Back to Journals » Clinical Pharmacology: Advances and Applications » Volume 13
Rupatadine Oral Solution Titration by Body Weight in Paediatric Patients Suffering from Allergic Rhinitis: A Population Pharmacokinetic Study
Authors Santamaria E, Izquierdo I , Valle M
Received 26 March 2021
Accepted for publication 7 May 2021
Published 8 June 2021 Volume 2021:13 Pages 115—122
DOI https://doi.org/10.2147/CPAA.S312911
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Arthur E. Frankel
Eva Santamaria,1,2 Iñaki Izquierdo,2 Marta Valle1,3
1Departament de Farmacologia, de Terapèutica i de Toxicologia, Universitat Autònoma de Barcelona, Barcelona, Spain; 2Clinical Development, Porfolio & Strategy, Biorhom SL Grupo Uriach, Barcelona, Spain; 3Pharmacokinetic/Pharmacodynamic Modeling and Simulation. Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau-IIB Sant Pau, Barcelona, Spain
Correspondence: Iñaki Izquierdo
Manager of Clinical Development & Medical Advise; Porfolio & Strategy Area, Biorhom, S.L. (Grupo Uriach) Polígon Ind. Riera de Caldes, Avinguda Camí Reial, 51-57, Palau-solità i Plegamans, Barcelona, 08184, Spain
Email [email protected]
Background: Allergic rhinitis (AR) and chronic urticaria, both are treated in children with doses of second generation of antihistamines that have been mostly based on extrapolation of data obtained in adults. The objectives of this work were to develop a model to explain the pharmacokinetics (PK) of rupatadine, a second generation antihistamine, administered to children 2− 11 years old and to calculate the non-compartmental PK parameters for two groups of age (2– 5 and 6– 11 years old) based on the individual Bayesian estimates from the selected model.
Methods: Data from two PK studies with rupatadine oral solution (1 mg/mL) were pooled: Study A, an extensive blood sampling study performed in 11 children (6– 11 years old) who received a single oral dose of rupatadine; and Study B, a sparse blood sampling study in 40 children (2– 5 years old) receiving multiple oral doses. A simultaneous population PK model was developed using data available for all children. Using individual Bayesian estimates from the selected model, steady-state plasma concentrations for both studies were simulated and the non-parametric PK parameters were calculated for two age groups: 2– 5 years (subgroup I) and 6– 11 years (subgroup II).
Results: A two-compartment model with first-order absorption and elimination with clearance depending on body weight, better described the PK of rupatadine for 2– 11 year old children. The plasma clearance dependence on weight was linear. The mean (SD) non-compartment PK parameters calculated using simulated plasma profiles at steady state were: Cmax, 2.54 (1.26) vs 1.96 (0.52) ng/mL; AUC0-24h, 10.74 (3.09) vs 10.38 (4.31) ng/mL/h; and t1/2, 12.28 (3.09) vs 15.94 (4.09) h, for children 6– 11 and 2– 5 years old, respectively.
Conclusions: The PK of rupatadine depends on the weight of paediatric patients but not on their age. The dosage strategy adjusted by body weight in children 2– 11 years old (2.5 mL if weight 10– 25 kg, and 5 mL if ≥ 25 kg) provides similar exposure between the two groups of age, and to that obtained in adults with the 10 mg dose tablet formulation.
Keywords: rupatadine, children, population pharmacokinetics
Introduction
Allergic rhinitis (AR) is an important and common condition that causes major morbidity in children and is a risk factor for the development of asthma. The successful treatment of AR in the pediatric population is essential to decrease the burden of this disease.1 Chronic spontaneous urticaria (CSU) is not frequent in children, and it is defined as the appearance of wheals on a recurrent basis, over 6 weeks. Unfortunately, well designed randomized controlled trials (RCTs) for children are lacking as suggested in a recent review.2 Second generation H1 antihistamines (SgH1) are effective and safe, and easy to administer in children and adolescents.
Rupatadine is an inverse agonist H1 receptor and PAF antagonist, approved by EMA, Health Canada, Japan and China regulatory agencies for the treatment of AR and CSU for adults and adolescents at the dose 10 mg tablets.3 Furthermore, rupatadine oral solution was authorized by the EMA, Health Canada and Israel agencies, more recently for children over 2 years at the same clinical indications.4–6
The pharmacokinetics (PK) of many paediatric drugs, including the first and second generation antihistamines used for the treatment of allergic diseases, has not been thoroughly investigated. Although dosage corrections based on weight are commonly performed for children, it should be borne in mind that a child is not a downscaled model of an adult, and that human development is not a linear process. During growth, there are changes in the composition of the body, as well as in the functional development of organs.7 PK data can support the drug development of paediatric formulations, and are particularly useful to decide dose adjustments during the process. To avoid frequent sampling in children, the US Food and Drug Administration (FDA) and European Medicines Agency (EMA) recommend using a population pharmacokinetic (popPK) modelling and simulation approach during drug development.8,9
In adults and adolescents, the model that best described the PK data of rupatadine tablets was an open two-compartment model with first-order absorption and elimination.10
A single published study described the PK in children between 6 and 11 years with an oral solution (1 mg/mL) of rupatadine specifically developed for this population.11 In the study, clearance was found to be related to body weight and PK parameters estimated for children within this age range, were very similar to those reported for adults taking 10 mg tablets, except for the volume of distribution.
In a second PK study,12 in 2–5 year-old children, we optimised the estimation of the PK parameters by using a popPK model with sparse sampling that minimised children’s discomfort during the study based on the hypothesis that there are no differences in the PK of rupatadine by age.
The aim of the present work was to describe the PK of rupatadine in paediatric patients overall, combining data from previous clinical trials (6–11 and 2–5 years) and to study whether the proposed doses are enough to assure a similar exposure in paediatrics than in adults. Finally, new non-compartmental PK parameters for each age subgroup are calculated and compared them with those in adults.
Methods
Population
We analysed data from two different clinical studies:
Study A11 was an open-label, single-centre study to evaluate the PK of an oral solution (1 mg/mL) of rupatadine. Eleven paediatric patients aged 6 to 11 yr with previous history of seasonal AR, who had to be symptomatic with a baseline of 5 symptoms score: T5SS (nasal congestion, sneezing, rhinorrhea, itchy nose, mouth, throat and/or ears and itchy, watery and red eyes score) ≥6 during each of the last 2 days before inclusion, were treated with an oral solution of rupatadine (1mg/mL) adjusted by body weight, over a period of 4 weeks in an open label design study.
Study B12 was a 28-day open-label, multicentre study in which children 2–5 years old weighing ≥10 kg were included and administered the same oral solution. Forty-four children were enrolled in this study, had to have a history of mild-moderate AR, defined as either intermittent or persistent according to ARIA guideline.13 Children had to be symptomatic with a baseline of 5 symptoms score: T5SS (nasal congestion, sneezing, rhinorrhea, itchy nose, mouth, throat and/or ears and itchy, watery and red eyes score) ≥6 during each of the last 2 days before inclusion, and allergen skin prick test positive wheal of 3 mm greater than the diluent control, or a positive (class 3 of positivity; ≥ 3.5–17.5 kU/L) on ImmunoCAP® test.
Further details of inclusion and exclusion criteria are described in previous PK studies referred.11,12
Both studies were approved by the corresponding Clinical Research Ethics Committee and Health authorities and parent/guardians provided signed consent for children to participate in the study. Study A, conducted in Australia, was approved by the Ethics Committee of the Royal Children’s Hospital and Murdoch Children’s Research Institute (Melbourne), and the Peninsula Private Hospital and Clinical Research Centre (Rivercity, in addition to the Department of Health and Ageing, Therapeutic Goods Administration of the Australian government [Study number 2006/593]). Study B was approved by the Ethics Committee for Clinical Pharmacology for Hungarian sites, the Pharma Ethics Ltd. Committee for South African sites and the pertinent Health authorities. Both studies were performed in compliance with the Declaration of Helsinki and were a part of the completed Paediatric Investigation Plan (EMEA-000582- PIP01-09).
Study Design, Dosing and Blood Sampling
Study A: Briefly, a complete concentration-time profile was obtained for each child after oral administration of rupatadine 1 mg/mL solution. In this study, 2.5 mL of oral rupatadine solution (1 mg/mL) were administered to children weighing between 10–25 kg, and 5 mL to those weighing ≥25 kg. A total of 8 blood samples were drawn at the following times: 30 min predose and 1, 2, 4, 8, 12 and 24 hours postdose (see Table 1).
 |
Table 1 Study Design: Sampling Times |
Study B: Data included in this analysis were part of a larger study.12 The design and sampling were based on the optimal design obtained in our previous study.11 Children received 2.5 mL of oral rupatadine solution (1 mg/mL) once daily for 28 days when weighed <25 kg and 5 mL when ≥25 kg. Forty-four children were randomized to one of the four sampling groups (approximately 10 children allocated to each group) described in Table 1.
Analytical Assay of Rupatadine
In both studies, rupatadine concentrations in plasma were determined as previously described.10 Blood samples (4 mL) were collected in lithium-heparin tubes at each time point. The samples were then centrifuged at 3000 rpm for 10 min at –4°C. Supernatant plasma was then separated in 2 aliquots of 1 mL each and frozen at –20°C until analysis. Plasma levels of rupatadine were measured using a validated liquid chromatography–mass spectrometry (LC/MS) analytical method. The linear range of the assays was between 0.1 and 10 mg/L, 0.1 mg/L being the lower limit of quantification. The within- and between-run precision error was lower than 12.71%. Accuracy-related errors were within ±12.83% for this rupatadine-selective method.
Population PK Model
One- and two-compartment disposition models with first-order absorption were fitted to the data with the first-order conditional estimation (FOCE) method using the NONMEM software (version 7.3)14 with a Fortran compiler (version 6).
Models were parameterised in terms of volume of distribution of central compartment (Vc/F); volume of distribution of the peripheral compartment (Vp/F); total body clearance (CL/F); intercompartmental clearance (CLd/F); first-order absorption rate constant (ka); and absorption lag time (Tlag).
Interindividual variability was estimated assuming a proportional variance model, with exponential errors following a log-normal distribution, as given by the following equation:
where 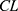 i is the true PK parameter of the i th individual,
i is the true PK parameter of the i th individual, 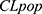 the population value for the typical individual,
the population value for the typical individual, 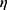 i is the interpatient random effect with a mean of 0 and variance
i is the interpatient random effect with a mean of 0 and variance 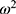 .
.
To quantify the residual variability of the model additive, proportional or combined (residual + proportional) error models were evaluated. Although patients were recruited at different centres, the same analytical method was applied to all samples since they were determined in the same laboratory with identical procedures. We investigated whether the following covariates correlated with any of the above described parameters: age, gender, weight, height and body mass index (BMI). Age was evaluated as continuous and categorical covariate according to the following groups: group I (2–5 years) and group II (6–11 years). As the number of available covariates was low, the evaluation of the potentially influential covariates was explored graphically first, and those showing a possible relationship with one or more parameters were tested for significance in NONMEM, unless the covariates were correlated between them. In such a case, the covariate with the more possible clinical application was selected for further testing with NONMEM. A forward-inclusion and backwards-elimination approach was planned for selecting the covariates of the model, although during the procedure we only used the forward-inclusion step. Covariate inclusion was based on the precision of parameter estimates, goodness-of-fit plots, and the minimum value of the objective function (OF) provided by NONMEM. A covariate was introduced if it decreased the OF by at least 3.84 points (P < 0.05).
A model was considered superior to other nested models on the basis of the following aspects: the OF value was reduced by at least 3.84 points (P < 0.05, one degree of freedom; approximate χ2 distribution), an estimation error of the parameters was lower, goodness-of-fit of graphical representations, the convergence of the model, and the covariance matrix.
Model Evaluation
The final model was evaluated using Monte Carlo simulations. A thousand individual concentration-time profiles of rupatadine were simulated after the administration of single daily doses of 2.5 mg or 5 mg for 28 days to test if the final model adequately described the observations. The proportion of individuals receiving each dose corresponded to that observed in the analysis dataset. Monte Carlo simulations were generated using fixed and random population estimates obtained from the final selected model. The mean profile and the intervals including 90% of the simulated concentrations were plotted together with the raw data. The agreement between simulations and observations was assessed visually.
PK Parameters Calculation
We obtained complete plasma concentration profiles of rupatadine for 24 hours using individual Bayesian estimated parameters. Sampling times were those used in the study with children 6–11 years old (study A), so that we could compare the Area Under the Curve (AUC0-24) between predicted and observed concentrations. The following PK parameters were calculated for each child after a single oral dose of rupatadine: maximum concentration (Cmax), time of maximum concentration (Tmax), the AUC0–24 calculated with the log-linear trapezoidal rule, and the half-life (t1/2). These parameters were based on simulated concentrations and calculated using a non-compartmental approach as implemented in the WinNONLIN software (Professional Edition, Version 2.1, Scientific Consulting, Pharsight, Mountain View, CA, USA).
Results
Data from 11 subjects (73 samples) from Study A were analysed. In study B, 44 children were initially recruited, but data from 40 were analysed, because four children did not have samples with concentrations above the limit of quantification. During the data analysis, one individual was excluded because data on the time of the last dose prior to blood extraction was missing. In summary, 120 samples were available for this study and 193 plasma concentrations of rupatadine were used for the data analysis. The demographics of the patients included in the data analysis are summarized in Table 2.
 |
Table 2 Characteristics of the Patients Included in the Analysis |
Population Pharmacokinetic Model
The structural model that best fitted to the data was a two-compartment model with elimination from the central compartment. The absorption phase was described by a first-order function associated with the Tlag. During model development, data from one patient was excluded because the time of the last dose was missing, and the model did not reach convergence. Data showed high interindividual variability, but only supported the estimation of interindividual variability in clearance (%) and central volume of distribution (%). The addition of interindividual variability in the absorption phase decreased the OF value but increased the estimation errors and the shrinkage of the random parameters. Residual variability was explained using an additive error model. No differences between the study populations (Study A vs Study B) were found for any of the fixed parameters. An allometric scaling model was tested; however, the fit was better when weight was included using a linear function. This last model decreased 15 points the OF value and reduced the estimation error in all the parameters except for the Tlag. None of the other available covariates (age, gender, height and BMI) showed a statistical correlation with the parameters. Table 3 lists the population estimates of the final selected model.
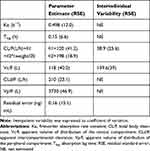 |
Table 3 Population Parameter Estimates from the Final Model |
Model Evaluation
The prediction potential of the final selected model was evaluated using Monte Carlo simulations. The 90% prediction interval of the 1000 simulated profiles comprised most empirical observations from the whole dataset of, and slightly over predicted the interindividual variability in the final part of the graph (Figure 1). Therefore, we considered that the model accurately predicted plasma concentrations in paediatric patients.
Non-Compartmental PK Parameters
Using individual Bayes estimates of the parameters, a full profile was simulated for each participant included in the data analysis. We obtained the parameters for each dosing schedule: 2.5 mL or 5 mL of rupatadine oral solution (1 mg/mL) for children weighing 10 – ≤25 or 25 kg, respectively. The median Tmax was 1 and 0.5 h, mean Cmax (SD) was 1.96 (0.52) and 2.54 (1.26) ng/mL, AUC0-24 was 10.38 (4.31) and 10.74 (3.09) ng/mL/h, and mean t1/2 was 15.94 (4.09) and 12.28 (3.09) h for children 2–5 and 6–11 years old, respectively.
Discussion
Data from Studies A and B resulted in a complete PK study of rupatadine in a paediatric population by using a popPK approach. This allowed us to propose dose recommendations for children between 2 and 11 years, considering a combination of two different experimental conditions (sparse and full blood sampling).
In Study A, 11 children 6–11 years were enrolled and a limited population PK model was developed that enabled the optimisation of the design in a subsequent PK study (Study B) in younger children, including a sparse sampling strategy as recommended by several authors.15 Therefore, in the popPK model presented in this work we have included overall data from 51 patients aged between 2 and 11 years. The developed model was structurally identical to that previously described for older children.11
This article supports the finding that the PK of rupatadine does not depend on age, at least from 2 years old to adults.10,11 The PK of rupatadine followed a two-compartment model, as previously observed in older children.11 The inclusion of 40 new children in the current analysis reinforces the consistency of our results. The only covariate that influenced the PK of rupatadine was body weight. When weight was considered in the model, our simulations predicted a comparable exposure in children 2–5 and 6–11 years old or in adolescents and adults.8,10 Paediatric PK studies usually cover a broader range of weights and heights than those performed in adults. As the physiological hepatic elimination of drugs depends on body size, weight, BMI or body area are expected covariates in the kinetic model in paediatrics.16 This result confirms the adequacy of adjusting the dosage of rupatadine by weight, as observed with other antihistamines.
The effect of body weight on the PK of drugs in paediatric patients has been assessed using either allometric17 or empirical models.18 Although both present advantages, the main strength of empirical size adjustments for PK parameters using body weight (especially if they have a simple structure), is their easy translation into body weight based dosage recommendations that are more familiar to clinicians, such as milligrams per kilogram of body weight.16 In our case, the incorporation of body weight in the model (modelled as a linear relationship between clearance and weight) allowed us to recommend two different doses for children older than 2 years depending on their weight (< 25 kg or ≥25 kg).
The mean parameters estimated by a non-compartmental analysis showed no differences in AUC0-24, Cmax or tmax at steady-state in paediatric subjects between 2 and 11 years of age based on the simulated results. With respect to adults, rupatadine elimination half-life (t1/2) seemed slightly longer in children than in adults;19 however, this difference does not likely have a clinical impact on the response (efficacy or safety) to rupatadine and is probably due to the calculation of the t1/2 in adults based on shorter sampling observations and not on predictions up to 24 hours. In fact, safety and efficacy results for both groups of age (2–5 and 6–11 years old) with the proposed dosing schedule were similar to those in the adult population.3
A deeper analysis of the estimated parameters revealed that the volume of distribution was half for a child than for an adult.20 However, when the volume of distribution is normalised by weight (kg), we found no differences between adults and children; and similar results have been previously reported for desloratadine, epinastine or levocetirizine.21–23
Drug clearance by CYP3A4, the enzyme that metabolizes rupatadine, is considered age-dependent. It is not surprising that clearance did not depend on age in our study, since a recent evaluation of the ontogeny function of CYP3A4 found that the maturation takes places before 2 years of age.24 Finally, since the active metabolites of rupatadine, desloratadine and its hydroxylated dihydroxy-desloratadine hydrochloride, maintain a similar exposure ratio in children and adults (data on file Uriach), the concentrations of these metabolites were not included (although determined) in the development of the model for the sake of simplicity.
As limitation of study, we could not estimate the interindividual variability of Ka, nor that of lag time, although data showed high interindividual variability in the absorption phase. As parameter estimation is highly dependent on sampling times, it is likely that the previous model developed with only 11 children did not have sufficient information to estimate the interindividual variability associated with the absorption phase. However, this cannot yet be clarified due to the lack of PK studies other than the one published by Peña et al in 2008, using an adult population.10 Nonetheless, the absorption rate constant in children was similar to that reported for adults, despite the fact that adults received tablets and children oral solution. With our model, we have demonstrated that the optimal design of sampling can be effective in obtaining sufficient information for studying the PK of a drug without compromising the well-being of children.
In conclusion, this study shows that paediatric drug development through popPK modelling can be useful to comply with the Ethical Considerations for Clinical Trials performed in children since it enables a simplified study design that prioritises the children’s well-being and comfort. This is achieved by the careful selection of more informative sampling times while allowing reasonably accurate estimates of the drug PK. This study further supports the initial dose schedule recommended for rupatadine based on weight (2.5 mg in children weighing < 25 kg, and 5 mg in children weighing ≥25 kg) in order to attain similar plasma concentrations than in adults and adolescents, thereby obtaining a similar pattern of efficacy and safety margins.
Acknowledgments
We would like to thank Dr. Noel Cranswick and Prof Paul Potter, who were involved as clinical investigator and coordinator in Studies A and B, respectively. We would also like to thank all the patients who participated in the studies and the staff of the hospitals where the patients were treated.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Funding
Marta Valle was supported by a grant (FIS CP04/00121) from the Spanish Health Ministry in collaboration with Institut de Recerca de l’Hospital de la Santa Creu i Sant Pau, Barcelona; she is a member of CIBERSAM (funded by the Spanish Health Ministry, Instituto de Salud Carlos III).
Disclosure
ES and II were full-time employees at Grupo Uriach at the time the clinical trials were performed. ES is currently affiliated with Novella Clinical Ltd and Marta Valle is currently the scientific director of Clinical Pharmacology, Modeling and Simulation of Parexel International SL. The authors report no other conflicts of interest in this work.
References
1. Barr JG, Al-Reefy H, Fox AT, Hopkins C. Allergic rhinitis in children. BMJ. 2014;349:g4153. doi:10.1136/bmj.g4153.
2. Cornillier H, Giraudeau B, Munck S, et al. Chronic spontaneous urticaria in children - a systematic review on interventions and comorbidities. Pediatr Allergy Immunol. 2018;29(3):303–310. doi:10.1111/pai.12870
3. Mullol J, Bousquet J, Bachert C, et al. Update on rupatadine in the management of allergic disorders. Allergy. 2015;70(Suppl 100):1–24. doi:10.1111/all.12531
4. Summary of product characteristics (SPC). Rupatadine oral solution (1 mg/mL). Available from: https://www.medicines.org.uk/emc/product/9888/smpc#gref. Accessed April 2021.
5. Potter P, Maspero JF, Vermeulen J, et al. Rupatadine oral solution in children with persistent allergic rhinitis: a randomized, double-blind, placebo-controlled study. Pediatr Allergy Immunol. 2013;24(2):144–150. doi:10.1111/pai.12036
6. Potter P, Mitha E, Barkai L, et al. Rupatadine is effective in the treatment of chronic spontaneous urticaria in children aged 2-11 years. Pediatr Allergy Immunol. 2016;27(1):55–61. doi:10.1111/pai.12460
7. Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology - drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi:10.1056/NEJMra035092
8. European Medicines Agency. EMEA/CHMP/EWP/147013/2004. Guideline on the role of pharmacokinetic in the development of medicinal products in the paediatric population. 2006. Available at: https://www.ema.europa.eu/documents/scientific-guideline/guideline-role-pharmacokinetics-development-medicinal-products-paediatric-population_en.pdf.
9. FDA. E11 and its addendum E11(R1). Clinical Investigation of Medicinal Products in the Pediatric Population. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e11-clinical-investigation-medicinal-products-pediatric-population.
10. Peña J, Carbo ML, Solans A, Nadal T, Izquierdo I, Merlos M. Antihistaminic effects of rupatadine and PKPD modelling. Eur J Drug Metab Pharmacokinet. 2008;33(2):107–116. doi:10.1007/BF03191027
11. Santamaría E, Estévez JA, Riba J, Izquierdo I, Valle M. Population pharmacokinetic modelling of rupatadine solution in 6–11 year olds and optimisation of the experimental design in younger children. PLoS One. 2017;12(4):e0176091. doi:10.1371/journal.pone.0176091.
12. Santamaría E, Izquierdo I, Valle M, Vermeulen J, Potter P. Rupatadine oral solution for 2-5-year-old children with allergic rhinitis: a safety, open-label, prospective study. J Asthma Allergy. 2018;11:225–231. doi:10.2147/JAA.S164632
13. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A. et alAllergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen. Allergy. 2008;63(Suppl 86):8–160.
14. Beal SL, Sheiner LB. NONMEM Users Guides. Maryland,USA: Icon Development Solutions, Ellicott City; 1989:98.
15. Mentré F, Dubruc C, Thenot J-P. Population pharmacokinetic analysis and optimization of the experimental design for mizolastine solution in children. J Pharmacokinetics Pharmacodynamics. 2001;28(3):299–319. doi:10.1023/A:1011583210549
16. Meibohm B, Laer S, Panetta JC, Barrett JS. Population pharmacokinetic studies in pediatrics: issues in design and analysis. AAPS J. 2005;7(2):E475–87. doi:10.1208/aapsj070248
17. de Kock M, Tarning J, Workman L, et al. Population pharmacokinetic properties of sulfadoxine and pyrimethamine: a pooled analysis to inform optimal dosing in african children with uncomplicated malaria. Antimicrob Agents Chemother. 2018;62(5):e01370–17. doi:10.1128/AAC.01370-17.
18. Hussein Z, Pitsiu M, Majid O, et al. Retrospective population pharmacokinetics of levocetirizine in atopic children receiving cetirizine: the ETAC study. Br J Clin Pharmacol. 2005;59(1):28–37. doi:10.1111/j.1365-2125.2005.02242.x
19. Solans A, Carbó ML, Peña J, Nadal T, Izquierdo I, Merlos M. Influence of food on the oral bioavailability of rupatadine tablets in healthy volunteers: a single-dose, randomized, open-label, two-way crossover study. Clin Ther. 2007;29(5):900–908. doi:10.1016/j.clinthera.2007.05.004
20. Keam SJ, Plosker GL. Rupatadine: a review of its use in the management of allergic disorders. Drugs. 2007;67(3):457–474. doi:10.2165/00003495-200767030-00008
21. Simons FER, Simons KJ. Levocetirizine: pharmacokinetics and pharmacodynamics in children age 6 to 11 years. J Allergy Clin Inmunol. 2005;116(2):355–361. doi:10.1016/j.jaci.2005.04.010
22. Sarashina A, Tatami S, Yamamura N, Tsuda Y, Igarashi T. Population pharmacokinetics of epinastine, a histamine H1 receptor antagonist, in adults and children. Br J Clin Pharmacol. 2005;59(1):43–53. doi:10.1111/j.1365-2125.2005.2250
23. Gupta S, Khalilieh S, Katensaria B, Banfield C. Pharmacokinetics of desloratadine in children between 2 and 11 years of age. Br J Clin Pharmacol. 2006;63(5):534–540. doi:10.1111/j.1365-2125.2006.02810.x
24. Salem F, Johnson TN, Abduljalil K, Tucker GT, Rostami-Hodjegan A, Re-evaluation A. Validation of Ontogeny Functions for Cytochrome P450 1A2 and 3A4 Based on In Vivo Data. Clin Pharmacokinet. 2014;53:625–636. doi:10.1007/s40262-014-0140-7
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.