Back to Journals » Research and Reports in Urology » Volume 12
Sacral Neuromodulation in a Patient with Wolff-Parkinson-White Syndrome: A Case Report
Authors Almutairi S
Received 15 April 2020
Accepted for publication 8 June 2020
Published 17 June 2020 Volume 2020:12 Pages 193—197
DOI https://doi.org/10.2147/RRU.S258403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jan Colli
Sulaiman Almutairi
Department of Urology, College of Medicine, Majmaah University, Al-Majmaah, Saudi Arabia
Correspondence: Sulaiman Almutairi
Department of Urology, College of Medicine, Majmaah University, P.O. Box 11952, Al-Majmaah 11952, Saudi Arabia
Tel +966504897956
Fax +96614321218
Email [email protected]
Abstract: Sacral neuromodulation has gained widespread use for bladder overactivity, frequency, urgency, fecal incontinence, and nonobstructive urinary retention; hence, implantations of this device in patients with comorbid cardiac conduction diseases have increased. Theoretically, there are some concerns regarding the use of sacral neuromodulation implants in patients with Wolf-Parkinson-White syndrome and cardiac conduction diseases because of the risk of interference with electrical impulses. This study aimed to describe the safety of using sacral neuromodulation to treat nonobstructive urinary retention in patients with a cardiac conduction disease. We report a case in which sacral neuromodulation was performed to treat nonobstructive urinary retention in a 25-year-old woman with Wolf-Parkinson-White syndrome who was receiving antiarrhythmic medication. The patient underwent magnetic resonance imaging of the spine and urodynamic studies after presenting with urinary symptoms at a urology clinic. She was then diagnosed with nonobstructive urinary retention. She underwent two-staged InterStim therapy, which involved implanting a permanent tined lead through the S3 foramen in the first stage and an implantable pulse generator in the second stage. The patient responded well to the therapy, and the frequency of clean intermittent catheterization was reduced from 6 times a day to once daily with only 250 mL drained per day. The cardiology team recommended intraoperative cardiac monitoring and postoperative electrocardiogram monitoring. No interference was observed between the implantable pulse generator and the cardiac rhythm on electrocardiography. She experienced no exacerbation of her cardiac symptoms. Sacral neuromodulation in a patient with Wolf-Parkinson-White syndrome appears to have been safe. Further, prospective and randomized studies with larger study samples are required to investigate the safety of these implants in WPW patients.
Keywords: sacral neuromodulation, Wolff-Parkinson-White syndrome, nonobstructive urinary retention, idiopathic urinary retention, cardiac arrhythmia
Introduction
Wolff-Parkinson-White (WPW) syndrome is a cardiac condition in which the atrial impulses reach the ventricle through an accessory pathway in addition to normal atrioventricular conduction. Diagnostic electrocardiogram (ECG) findings of WPW syndrome include a delta wave, a wide QRS complex, and a short PR interval. WPW symptoms typically include palpitations and a history of syncope. WPW can lead to sudden unexpected death. The reported prevalence of WPW varies widely in literature and is estimated to be 0.36/1000 with a peak of 0.61/1000 in ages 20–24 years.1 Treatment options for this condition range from antiarrhythmic medications to ablation of the accessory pathway, or a combination of the two. Treatment goals include symptom control and reducing the risk of sudden death.2
The United States Food and Drug Administration (FDA) approved the use of sacral neuromodulation (SNM) InterStim therapy (Medtronic Inc., Minnesota, USA) for refractory bladder overactivity and urge incontinence in 1997 and for nonobstructive urinary retention in 1999.3
The mechanism of action of SNM in the bladder is poorly understood; however, it modulates the reflex arc of the pelvic floor neuromuscular complex, which controls the voiding function of the bladder, via afferent pathways.3
Because of a theoretical risk of interference with electrical impulses, there are some concerns regarding the use of SNM implants in patients with cardiac pacemakers. To the best of our knowledge, there is no report on the use of SNM in a patient with WPW syndrome. Here, we present a case report detailing the successful use of SNM for nonobstructive urinary retention in a patient with WPW syndrome.
Case Report
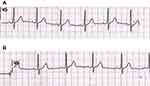
A 25-year-old woman with WPW syndrome (Figure 1) presented to our urology clinic complaining of a nine-year history of nonobstructive urinary retention. Three years prior, she had undergone cardiac ablation, which was unsuccessful, and she was prescribed a 250-mg daily dose of verapamil (Pfizer co, New York, USA) an antiarrhythmic medication to normalize her cardiac rhythm. She had no other medical conditions or family history of urinary retention. She reported multiple visits to the emergency department for urgent decompression by urethral catheterization and having attended multiple urology clinics over the last nine years and undergone multiple diagnostic ultrasounds for the investigation of her urinary retention. The ultrasound images showed an unremarkable upper urinary tract and a smooth bladder with a volume of one liter. Urodynamic studies revealed no measurable detrusor activity. Despite a bladder volume greater than 487 mL, the patient had no urge to void, and her electromyographic study was normal (Figure 2). She was unable to void after the urodynamic study. As a result, she was diagnosed with nonobstructive urinary retention and was prescribed clean intermittent catheterization four times daily. In addition to her nine-year history of nonobstructive urinary retention, the patient had also experienced constipation over the same time period. She had no other past urologic history and no known neurologic disorders.
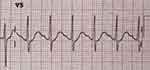 |
Figure 1 The electrocardiogram prior to antiarrhythmic treatment. The electrocardiogram shows a short PR interval, a delta wave, wide QRS complex, and ST depression. |
 |
Figure 2 The urodynamic study. A summary of the urodynamic study showing a postvoid residual volume of 487 mL with a voided volume of only 1.4 mL and almost no measurable detrusor pressure. |
The patient was referred to the author’s clinic for further investigation and treatment. A one-liter postvoid residual volume was drained through a Foley’s catheter (Sterimed Group, India). We attempted a two-week trial of tamsulosin hydrochloride 0.4 mg daily (omnic ocas, SAJA CO, Jeddah SA). However, this was discontinued due to serious side-effects (palpitations and orthostatic hypotension), and the patient was advised to continue with the clean intermittent catheterization every four to six hours. This posed no problems, aside from a few urinary tract infections, which were treated according to the urine cultures and sensitivities with oral antibiotics. For the last nine years, intermittent catheterization had not succeeded in achieving any improvement in symptoms or spontaneous voiding. We ordered an outpatient magnetic resonance imaging (MRI) of the spine and pelvis one week after her initial consult at the author’s clinic, where no neurologic, urinary, or gynecological abnormalities were observed. Based on the surgeon’s preference, we then recommended two-stage SNM as a definitive treatment for her urinary retention.
The patient gave consent for a two-staged SNM procedure. Under local anesthesia, conscious sedation, and fluoroscopic guidance, the first SNM stage was performed using the Medtronic unit InterStim therapy (Medtronic Inc., Minnesota, USA). The patient was placed in the prone position with both feet at the edge of the operating table to evaluate hallux movement with stimulation. After marking the sacral spine, local anesthesia with lidocaine 2% (AstraZeneca Pharmaceuticals India Ltd, India) was administered in the S3 region. The sacral neuromodulation kit was used including spinal needles, an introducer, a tined permanent lead, a portable generator, a disposable wire, and connector (Medtronic Inc., Minnesota, USA). Thereafter, a spinal needle was inserted into the left S3 foramen, and we tested for contractions of the levator ani and movement of the hallux. An introducer then replaced the spinal needle, 2 cm deep to the sacral foramen. Finally, the components of the InterStim (Medtronic Inc., Minnesota, USA) were implanted by feeding a tined permanent lead over the introducer and connecting it to a portable generator using a different incision site, disposable wire, and a connector. The procedure was performed under continuous ECG monitoring. The patient was discharged home with a portable generator the following day.
The patient presented for her first follow-up appointment one week after the first stage of the implantation. At that time, she reported spontaneous voiding four times daily and a reduction in the frequency of catheterization to once daily with a urinary drainage of only 200 mL. The patient tolerated the first stage well, with no reported side-effects; therefore, we planned the second-stage SNM to be implanted ten days later. This consisted of the implantation of an implantable pulse generator (IPG) from InterStim (Medtronic Inc., Minnesota, USA) in the left buttock under sterile technique and local anesthesia. At her six-week follow-up appointment, the patient reported a consistent reduction in the frequency of clean intermittent catheterization from six times to once daily with a urinary drainage of only 250 mL.
We consulted the Cardiology team both pre- and postoperatively. They requested continuous intraoperative cardiac monitoring and a postoperative ECG. The ECG did not show any significant changes, and there was no exacerbation of her cardiac symptoms. Furthermore, the one-month postoperative ECGs, which were performed when the SNM device was on and then when the device was off, showed no changes in her cardiac rhythm (Figure 3). This study was approved by the Institutional Review Board at King Fahad Medical City (IRB Log No. 19-636E) and was performed in accordance with the Declaration of Helsinki. Written informed consent for publication of data was obtained from the patient.
Discussion
Nonobstructive urinary retention is an indication for SNM with InterStim therapy. To the best of our knowledge, this is the first report to discuss a very rare coincidence of SNM implantation for nonobstructive urinary retention in a patient with WPW syndrome. Jonas et al4 reported the efficacy of SNM in 177 patients with refractory nonobstructive urinary retention. Sixty-eight of the 177 patients qualified for SNM implantation based on a percutaneous testing trial. Thirty-seven patients were randomly assigned for SNM administration, while 31 were designated to be the control group, where the implantation of the SNM device was delayed for six months. The authors reported that 69% of the patients in the SNM group had successful spontaneous voiding and were free of intermittent catheterization, and 14% of the participants reported a 50% reduction in bladder residual volume. The postvoid residual volume decreased in 83% of patients in the SNM group, compared to only 9% in the control group, at six months. These results were deemed statistically significant, and the authors concluded that SNM implants should be offered to patients with chronic urinary retention. However, none of the patients in this study had any cardiac conditions (listed as an exclusion criterion), including WPW syndrome.5
Ghazi et al5 studied the safety of SNM in three patients with a cardiac pacemaker. Using the Medtronic unit InterStim (Medtronic Inc., Minnesota, USA), these patients underwent the first trial using percutaneous nerve evaluation (PNE) in the S3 foramen using a temporary lead. All three patients reported a more than 50% improvement in their bladder-overactivity symptoms (mainly urgency and urge incontinence), and a SNM device was implanted in each patient. One of these patients had her SNM implant removed after two months for a non-cardiac reason, while the other two were followed-up for six months, and they maintained their voiding improvement with no cardiac symptoms. Postoperative programming was done under ECG monitoring by a cardiologist. No interference was observed between the InterStim (Medtronic Inc., Minnesota, USA) implantable pulse generator and the cardiac pacemaker. The safety of SNM in the presence of a cardiac pacemaker was reported even when testing with maximum SNM stimulation.
In 2007, Wallace et al6 reported on the safety of implanting SNM implants in three patients with a cardiac pacemaker for urge incontinence and an overactive bladder. The SNM implants were implanted under intraoperative and postoperative cardiac monitoring. All three patients reported a greater than 50% improvement in their voiding symptoms, and there was no evidence of interference from the SNM in these patients.
Conclusion
The efficacy of SNM in the management of nonobstructive urinary retention is well established; however, the safety of SNM in patients with WPW syndrome on antiarrhythmic medication has not been previously investigated in the literature. To the best of our knowledge, this is the first case report in the literature to explore this. The limitation of this study is that it is a case report on a single patient; hence, further prospective and randomized studies with larger study samples on the safety of these implants in WPW patients are highly recommended.
Abbreviations
WPW, Wolff-Parkinson-White; ECG, electrocardiogram; FDA, Food and Drug Administration; SNM, sacral neuromodulation; MRI, magnetic resonance imaging; IPG, implantable pulse generator; PNE, percutaneous nerve evaluation.
Acknowledgments
The author would like to thank Editage for English language editing.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Lu C-W, Wu M-H, Chen H-C, Kao F-Y, Huang S-K. Epidemiological profile of Wolff–Parkinson–White syndrome in a general population younger than 50years of age in an era of radiofrequency catheter ablation. Int J Cardiol. 2014;174(3):530–534. doi:10.1016/j.ijcard.2014.04.134
2. Blomström-Lundqvist C, Scheinman MM, Aliot EM, et al. ACC/AHA/ESC guidelines for the management of patients with supraventricular arrhythmias—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients with Supraventricular Arrhythmias) developed in collaboration with NASPE-Heart Rhythm Society. J Am Coll Cardiol. 2003;42:1493–1531. doi:10.1016/j.jacc.2003.08.013
3. Siegel SW, Moeller SE. Sacral neuromodulation for the treatment of overactive bladder (OAB). In: Raz S, Rodriquez L, editors. Female Urology. Philadelphia: WB Saunders Company; 2008:266–276.
4. Ghazi AA, Elterman DS, Hassouna M. Sacral neuromodulation in patients with a cardiac pacemaker. Int Neurourol J. 2016;20:270–272. doi:10.5213/inj.1632536.268
5. Jonas U, Fowler CJ, Chancellor MB, et al. Efficacy of sacral nerve stimulation for urinary retention: results 18 months after implantation. J Urol. 2001;165:15–19. doi:10.1097/00005392-200101000-00004
6. Wallace PA, Lane FL, Noblett KL. Sacral nerve neuromodulation in patients with cardiac pacemakers. Am J Obstet Gynecol. 2007;197:94–e13. doi:10.1016/j.ajog.2007.04.017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.