywords: processed skin cell proteins, PSP®, skin cream, eye cream, eyelid surgery, Lumière
Introduction
Blepharoplasty is one of the most common surgical procedures performed on the face that can not only rejuvenate one’s appearance through excision of excess eyelid tissue but can often provide a functional improvement of the superior visual field. A surgical incision made in the natural lid crease is well disguised and rarely results in a visible scar. However, this incision can take weeks to months to heal, with variable persistence. If left visible, the incision can be unsightly and troubling to patients. Different approaches to creating the incision, such as using a monopolar cautery needle-tip, radiofrequency, and carbon dioxide laser, have been studied without any significant difference in healing or scar appearance.1,2 Moreover, many techniques have been used in attempts to minimize scarring postoperatively, such as steroid creams, injections, and laser resurfacing of the affected area.3,4 Growth factors and cytokines are recognized to have a beneficial effect on wound repair and regulation of the wound healing process and have been shown to play a role in improvement of wrinkle appearance, reversal of photodamage, and skin rejuvenation.4–8 Not often are topical eye creams recommended postoperatively as a means to reduce scar formation. An eye cream has been introduced containing a proprietary mixture of human growth factors and cytokines, known as processed skin cell proteins (PSP®; Neocutis Inc., San Francisco, CA, USA). The mixture is synthesized biotechnologically using cultured human fetal skin cells from a dedicated cell bank.9 This cream, currently marketed as Lumière Bio-Restorative Eye Cream (Neocutis Inc., San Francisco, CA, USA), is proven to be safe and effective for periorbital skin rejuvenation.5 The purpose of this study was to determine the safety and efficacy of Lumière Bio-Restorative Eye Cream in improving the restoration process (ie, scar management) and overall appearance of the upper eyelid incision after upper eyelid blepharoplasty.
Methods
This is a single-center, prospective, randomized, single-blind, and split-face study on 28 consecutive patients who underwent bilateral upper eyelid blepharoplasty. The protocol of the study was approved by the University of Miami Miller School of Medicine Institutional Review Board. The study was conducted in accordance with the provisions of the Declaration of Helsinki and was performed in compliance with the Health Insurance Portability and Accountability Act.
Subjects with the age between 18 and 80 years and good general health who agreed to wear sun protection (ie, hats, sunglasses) when outdoors, and also agreed to use a medically acceptable method of contraception if they were a female of childbearing potential, were eligible for and scheduled by the investigator to undergo bilateral blepharoplasty of the upper eyelids. Subjects were excluded if they were lactating, pregnant, or planning a pregnancy during the course of the study, using any skin care products on the upper eyelids that could interfere with the study within the 2 weeks prior to enrollment (such as topical retinoids, retinols, glycolic acid, hydroquinone eye cream), undergoing treatments, taking medications, or with medical conditions that could interfere with the study results as determined by the investigator, known to be hypersensitive to any of the ingredients in the study product, unwilling or unable to comply with the study visit schedule, have a history of uncontrolled allergies that may affect the periorbital area, or have any ocular infection. Informed consent was obtained from each subject, and preoperative photographs were taken in a standardized method.
On day 0, the patients underwent bilateral upper eyelid blepharoplasty including excessive preaponeurotic fat removal by a single surgeon. The subjects then returned for a routine week 1 postoperative follow-up visit to monitor incision healing, remove sutures, and record any adverse events per current standard of care.
The subjects then returned for a week 2 postoperative follow-up visit, during which each subject was randomized to receive treatment of Lumière Bio-Restorative Eye Cream on one upper eyelid and no treatment on the other upper eyelid. We conducted a split-face study, and the side that the study product was applied to (right or left) was randomized. Each subject received the study product as well as complete study instructions for cleansing the upper eyelid area with Neocutis Neo-Cleanse Gentle Cleanser and application of the study product in the morning and at least 30 minutes prior to bedtime to mitigate the potential for product spreading. Subjects were instructed to apply carefully study product only to the appropriate upper eyelid and not to use any other product on the other eye. Each subject applied the study product twice a day for 12 weeks total, starting 2 weeks after the blepharoplasty. Study products were replenished as needed. The subject was instructed to complete a study product diary that recorded any missed applications during the study.
For the first 2 weeks after starting the study product (week 2 through 4), all subjects completed a diary to assess common effects of the study product on a 4-point scale (none =0, mild =1, moderate =2, and severe =3), including pain, itching, redness, and peeling. Any other effects were also assessed on this scale.
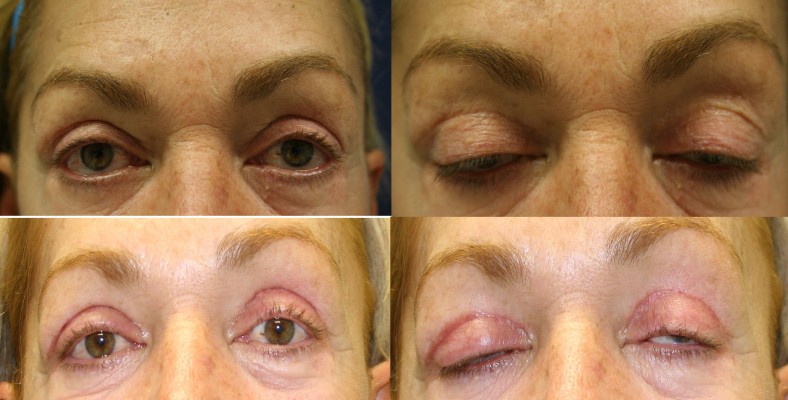
Subjects returned at weeks 6, 10, and 14 (postblepharoplasty). At each visit, standardized photographs were taken, subject and investigator assessments were completed, and subject diaries were reviewed and collected. Study personnel reviewed all adverse events, concomitant medications, and concurrent procedures.
The principal investigator remained blinded to the randomized eyelid receiving the study product and completed a live clinical assessment at each visit to visually and tactilely evaluate three categories in the clinic: scar appearance, the overall appearance of the upper eyelid skin, and direct comparison between a single subject’s eyelids. Scar appearance was assessed for each eye separately on a 6-point scale by erythema (0= no erythema, 1= mild erythema, 2= mild-to-moderate erythema, 3= moderate erythema, 4= moderate-to-severe erythema, and 5= severe erythema), pigmentation (on a graded scale from 0= no pigmentation to 5= marked pigmentation), and scar thickness (on a graded scale from 0= normal thickness to 5= abnormally thickened). The overall appearance of upper eyelid skin was assessed separately (right and left) on a 6-point scale by fine wrinkles (on a graded scale from 0= no wrinkles to 5= severe fine wrinkles) and texture (where 0= a normal texture and 5= a markedly heterogeneous texture). Direct comparison between the right and left sides was used to assess which scar and which eyelid had a better overall appearance (right, left, or same).
Each subject completed a self-assessment with a 4-point scale (0= none, 1= mild, 2= moderate, and 3= severe) to evaluate for pain/soreness, tenderness, itching, swelling, redness, and eye irritation for both the eyelid treated with the study product and the eyelid treated with no product. Subjects also evaluated the appearance of the scar and overall eyelid (scar/eyelid treated with the product, scar/eyelid not treated with the product, or the same). At the study exit visit, after treating one eyelid with the study product for 12 weeks, the subjects were asked if they would recommend the study product to a friend to use after blepharoplasty (1= I would strongly recommend this product, 2= I would recommend this product, 3= I am unsure if I would recommend this product or not, 4= I would never recommend this product, 5= I would strongly recommend against this product). Statistical analysis was performed using SPSS Version 17 (SPSS Inc., Chicago, IL, USA) and a P-value of 0.05 or less was considered statistically significant.
Results
A total of 28 patients were recruited. Eight subjects could not complete the study due to product adverse effects (three patients), and noncompliance or lost follow-up (five patients). A total of 20 participants successfully completed the study. The mean age of the patients was 66.3±9.2 (range of 45–79) years, and 18 patients were female. No subjects missed more than two applications within the study duration.
Adverse events
Three subjects reported adverse events likely related to the study product use that included itching and redness in the periorbital treated area. These symptoms resolved after discontinuation of the study product. Two of these subjects reported ocular irritation when the cream inadvertently entered the eye. All three subjects were withdrawn from the study. No subjects reported severe itching, redness, or skin peeling.
Subject self-assessment
At 2 weeks postoperatively, before the study product was applied, ten out of 20 subjects thought the scar looked better in the eyelid that was randomized to the product (50%), and nine patients believed that this eye overall looked better (45%), while these values increased to 13 eyes (65%) and eleven eyes (55%), respectively at the last follow-up, which meant a statistically significant improvement. Details are summarized in Table 1.
  | Table 1 Subjects self-assessment of the eyelid scar and overall appearance. The patients were asked to choose which eyelid had a better appearance |
Investigator assessment
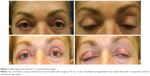
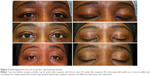
The investigator assessment of scar appearance including erythema and pigmentation of the operated eyelids revealed a marked improvement from grade 3 of erythema and a mild degree of pigmentation in most patients at the second postoperative week to grade 0 at the last visit (Figures 1 and 2). However, the difference between two eyelids was not statistically significant (Tables 2 and 3).
  | Table 2 Grading of erythema in product and control groups |
  | Table 3 Grading of pigmentation in product and control groups |
There was not a significant difference between the two eyelids in terms of the scar and overall eyelid appearance before the study product was started, whereas, at the visit of week 10, the investigator could significantly discriminate between two eyelids in favor of the product-treated eyelids (P=0.04). Again at the last visit, the investigator could not significantly discriminate between the two eyes (P=1.00, Table 4).
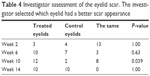  | Table 4 Investigator assessment of the eyelid scar. The investigator selected which eyelid had a better scar appearance |
Two-thirds (66.6%) of the eyelids treated with the study product were graded to have a better scar appearance than the control eyelid at the start of the study and upon study completion. The investigator evaluated no difference in the scar appearance between the two eyelids in 5/13 (38.5%) of eyelids from the start of the study till its completion.
Product recommendation
After completion of the study, 12 out of 20 subjects would strongly recommend the product to a friend undergoing blepharoplasty (60%), five would recommend the product to a friend (25%), and three were not sure if they would recommend the product (15%).
Subject–investigator concordance
Subjects and investigator assessments were concordant 38 out of 80 times (47.5%) regarding scar appearance, while they were concordant 21 out of 80 times regarding overall eyelid appearance.
Discussion
A multitude of patient-related and procedure-related factors contribute to the scar appearance and ultimate aesthetic result of upper eyelid blepharoplasty including skin type, the intensity of wound healing response, surgical instrumentation, the location of incisions, tissue dissection and removal techniques, hemostasis, suture material, and suturing technique.1–4 This is the first randomized, controlled, observer-blinded evaluation study to determine the safety and efficacy of Lumière eye cream in scar management and eyelid appearance following upper eyelid blepharoplasty. The cream product is a mixture comprising 16 growth factors as well as a number of cytokines known as PSP®, which are involved in the regulation of cutaneous wound healing. Other key ingredients include caffeine, hyaluronic acid, and bisabolol.5,9
The role of growth factors and cytokines in the process of wound healing and scar formation is well known.4,10 Bowes et al11 have shown that transforming growth factor-beta 2-treated wounds have a significantly higher degree of collagen fiber orientation, which may affect the eventual scar. In another study, it was demonstrated that placenta-derived mesenchymal stem cells promoted skin wound healing in a mouse model through the release of proangiogenic factors such as vascular endothelial growth factor, increase healing promoting factors such as integrin-β1 and integrin-β3, and alteration of other tissue cytokines.12 However, clinical applications of commercial products containing growth factors and cytokines have been studied only in recent years.5–9,13
The efficacy of the PSP® products on the improvement of facial and periorbital wrinkles has been reported in two earlier reports.5,9 Gold et al9 reported a significantly improved facial wrinkles up to 20% following 2 months of twice-daily Bio-Restorative Skin Cream application. Their evaluations included photographic assessment, investigator assessment, and subjects’ self-assessment. The highest success rate in their study was reported from the periocular area. Lupo et al5, by the same methods to assess the efficacy of the product, reported that clinical signs for wrinkles, lower eyelid bags or sagging, dark circles, and skin texture significantly improved after 6 weeks of twice-daily Lumière eye cream application.
The results of the present study demonstrate that at all time points, the subjects evaluated the eyelids treated with Lumière with a better scar, and overall appearance than eyelids receiving no topical treatment. However, it was true in terms of the investigator assessments only in some points of study. The higher success rate reported by the subjects was also documented in the study by Lupo et al.5 They reported wrinkle improvements of 19%–37% by the subjects’ self-assessment versus 14%–28% improvement based on the investigator assessment. This higher success rate reported by the patients may be subject to bias in our study because the patients (in contrast to the investigator) were aware which eyelid was treated by the product.
A higher percentage (although not statistically significant in most parameters) of eyelids treated with the study product had a better scar appearance and grading of the erythema and pigmentation than the untreated eyelid, as graded by the investigator, at the 6-week and 10-week time points (Tables 2–4). However, these positive effects seem to be temporary, with the vast majority of cases showing improvement or resolution of erythema and pigmentation in both eyes and a more similar appearance by the end of the study. These outcomes show that, although both the eyelids achieved similar appearances 14 weeks following surgery, the treated side seemed to achieve final appearance faster. Some authors believe that high molecular weight compounds including cytokines and growth factors may not simply penetrate a healthy skin, although very low concentrations of these molecules are usually sufficient to reach the receptor sites.9,14 Disrupted skin surface of the surgical wound may facilitate the penetration of the active ingredients and may be responsible for the more effects of the product in the first weeks of application. Taken together, these outcomes suggest that regular topical application of Lumière, although not very strongly, might contribute to a faster resolution of the temporary effects after upper eyelid blepharoplasty such as erythema, pigmentation, and scar appearance.
Lumière eye cream showed to be a safe product in our study. Adverse side effects including itching, redness, and ocular irritation were noted in three subjects and all complaints improved after discontinuation of the product. No serious or permanent side effects were noted in our series. Similarly, in the series by Lupo et al,5 all patients tolerated the eye cream well and no significant side effects were reported.
Study limitations include a subject bias since they were not blinded to the treatment side, the subtlety of scar, and overall eyelid appearances that can be difficult to assess objectively and a relatively low sample size. It is the subtlety of the eyelids and scar appearance that influence patient satisfaction, thus determining the success of aesthetic procedures. The significance of the differences between the treated and control eyelids might be strengthened by a higher number of patients.
In summary, regular topical application of Lumière may be particularly beneficial in hastening the wound healing process and reducing the intensity of the temporary postoperative scar erythema and pigmentation after upper eyelid blepharoplasty, particularly early on in the first few weeks following the procedure. We conclude that Lumière is relatively safe and slightly effective in scar management following upper eyelid blepharoplasty and may lead to hastened recovery. A larger study with more participants and longer follow-up is required to confirm these results.
Acknowledgment
Patient consent was obtained for using patients’ photos.
Disclosure
The authors report no conflicts of interest in this work.
References
Kashkouli MB, Kaghazkanai R, Mirzaie AZ, Hashemi M, Parvaresh MM, Sasanii L. Clinicopathologic comparison of radiofrequency versus scalpel incision for upper blepharoplasty. Ophthal Plast Reconstr Surg. 2008;24(6):450–453. | ||
Niamtu J. Radiowave surgery versus CO laser for upper blepharoplasty incision: which modality produces the most aesthetic incision? Dermatol Surg. 2008;34(7):912–921. | ||
Tian WC. Savior of post-blepharoepicanthoplasty scarring: Novel use of a low-fluence 1064-nm Q-switched Nd:YAG laser. J Cosmet Laser Ther. 2016;28(2):69–27. | ||
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. | ||
Lupo ML, Cohen JL, Rendon MI. Novel eye cream containing a mixture of human growth factors and cytokines for periorbital skin rejuvenation. J Drugs Dermatol. 2007;6(7):725–729. | ||
Ehrlich M, Rao J, Pabby A, Goldman MP. Improvement in the appearance of wrinkles with topical transforming growth factor beta(1) and l-ascorbic acid. Dermatol Surg. 2006;32(5):618–625. | ||
Fitzpatrick RE, Rostan EF. Reversal of photodamage with topical growth factors: a pilot study. J Cosmet Laser Ther. 2003;5(1):25–34. | ||
Hussain M, Phelps R, Goldberg DJ. Clinical, histologic, and ultrastructural changes after use of human growth factor and cytokine skin cream for the treatment of skin rejuvenation. J Cosmet Laser Ther. 2008;10(2):104–109. | ||
Gold MH, Goldman MP, Biron J. Efficacy of novel skin cream containing mixture of human growth factors and cytokines for skin rejuvenation. J Drugs Dermatol. 2007;6(2):197–201. | ||
Vogt PM, Drücke D, Mühlberger T, Homann HH, Steinau HU. Clinical application of growth factors and cytokines in wound healing. Zentralbl Chir. 2000;125(Suppl 1):65–68. | ||
Bowes LE, Jimenez MC, Hiester ED, et al. Collagen fiber orientation as quantified by small angle light scattering in wounds treated with transforming growth factor-beta2 and its neutralizing antibody. Wound Repair Regen. 1999;7(3):179–186. | ||
Abd-Allah SH, El-Shal AS, Shalaby SM, Abd-Elbary E, Mazen NF, Abdel Kader RR. The role of placenta-derived mesenchymal stem cells in healing of induced full-thickness skin wound in a mouse model. IUBMB Life. 2015;67(9):701–709. | ||
Ehrlich HP, Freedman BM. Topical platelet-derived growth factor in patients enhances wound closure in the absence of wound contraction. Cytokines Cell Mol Ther. 2002;7(3):85–90. | ||
Bos JD, Meinardi MM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Exp Dermatol. 2000;9(3):165–169. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.