Back to Journals » Clinical Ophthalmology » Volume 13
Rotational stability of a new multicomponent intraocular lens
Authors Uy HS , Tesone-Coelho C
Received 7 May 2019
Accepted for publication 22 August 2019
Published 26 September 2019 Volume 2019:13 Pages 1897—1907
DOI https://doi.org/10.2147/OPTH.S214835
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Harvey S Uy,1,2 Carolina Tesone-Coelho3
1Cataract and Refractive Service, Peregrine Eye and Laser Institute, Makati, Philippines; 2Department of Ophthalmology and Visual Sciences, University of the Philippines, Manila, Philippines; 3Department of Research & Development, InfiniteVision Optics, Strasbourg, France
Correspondence: Harvey S Uy
Cataract and Refractive Service, Peregrine Eye and Laser Institute, 50 Jupiter St., Makati, Metro Manila 1209, Philippines
Tel +63 2 890 0115
Fax +63 2 511 8505
Email [email protected]
Purpose: To evaluate the rotational stability of the Precisight multicomponent intraocular lens (MCIOL) following primary implantation and after enhancement procedures.
Patients and methods: Prospective, single-center study of eyes that underwent routine cataract surgery with implantation of a non-toric MCIOL, (Precisight, InfiniteVision, Optics, Strasbourg, France). The axis of the MCIOL was measured with a line bisecting the two dialing holes in the front lens. Intraoperative orientation was determined using a digital surgical guidance system while the postoperative orientation was determined using slit-lamp imaging. Two populations were analyzed: eyes that only underwent cataract surgery (PRIM) and eyes that also underwent enhancement (ENH), consisting of surgical front optic exchange. Both populations had 3 observation visits: first implantation (P-Op); 3 months (3mo) and 6 months (6mo) after primary surgery. The ENH group had an additional fourth visit that corresponded to the enhancement surgery (E-Op). The main outcome measure was mean absolute change in MCIOL orientation (degrees). The effects of axial length (AL) and anterior chamber depth (ACD) on IOL rotational stability were examined.
Results: Thirty-three eyes received MCIOL of which 29 had usable orientation images. Of these, 12 were in the PRIM group and 17 underwent ENH. Regarding the mean absolute rotation, among PRIM eyes, P-Op to 3mo was 3.03±2.45 degrees; P-Op to 6mo, 2.28±1.54 degrees; and 3–6mo, 2.37±1.56 degrees. Among the ENH eyes, P-Op to 3mo was 3.09±1.68; E-Op to 6mo, 2.71±3.30 and P-Op to 6mo, 3.62±3.42. There were no significant differences in the IOL rotation. There were no statistical differences in rotational stability between the ENH and PRIM groups. There was no correlation between IOL rotation and AL or ACD.
Conclusion: Precisight appears to be rotationally stable. The enhancement procedure does not affect rotational stability.
Keywords: rotational stability, cataract, piggyback lens, multicomponent intraocular lens, intraocular lens exchange
Introduction
Premium cataract surgery is considered a refractive procedure since an increasing number of patients expect to achieve spectacle independence after the removal of the crystalline lens. A variety of intraocular lenses (IOL) are available for the correction of preoperative myopia, hyperopia, astigmatism and presbyopia. In addition, surgical techniques such as corneal and limbal relaxing incisions (CRI, LRI) can reduce low to moderate corneal astigmatism by altering the corneal curvature.1–3 Although relaxing incisions are safe and easy to perform, peer-reviewed literature has shown that they are limited in terms of the magnitude of correctable astigmatism, long-term stability and consistent predictability of outcomes.1–5
Toric IOL’s (TIOL) are used widely in cataract surgery for the correction of pre-operative astigmatism and demonstrate more stable and predictable postoperative refractive results, as well as provide a wider range of correction.6 To maximize TIOL efficacy for astigmatism reduction, it is critical to align the TIOL cylindrical power with the steep corneal meridian. It has been shown that every degree of off-axis orientation results in a loss of 3.3% of the IOL cylindrical power. If the IOL rotates 30 degrees, the cylindrical correction is completely lost.4 TIOL rotational stability is then a critical property that determines long-term success of astigmatism correction and visual function. IOL rotation may be observed as early as 1 hr after surgery. The majority of rotations are observed within the first 10 days following surgery. There are several causes of early postoperative IOL rotation such as incomplete ophthalmic viscosurgical device (OVD) removal or insufficient haptic force on the capsular bag. Late postoperative rotation is influenced by the interaction of the IOL architecture and its interaction with the capsular bag.6–8 An increased incidence of rotation occurs in cases in which the IOL is implanted in the vertical orientation (with-the-rule astigmatism).9
Small amounts of astigmatism and spherical correction may also be performed after intraocular surgery. For combined surgeries, such as trabeculectomy or penetrating keratoplasty (PKP), a significant, unpredictable amount of post-operative spherical and cylindrical errors is expected.10–13 The surgeon may implant an initial non-toric, monofocal IOL to correct the bulk of the refractive error and then utilize a subsequent procedure such as a secondary sulcus-supported IOL, also known as supplementary or piggyback IOL, to correct the residual refractive error.11 Recent piggyback IOL models are capable of correcting both spherical and cylindrical refractive errors. While piggyback IOLs demonstrate generally good postoperative rotational stability, compared to bag-fixated IOL’s, sulcus placement may be relatively unstable in the long term because of the lack of fibrosis around the IOL.11,14 In cases of myopic eyes or eyes with keratoconus, piggyback IOLs may rotate significantly and in these cases a sulcus suture might be utilized to improve stability.15 The specific dioptric correction needed to correct all degrees of astigmatism may not be always available with currently available commercial products.
In a recent study, we described the refractive outcomes of enhancement procedures utilizing a new generation of multicomponent IOLs (MCIOLs).16,17 The MCIOL (Precisight, InfiniteVision Optics, Strasbourg, France) is composed by a hydrophobic base lens that serves as a docking station and an exchangeable hydrophilic front lens that is connected to the base lens by bilateral bridge openings (Figure 1). With this two component IOL, the surgical exchange of the front lens component (enhancement procedure) was shown to be safe and effective for correcting residual refractive errors. Because both optical components are mechanically coupled together and because the base component becomes permanently fixed by fibrosis in its original position within the capsular bag, the chance for post-enhancement IOL rotation is minimized. This feature is particularly important for the development of future toric models of this new MCIOL. In this study, we first report the rotational stability analyses of patients implanted with this novel MCIOL after cataract and enhancement surgeries.
Patients and methods
This is a prospective, interventional, non-comparative case series. The study protocol and informed consent forms were reviewed and approved by the Peregrine Eye and Laser Institute Institutional Review Board (PELI-IRB). The tenets of the Declaration for Helsinki were followed in this research and all patients have signed and received a copy of the written informed consent form.
We included eyes of adults (>21 years old) with visually significant cataracts and preoperative corneal astigmatism of 1.0 Diopter or less. We excluded eyes with concomitant corneal pathology, glaucoma, retinal disease, uveitis, prior ocular surgery or trauma as well as patients who were unable to complete the six-month follow up visit. During the screening visit, all patients underwent history, review of systems, comprehensive eye examination, manifest refraction, intraocular pressure (IOP) measurement, dilated retinal examination, corneal topography (Pentacam® HD, OCULUS Optikgeräte GmbH, Wetzlar, Germany), and optical biometry (IOL Master 700, Carl Zeiss Meditec, Jena, Germany). Eligible eyes underwent cataract surgery by phacoemulsification (PHACO) and unilateral implantation of the Precisight MCIOL. All surgeries were performed under topical anesthesia by the same surgeon (HSU) at the Peregrine Eye and Laser Institute, Makati City, Philippines between February 2017 and March 2018.
Description of surgical procedure
A 1.4 mm blade was used to create two side port incisions through which an unpreserved mixture of intracameral epinephrine and 1% unpreserved lidocaine was injected. Then 1.4% sodium OVD (Z-hyalin, Zeiss Meditec, Jena, Germany) was injected to fill the anterior chamber. A 2.2 mm keratome was then used to create a temporal clear corneal incision (CCI). A 6.5 mm capsulorrhexis was created using Gianetti forceps (E. Janach, Como, Italy) followed by hydrodissection and hydrodelineation. Nuclear disassembly and removal was completed using a single PHACO machine (Centurion, Alcon Surgical, Fort Worth, TX, USA) with a PHACO chop technique. After aspiration of cortical material, the capsular bag and anterior chamber were again filled with OVD.
The Precisight MCIOL was preassembled outside of the eye by placing the two front optic tabs into the bridges of the base component followed by gently tapping the front optic into position inside the collar of the base lens (Figure 1). The assembled MCIOL was then placed into a disposable butterfly cartridge, which is then folded and placed onto a disposable 2.2 injector system (Accuject, Medicel AG, Altenrhein, Switzerland). The preassembled MCIOL was then injected through the 2.2 mm CCI using a wound assisted injection technique, and placed within the capsular bag. Specially designed Sinskey hooks were used to position the MCIOL to ensure complete in-the-bag placement. The OVD was removed from the anterior chamber and behind the MCIOL using a standard irrigation and aspiration handpiece. OVD was removed from the interface between the front and base lens components of the MCIOL with a specially designed 30g right angle cannula. Finally, the anterior chamber was reformed, if necessary, using balanced saline solution. Antibiotic and corticosteroid eye drops were applied postoperatively.
Follow-up visits
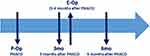
All patients were followed up on postoperative Day 1, Day 7–14, and Month 3. Primary (PRIM) patients underwent an additional Month 6 visit. At each visit, visual acuity testing, slit lamp examination and intraocular pressure measurement were performed. Manifest refraction (MR) was performed at the Month 3 visit and thereafter. Three months after cataract surgery, eyes with MR spherical equivalent (MRSE) less than ±0.75 D were classified into the primary cataract surgery group (PRIM) and did not undergo enhancement surgery but continued on to a Month 6 follow up visit. Eyes with MRSE greater than ±0.75 D were classified into the enhancement group (ENH) and underwent the enhancement procedure if the patient desired further improvement of uncorrected visual acuity.
The enhancement procedure is a minimally invasive surgical exchange of the front lens component with a different front lens with the corrected refractive power.16 In order to examine the rotational stability, both from the primary and the enhancement surgery procedures, the eyes were divided in two groups: (i) those who underwent only the primary cataract surgery with the implantation of the Precisight MCIOL, PRIM group; and (ii) those who underwent the enhancement procedure 3 months after the PHACO, ENH group.
There were 3 sequential observation periods when the MCIOL orientation was evaluated: at the end of primary cataract surgery (P-Op); at the 3 months postoperative visit (3 mo); and 6 months postoperative visit (6 mo). An additional fourth measure was done for the ENH group at the end of the enhancement procedure (E-Op). The timeline of visits is depicted in the Figure 2.
Determination of MCIOL orientation
At the pre-operative screening, each eye underwent optical biometry (IOL Master 700, Carl Zeiss Meditec, Jena, Germany) which generated keratometric and biometric data for toric IOL calculation and a reference image which established the principal meridians of the eye. These data were combined and uploaded into a digital surgical guidance system (Callisto Eye Assistance, Carl Zeiss Meditec, Jena, Germany). At the start of cataract surgery, the surgical microscope view of the eye was registered into the Callisto software which compared the ocular position against the reference image. The software then determined the horizontal (0–180 degree) orientation of the eye as well as IOL orientation. These guidance images are displayed as an overlay on surgeon’s eyepiece and permitted real time tracking of ocular movements and IOL orientation. The methods used to determine MCIOL orientation of the Precisight lens are described below:
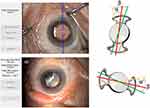
- During PHACO (P-Op) and enhancement surgery (E-Op): the Callisto Eye Assistance (Carl Zeiss Meditec, Jena, Germany) was used to define the horizontal orientation of the eye (Yellow line) and the IOL orientation Figure 3A. The IOL axis is based on a line crossing the two opposite optical manipulation holes of the front lens (Red line). The IOL orientation is given by the angle formed by the intersection of the IOL axis and horizontal axes (α). Then, the examiner chooses a reference axis (eg, limbal vessel or scleral pigment, Green line) to determine the β angle, between the horizontal and reference axes.
- Post-operative visits (3mo and 6mo): a digital slitlamp image was taken at a maximum pupil dilation. The subject’s head is positioned in the straight and upright position on the chin rest of the slitlamp, with an axial fixation object to ensure repeatable eye and head alignment and to minimize the cyclorotation effects. In postoperative images, first the examiner fixes the IOL axis by crossing a line between both manipulation holes of the front lens (Red line, Figure 3B). Then, the same reference, as was chosen in P-Op and E-Op, is selected and the reference orientation is traced (Green line). The β angle was constant for all visits and for that reason the postoperative IOL orientation (α’) is automatically obtained (Figure 3B)
The IOL rotation is the difference between the IOL axes of two given visits (α’ – α). The method used to determine the IOL rotation is independent of the cyclorotation of the eye, because the limbal vessels or pigmented spots on the sclera were used as reference points in all analyzed pictures. The IOL axes were obtained automatically by the image analysis using a specific script of the Matlab R2014b software (Mathworks, Natick, MA, USA). Internal repeatability and reproducibility tests have shown a precision of ±3° for operative and postoperative analyses which is satisfactory to meet the regulatory standards and clinical requirements. Standard requirements prescribes that IOL rotation when compared to Day 0 (the day of surgery) and the examination at 6 months postoperative shall be less than 10° in 90% of the cases, less than 20° in 95% of the cases, and less than 30° in 99% of the cases.18
The IOL rotation was analyzed in raw and absolute values (Table 2).
 |
Table 1 Raw data of image analyses in the different follow up visits of two the investigational groups: ENH and PRIM |
 |
Table 2 Difference between two analyzed visits in the two groups: ENH and PRIM. Values are Means ± standard deviation (minimum to maximum values) |
The pre-operative axial length (AL) was obtained by optical biometry (IOL Master 700, Carl Zeiss Meditec, Jena, Germany) and the anterior chamber depth (ACD) of the follow-up visits was assessed by Scheimpflug imaging (Pentacam® HD, OCULUS Optikgeräte GmbH, Wetzlar, Germany). To minimize possible errors and artifacts, the pseudophakic ACD was not determined automatically, but was determined by using the Pentacam digital caliper to measure the distance from the central corneal endothelium to the IOL anterior surface.
Data were analyzed by one examiner (CT-C) using Statistica 10 (StatSoft Inc., Tulsa, OK, USA) for statistical analyses. The IOL orientation at each visit was analyzed by repeated-measures Analysis of variance (ANOVA). Parametric variables regarding the rotation angles were analyzed using Student's t-test. When the data distribution did not follow the Gaussian distribution, Mann-Whitney tests were performed. Spearman correlations between the IOL rotation and the biometric parameters (AL and ACDs) were also performed. In all cases statistical significance was set to α<0.05.
Results
Thirty-three eyes underwent uncomplicated cataract surgery and MCIOL implantation. Of these, 13 were classified as PRIM and 20 eyes as ENH. The mean age was 67.8±6.6 (55–78) for the PRIM group and 66.7±7.4 (52–81) for the ENH group (P=0.68). We disqualified 4 eyes with insufficient image quality for defining critical IOL and anatomic details. For the final analysis, we included 12 eyes in the PRIM group and 17 eyes in the ENH group. The IOL axes of eyes used in this analysis are shown in Table 1. The mean MCIOL orientation in P-Op was 140.8±29.9° (46.3°–164.7°) for the PRIM group and 138.2±47.2° (29.3°–180°) for the ENH group. Of all patients, only four (14%) had a primary implantation angle between 0 and 120°. The mean and mean absolute rotational changes in MCIOL orientation at the different observation periods are reported in Table 2.
Rotational stability in ENH group
Repeated-measures ANOVA did not reveal any statistically significant differences regarding the IOL orientation during the four visits (P-Op, 3 mo, E-Op, 6 mo) of the ENH group (F3,48=2.24, p=0.1). Overall, the enhancement procedure did not significantly change IOL orientation and the IOL remains stable during the postoperative visits. The mean difference between the IOL orientation in the pre-enhancement visit (3mo) and E-Op was 2.35±4.08° (−4.0°–10.7°). As no preferential direction of IOL rotation was observed, we also evaluated the absolute value of rotation which was 3.44±3.32° (0.4°–10.7°). In only one eye, the difference in IOL orientation observed before and at the end of the enhancement surgery was greater than ±10° (10.7°). Regarding the rotational stability after ENH, the IOL orientation rotated an average of −0.04±3.51° (−8.7°–6.1°). Again no trends in the direction of IOL rotation were observed. The mean absolute rotation was 2.71±2.3° (0.1°–8.7°). An IOL rotation greater than ±5° was observed in three eyes after the enhancement procedure (compared to the day of the surgery). For further details see Table 2.
Rotational stability comparison between ENH and PRIM groups
Since the E-Op IOL orientation was not significantly different from the initial orientation of implantation, we then compared the rotational stability over time in the two groups. Regarding the differences in IOL orientation among the P-Op and the two follow-up visits in both ENH and PRIM groups, factorial repeated-measures ANOVA did not reveal any significant differences. No significant differences were also observed regarding the factor Group (F1,27=0.04, p=0.85) nor the factor Visit (F2,54=1.19, p=0.31). No interaction was observed between the factors as well (F2,54=1.11, p=0.34). No preferential direction of IOL rotation was observed at 6mo compared to P-Op (Figure 4). The average raw and absolute IOL rotation at the different time points are shown in Table 2. At 3 months post-cataract surgery (3mo), 88% of ENH eyes and 83% of PRIM eyes had an IOL rotation ≤ ±5°. In both groups, 100% of the eyes had an IOL rotation ≤ ±10° compared to the P-Op data. At 6 months post-cataract surgery (6mo), these frequencies remain stable for the PRIM group when compared to the 3mo postoperative visit values. For the ENH, the results are slightly different. Seventy-six percent (76%) of patients presented an IOL rotation ≤ ±5° and 94% presented ≤ ±10°. One eye had 13.8° of rotation when compared to the P-Op-value. It is noteworthy to mention that this eye is the one which presented 10.7° of difference between the 3mo and the E-Op visits.
IOL rotation and biometry factors
The axial length (AL) was studied in relation to the IOL rotation at 5–7 months after cataract surgery (compared to the baseline). No significant correlation was found between these two variables in any group (PRIM group r=0.049 and ENH group r=0.23, Figure 5A).
IOL rotation (relative to the baseline) was also studied in relation to the pseudophakic anterior chamber depth (ACD) at 3mo (PRIM group r=0.11 and ENH group r=0.44) and 6mo (PRIM group r=0.34 and ENH group r=0.28). No significant correlation was observed in any of the visits (Figure 5B and C).
Discussion
In the current study, the Precisight MCIOL system showed good long-term rotational stability over a 6 month follow-up period after primary cataract surgery. In the 2 follow-up visits (Month 3 and Month 6 postoperatively), 83% of patients had less than ±5° of rotation and none were greater than ±8°. Between the two postoperative visits, the absolute average rotation was minimal (2.36±1.56°), indicating that the MCIOL was stable at three months postoperatively. If a toric optic were used, these minimal rotational changes are expected to decrease the efficiency of astigmatism reduction by approximately 10% which would probably not of significant impact. It is likely that some of these minimal rotational changes would be due to variability in measurement rather than actual IOL rotation.
For an IOL entirely sequestered in the capsular bag, an off-orientation rotation may occur when there is a resultant torque acting on the IOL. Coincidence of center of the torque with the center of the lens generates independent rotation. If this is not the case, then decentration would be expected as well.19 Depending on the design of the IOL,8 the generation of capsule fibrosis and other biomechanical changes such as asymmetric shrinkage of the capsular bag, increases the risk of IOL rotation and decentration.20 Patel and colleagues have elegantly demonstrated that the plate haptic IOLs show greater rotational stability than the loop haptics. Open-loop haptic IOLs usually rotate counter clockwise after two weeks of implantation.8 The asymmetric capsule shrinkage in the open loop lens may explain this rotation.
The Precisight MCIOL has a symmetric haptic design, similar to that found in plate IOLs. This type of closed-loop haptic has been shown to be highly stable at 120 days after surgery,21 even in cases in where the risk of early IOL rotation is increased, eg myopic eyes.22 Myopic eyes require extra attention because they might have larger capsular bags and for that reason, the risk for early rotation of the IOL is greater. Furthermore, the base component of Precisight MCIOL, which is in contact with the capsular bag, consists of a hydrophobic acrylic material with 4% water content. Acrylic hydrophobic IOLs have been shown to have better rotational stability when compared to hydrophilic IOLs.23
In this study, when the enhancement procedure was performed 3–4 months after the primary cataract surgery, there was an increased, but not statistically significant, absolute rotation between the pre-enhancement visit (3mo) and the enhancement (E-Op) operative day (3.44±3.32°). This may be explained by the enhancement procedure itself. At the time of the enhancement surgery, the shrinkage of the capsular bag may not be complete in all patients. This could explain the 10° rotation of the base lens haptics observed in one patient during the front lens exchange. Although this instance was not statistically significant, it affected the amount of mean absolute IOL rotation between both follow-up visits (4.52±3.85) and between the primary cataract surgery and the last follow-up visit (3.62±3.42) in the ENH group. We may still conclude that Precisight is equally stable with or without enhancement procedure because after the enhancement surgery, the lens rotation was minimal (2.71±3.30). Allowing more time to pass between the primary surgery and the enhancement might avoid this problem by allowing greater capsular fixation. It should be emphasized that since non-toric lenses were used in this study, no attempt to check the toric orientation at the time of enhancement was made. Had toric lenses been used, some of the rotation seen during the enhancement procedure could possibly have been avoided by correcting any rotation that might have occurred.
Although Shah and colleagues24 found a strong correlation between rotational stability and AL six months postoperatively, we found no significant correlation between these two parameters. Other studies also failed in showing this correlation.22,23 A possible explanation of our observation is the limited AL range in the present study (22.25–24.33 mm). A correlation between AL and rotational stability might be found by analyzing a wider range of AL, as was the case with Shah and colleagues (19.5–29.03 mm). Also, no correlation was found between ACD and rotational stability, which confirms the findings of previous studies.25,26 A deeper anterior chamber should be expected to have a less crowded anterior segment and might allow more space for IOL rotation.15 Precisight is composed of two optics with a constant space between them making this lens thicker than the standard in-the-bag IOLs. As a consequence, the ACD is likely to be slightly smaller, which could help with the lens stability. The fan-shaped haptics may also contribute to better rotational stability.
It noteworthy to mention that the IOL orientation selected by the surgeon during PHACO was not completely random. Only four patients had an orientation less than 120° because the surgeon realized that an orientation greater than 120° facilitates a temporal approach enhancement procedure. If the enhancement procedure is needed in a patient with IOL orientation greater than 120°, only the proximal lens tab needs to be detached from the base component bridge. The direct pulling movement to remove the primary front lens will complete the lenses disassembly. The manipulation during the assembly of the enhancement front lens is also facilitated by this orientation position.
Study limitations include the small sample size which limits the possibility of extensive statistical interpretation. In the future, additional patients with longer follow-up times should be done to confirm the results found in the current study. The interpretation of images was also challenging. Other studies have also demonstrated problems with digital images obtained during the surgery and follow-up visits.25,27,28 This confirms the importance of high quality images for objective analysis of IOL rotational stability. In future studies, where toric optics were used, sequential wavefront aberrometry may provide objective measures of IOL rotation.
Conclusion
This study demonstrated that the Precisight MCIOL exhibits good rotational stability over a period of six months after cataract surgery and that the enhancement procedure does not significantly change IOL orientation. These findings provide evidence that this novel IOL platform is suitable for supporting toric optics for the management of pre-existing corneal astigmatism.
Acknowledgment
We thank Quentin Colman for his technical assistance.
Author contributions
HSU contributed to the data acquisition, interpretation, writing and critically revising the paper. CT-C contributed to the conception, design, and data analysis, drafting, and paper composition. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
HSU is an investigator and shareholder of InfiniteVision Optics. CT-C is an InfiniteVision Optics employee. The authors report no other conflicts of interest in this work.
References
1. Budak K, Yilmaz G, Aslan BS, Duman S. Limbal relaxing incisions in congenital astigmatism: 6 month follow-up. J Cataract Refract Surg. 2001;27(5):715–719. doi:10.1016/s0886-3350(00)00687-8
2. Monaco G, Scialdone A. Long-term outcomes of limbal relaxing incisions during cataract surgery: aberrometric analysis. Clin Ophthalmol. 2015;9:1581–1587. doi:10.2147/OPTH.S89024
3. Roberts HW, Wagh VK, Sullivan DL, Archer TJ, O’Brart DPS. Refractive outcomes after limbal relaxing incisions or femtosecond laser arcuate keratotomy to manage corneal astigmatism at the time of cataract surgery. J Cataract Refract Surg. 2018;44(8):955–963. doi:10.1016/j.jcrs.2018.05.027
4. Novis C. Astigmatism and toric intraocular lenses. Curr Opin Ophthalmol. 2000;11(1):47–50.
5. Tehrani M, Dick HB. Incisional keratotomy to toric intraocular lenses: an overview of the correction of astigmatism in cataract and refractive surgery. Int Ophthalmol Clin. 2003;43(3):43–52.
6. Kaur M, Shaikh F, Falera R, Titiyal JS. Optimizing outcomes with toric intraocular lenses. Indian J Ophthalmol. 2017;65(12):1301–1313. doi:10.4103/ijo.IJO_810_17
7. Miyake T, Kamiya K, Amano R, Iida Y, Tsunehiro S, Shimizu K. Long-term clinical outcomes of toric intraocular lens implantation in cataract cases with preexisting astigmatism. J Cataract Refract Surg. 2014;40(10):1654–1660. doi:10.1016/j.jcrs.2014.01.044
8. Patel CK, Ormonde S, Rosen PH, Bron AJ. Postoperative intraocular lens rotation: a randomized comparison of plate and loop haptic implants. Ophthalmology. 1999;106(11):2190–2195; discussion 2196. doi:10.1016/S0161-6420(99)90504-3
9. Ruhswurm I, Scholz U, Zehetmayer M, Hanselmayer G, Vass C, Skorpik C. Astigmatism correction with a foldable toric intraocular lens in cataract patients. J Cataract Refract Surg. 2000;26(7):1022–1027. doi:10.1016/s0886-3350(00)00317-5
10. Ho Wang Yin G, Hoffart L. Post-keratoplasty astigmatism management by relaxing incisions: a systematic review. Eye Vis (Lond). 2017;4:29.
11. Meyer JJ, McGhee CN. Supplementary, sulcus-fixated intraocular lens in the treatment of spherical and astigmatic refractive errors in pseudophakic eyes after keratoplasty. Cornea. 2015;34(9):1052–1056. doi:10.1097/ICO.0000000000000506
12. Ong C, Nongpiur M, Peter L, Perera SA. Combined approach to phacoemulsification and trabeculectomy results in less ideal refractive outcomes compared with the sequential approach. J Glaucoma. 2016;25(10):e873–e878. doi:10.1097/IJG.0000000000000489
13. Tzu JH, Shah CT, Galor A, Junk AK, Sastry A, Wellik SR. Refractive outcomes of combined cataract and glaucoma surgery. J Glaucoma. 2015;24(2):161–164. doi:10.1097/01.ijg.0000435773.20279.56
14. Gundersen KG, Potvin R. A review of results after implantation of a secondary intraocular lens to correct residual refractive error after cataract surgery. Clin Ophthalmol. 2017;11:1791–1796. doi:10.2147/OPTH.S144675
15. Meyer JJ, Kim BZ, Ziaei M, McGhee CN. Postoperative rotation of supplementary sulcus-supported toric intraocular lenses. J Cataract Refract Surg. 2017;43(2):285–288. doi:10.1016/j.jcrs.2016.12.014
16. Portaliou DM, Grentzelos MA, Pallikaris IG. Multicomponent intraocular lens implantation: two-year follow-up. J Cataract Refract Surg. 2013;39(4):578–584. doi:10.1016/j.jcrs.2012.11.020
17. Portaliou DM, Kymionis GD, Pallikaris IG. Multi-component adjustable intraocular lenses: a new concept in pediatric cataract surgery. J Refract Surg. 2014;30(1):62–66. doi:10.3928/1081597X-20131023-01
18. NF EN ISO 11979-7. Ophthalmic Implants — Intraocular Lenses — Part 7: Clinical Investigations. Afnor; 2014.
19. Wasserman D, Apple DJ, Castaneda VE, Tsai JC, Morgan RC, Assia EI. Anterior capsular tears and loop fixation of posterior chamber intraocular lenses. Ophthalmology. 1991;98(4):425–431. doi:10.1016/s0161-6420(91)32274-7
20. Montes-Mico R, Cervino A, Ferrer-Blasco T. Intraocular lens centration and stability: efficacy of current technique and technology. Curr Opin Ophthalmol. 2009;20(1):33–36.
21. Buckhurst PJ, Wolffsohn JS, Naroo SA, Davies LN. Rotational and centration stability of an aspheric intraocular lens with a simulated toric design. J Cataract Refract Surg. 2010;36(9):1523–1528. doi:10.1016/j.jcrs.2010.03.047
22. Mencucci R, Favuzza E, Guerra F, Giacomelli G, Menchini U. Clinical outcomes and rotational stability of a 4-haptic toric intraocular lens in myopic eyes. J Cataract Refract Surg. 2014;40(9):1479–1487. doi:10.1016/j.jcrs.2013.12.024
23. Draschl P, Hirnschall N, Luft N, et al. Rotational stability of 2 intraocular lenses with an identical design and different materials. J Cataract Refract Surg. 2017;43(2):234–238. doi:10.1016/j.jcrs.2016.12.011
24. Shah GD, Praveen MR, Vasavada AR, Vasavada VA, Rampal G, Shastry LR. Rotational stability of a toric intraocular lens: influence of axial length and alignment in the capsular bag. J Cataract Refract Surg. 2012;38(1):54–59. doi:10.1016/j.jcrs.2011.08.028
25. Gyongyossy B, Jirak P, Schonherr U. Long-term rotational stability and visual outcomes of a single-piece hydrophilic acrylic toric IOL: a 1.5-year follow-up. Int J Ophthalmol. 2017;10(4):573–578. doi:10.18240/ijo.2017.04.12
26. Klamann MK, Von Sonnleithner C, Gonnermann J, Maier AK, Torun N, Bertelmann E. Influence of biometric parameters on rotational stability of toric IOLs. Eur J Ophthalmol. 2013;23(6):836–840. doi:10.5301/ejo.5000316
27. Weinand F, Jung A, Stein A, Pfutzner A, Becker R, Pavlovic S. Rotational stability of a single-piece hydrophobic acrylic intraocular lens: new method for high-precision rotation control. J Cataract Refract Surg. 2007;33(5):800–803. doi:10.1016/j.jcrs.2007.01.030
28. Wolffsohn JS, Buckhurst PJ. Objective analysis of toric intraocular lens rotation and centration. J Cataract Refract Surg. 2010;36(5):778–782. doi:10.1016/j.jcrs.2009.12.027
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.