Back to Journals » Clinical Ophthalmology » Volume 10
Role of the treating surgeon in the consent process for elective refractive surgery
Authors Schallhorn SC, Hannan SJ, Teenan D , Schallhorn JM
Received 22 August 2016
Accepted for publication 8 October 2016
Published 28 November 2016 Volume 2016:10 Pages 2391—2402
DOI https://doi.org/10.2147/OPTH.S120345
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Steven C Schallhorn,1–3 Stephen J Hannan,3 David Teenan,3 Julie M Schallhorn1
1Department of Ophthalmology, University of California, San Francisco, San Francisco, 2Roski Eye Institute, University of Southern California, Los Angeles, CA, USA; 3Optical Express, Glasgow, UK
Purpose: To compare patient’s perception of consent quality, clinical and quality-of-life outcomes after laser vision correction (LVC) and refractive lens exchange (RLE) between patients who met their treating surgeon prior to the day of surgery (PDOS) or on the day of surgery (DOS).
Design: Retrospective, comparative case series.
Setting: Optical Express, Glasgow, UK.
Methods: Patients treated between October 2015 and June 2016 (3972 LVC and 979 RLE patients) who attended 1-day and 1-month postoperative aftercare and answered a questionnaire were included in this study. All patients had a thorough preoperative discussion with an optometrist, watched a video consent, and were provided with written information. Patients then had a verbal discussion with their treating surgeon either PDOS or on the DOS, according to patient preference. Preoperative and 1-month postoperative visual acuity, refraction, preoperative, 1-day and 1-month postoperative questionnaire were compared between DOS and PDOS patients. Multivariate regression model was developed to find factors associated with patient’s perception of consent quality.
Results: Preoperatively, 8.0% of LVC and 17.1% of RLE patients elected to meet their surgeon ahead of the surgery day. In the LVC group, 97.5% of DOS and 97.2% of PDOS patients indicated they were properly consented for surgery (P=0.77). In the RLE group, 97.0% of DOS and 97.0% of PDOS patients stated their consent process for surgery was adequate (P=0.98). There was no statistically significant difference between DOS and PDOS patients in most of the postoperative clinical or questionnaire outcomes. Factors predictive of patient’s satisfaction with consent quality were postoperative satisfaction with vision (46.7% of explained variance), difficulties with night driving, close-up vision or outdoor/sports activities (25.4%), visual phenomena (12.2%), dry eyes (7.5%), and patient’s satisfaction with surgeon’s care (8.2%).
Conclusion: Perception of quality of consent was comparable between patients that elected to meet the surgeon PDOS, and those who did not.
Keywords: consent process, refractive surgery, laser vision correction, refractive lens exchange, quality of life outcomes
Introduction
Prior to elective surgery, patients need to be adequately informed of the benefits and inherent risks of the proposed treatment, possible outcomes, as well as surgical and nonsurgical alternatives to the recommended procedure.1,2 As the number of procedures and their degree of technical complexity grow, delivering the correct information to the patient is becoming increasingly difficult. On one hand, patients should be informed in detail about their surgical procedure; on the other hand, overloading patients with too much technical/medical information may have unintended negative effects by confusing patients, reducing their ability to retain information, and impairing their ability to provide an informed decision.1–3
Inadequate consenting process (combined with poor outcomes) is one of the most common reasons for malpractice litigation in ophthalmology.4–8 This problem is particularly hard to overcome, as many publications have shown that very little information presented during informed consent is actually retained by the patient.9–13 For this reason, numerous reports have studied the best way of communicating essential information to the patient.1–3 This includes verbal discussion, use of videotaped presentation, and use of leaflets and written consent forms, allowing patients to digest information at their own pace.14–17
In elective refractive surgery, such as laser vision correction (LVC), refractive lens exchange (RLE), or private cataract surgery, one of the issues is the role of the treating surgeon in the consent process, and whether medical personnel other than the treating physician can assist in the delivery of a high-quality and meaningful preoperative consent process. The ability to discuss a surgical procedure with patients of different backgrounds and different cognitive abilities can vary significantly from surgeon to surgeon.15–18 In some cases, the physician’s inability to explain a procedure in “plain language” results in poor understanding of the planned procedure, or an emotional barrier where patients might be reluctant or embarrassed to ask further questions.16 Several clinical practices over the world have adopted a model where the preoperative discussion is performed by qualified clinical personnel,19,20 with the aid of other tools, such as a video consent,16,17 and/or plain-language written information,14,17 with the intent to provide a uniform and thorough consent process. In this model, consent is a multistep process culminating with the surgeon.
The aim of the study was to investigate whether there was a difference in patient perception of their consent quality, clinical outcomes, and satisfaction after LVC and RLE between patients who met their treating surgeon on the day of surgery (DOS) and those who chose to have a discussion with the surgeon prior to the day of surgery (PDOS).
Patients and methods
This study was deemed exempt from full review by the Committee on Human Research at the University of California, San Francisco (CA, USA), because it used only retrospective, de-identified patient data. Informed consent to undergo laser in situ keratomileusis (LASIK), photorefractive keratectomy (PRK) or RLE procedure was obtained from all patients. All patients also provided consent to use their de-identified treatment data for research purposes and statistical analysis.
Refractive and visual outcomes and postoperative patient questionnaire outcomes were extracted from electronic database for patients who underwent LVC (3,972 patients) and RLE (979 patients) between October 2015 and June 2016. The inclusion criteria were corrected distance visual acuity 20/25 or better in each eye, attended 1 day and 1 month postoperative aftercare, and completed a patient experience questionnaire at both visits.
All patients underwent a preoperative consultation with an optometrist who was well instructed in the refractive surgery process. This consisted of a discussion as well as an examination and diagnostic testing. The discussion included assessing medical and ocular history, medications, employment, hobbies, lifestyle, refractive correction history, motivation for, and expectations of, refractive surgery. The examination consisted of a manifest and cycloplegic refraction, uncorrected (UDVA) and corrected distance visual acuity (CDVA), external ocular exam, ocular motility, confrontational visual fields, pupil diameter, detailed biomicroscopic exam (conjunctiva, tear film, cornea, anterior chamber, iris, crystalline lens), dilated fundoscopy (macula, optic nerve, and retinal periphery), and diagnostic tests (corneal topography: Pentacam; Oculus Optikgeräte GmbH, Wetzlar, Germany; wavefront aberrometry: iDesign Advanced WaveScan System; Abbott Medical Optics Inc., Santa Ana, CA, USA; autorefraction and non-contact tonometry: Tonoref II, Nidek Co. Ltd., Gamagori, Japan). If there were any unresolved issues, the patients were either scheduled for a follow-up examination or an appointment with the treating surgeon.
Based on the consultation, the optometrist determined whether the patient was a suitable candidate for refractive surgery and proposed a procedure (LVC or RLE) to best meet the patient’s needs. This was followed by a discussion of the benefits, risks, side effects, healing process, and alternatives as well as addressing any patient questions or concerns. The patient was provided an information pack consisting of a copy of the informed consent document, written information about the procedure, preparation for the surgery day, and information on what to expect on the day of surgery and after the procedure. All patients watched an educational video, which reiterated the benefits, risks, and possible side effects of the proposed surgery. Patients who were candidates for RLE were required to attend another consultation where further measurements (biometry for lens calculation: IOLMaster; Carl Zeiss Meditec AG, Jena, Germany; retinal optical coherence tomography: Cirrus 4000 OCT, Carl Zeiss Meditec AG; and specular microscopy: SP 2000P specular microscope; Topcon, Co., Tokyo, Japan) were performed. These patients had another detailed discussion with the optometrist about the choice of multifocal lenses and optical side effects.
All patients, if they desired to proceed with surgery, were encouraged to see their treating surgeon PDOS; this visit was made freely available to all who desired to schedule it and was without cost. Those who elected not to see their surgeon PDOS had a consultation with their surgeon prior to the procedure but on the DOS. In both cases, the surgeon confirmed the patient was suitable for the procedure and ensured they provided their informed consent. The consummation of the consent process was the signing of the written consent document by the patient and surgeon.
Postoperatively, patients were evaluated at 1 day, 1 week, 1 month, and 3 months and thereafter, as necessary. All patients were asked to complete a postoperative questionnaire. It was self-administered by the patient using a password-protected and secure computer terminal in an isolated area of the clinic and not accessible to any clinic personnel. The results of this questionnaire were not made available to their treating physician, and patients were informed of this to ensure that they knew that they had total confidentiality in their responses. The questionnaire responses were stored in the secured central database, which is compliant with ISO 27001 for information security management systems. All response fields used a Likert scale to obtain the patient’s preferences or degree of agreement. Questionnaires completed at postoperative day 1 and on 1 month postoperative visit were used for analysis (Table 1). A total of 78% of LVC patients and 81.2% of RLE patients also completed a preoperative questionnaire where they rated degree of difficulties with visual phenomena and dry eye symptoms (Question 7 from Table 1) with spectacle/contact lens correction preoperatively. The mean difference between preoperative and postoperative scores was used for analysis. All questionnaire outcomes were compared between patients who met the surgeon PDOS and DOS.
  | Table 1 Patient experience questionnaire |
Statistical analysis
Data tabulation and statistical operations were performed with SAS 9.3 (SAS Institute, Inc., Cary, NC, USA) and Microsoft Office Excel 7.0 (Microsoft Corporation, Redmond, WA, USA) software. Unpaired t-test was used to compare clinical data and questionnaire outcomes between patients who had met their surgeon on DOS and PDOS. Chi-square test was used to compare percentages.
Multivariate regression model was developed to find factors predictive of the outcomes of the Question 4 (Table 1): “Do you feel you were properly consented for surgery?” Stepwise generalized linear approach to the model creation was used, and patient’s demographics, clinical parameters as well as other questionnaire responses were considered as independent variables in the regression model. Standard assumptions required for the use of a multivariate regression model approach were tested and satisfied. Although different data transformations were explored, a linear relationship between the dependent and independent variables was deemed to be the most appropriate. Normality of all variables was tested and confirmed using Q–Q plots. A correlation matrix was used to conclude that there was little to no multicollinearity and independence was confirmed. An assumption of homoscedasticity was determined to be met using a White test to measure the homogeneity of variance of the residuals.
Results
Eight percent (8.0%) of LVC patients and 17.1% of RLE patients elected to meet their surgeon PDOS. Tables 2 (LVC) and 3 (RLE) compare preoperative and 1-month postoperative clinical parameters between patients who have met their surgeon on the DOS and PDOS. There was no statistically significant difference in patient demographics or clinical parameters between DOS and PDOS patients, apart from a tiny (0.03 D), but statistically significant, difference in postoperative cylinder between DOS and PDOS patients in LVC group (Table 2).
Postoperative day 1 questionnaire
On the first postoperative day, an equal proportion of DOS and PDOS LVC patients (99.4%) stated they were satisfied or very satisfied with the care provided by their surgeon (Question 1 from Table 1). For the same question, 99.4% of RLE DOS and 98.8% of RLE PDOS patients (P=0.42) were satisfied/very satisfied with surgeon’s care.
The vast majority of patients in the LVC group (98.8% DOS patients vs 99.4% PDOS; P=0.37) and RLE group (99.1% DOS and 98.8% PDOS; P=0.68) were satisfied or very satisfied with their surgeon answering all their questions (Question 2). Additionally, 98.0% DOS and 98.7% PDOS LVC patients (P=0.36) and 97.5% DOS and 98.8% PDOS RLE patients (P=0.32) felt the preoperative written information, including consent form, was useful and informative (Question 3).
One-month postoperative questionnaire
In the LVC group, 97.5% of DOS patients and 97.2% of PDOS patients indicated they were properly consented for surgery (Question 4; P=0.77). Similar outcomes were achieved in RLE group: 97.0% of DOS and 97.0% of PDOS patients stated their consent process for surgery was adequate (P=0.98).
Figure 1 shows the satisfaction with visual acuity (Question 5). One month postoperatively, 93.6% of DOS and 94.4% of PDOS LVC patients were satisfied or very satisfied with their vision. For RLE patients, the percentage of satisfied or very satisfied patients was 88.7% DOS and 88.6% PDOS. Satisfaction with vision was not statistically significant between DOS and PDOS patients for both, LVC and RLE (Figure 1).
  | Figure 1 One-month postoperative satisfaction with vision compared between patients that met their surgeon on the day of surgery (DOS) and prior to the day of surgery (PDOS). |
The percentage of patients that would recommend surgery to their friends or relatives (Question 6) was 96.5% and 97.8% for LVC DOS and PDOS patients, respectively (P=0.22). Of the RLE patients 95.3% of DOS and 93.4% of PDOS patients responded affirmative to Question 6 (P=0.30).
Table 4 shows the mean scores for visual phenomena and dry eye symptoms (Question 7). The mean postoperative score for starburst, glare, and halo was slightly higher in PDOS LVC patients compared to DOS patients, and this difference was statistically significant (Table 4). However, when comparing change between preoperative and postoperative mean score, the change in any of the visual phenomena symptoms was not statistically significant. RLE DOS and PDOS had comparable outcomes for visual phenomena (Table 4). RLE PDOS had statistically significantly higher postoperative dry eye symptoms, but the mean change between pre- and postoperative symptoms was not statistically significant.
Figure 2A (LVC) and B (RLE) plots the difficulties patients experienced with tasks related to night driving, close-up vision, outdoor or sport activities (Questions 8–10). There was no statistically significant difference between DOS and PDOS patients in any of the questioned activities.
Factors associated with patient’s perception of the quality of their consent
Table 5 summarizes the results of regression analysis to predict outcomes of question: “Do you feel you were properly consented for surgery?” Initially, two individual regression models for RLE and LVC were created. As the surgery type, age, and refraction were not significant factors in univariate analysis, and both models had similar outcomes, RLE and LVC data were combined, and only one final regression model was created. Additionally, as the incidence of the response variable was low (only 2.6% of patients felt not properly consented for surgery), the two datasets (LVC and RLE) were combined to create a larger, more powerful sample for regression analysis.
  | Table 5 Results of multivariate regression analysis to predict outcomes of Question 4: “Do you feel you were properly consented for surgery?” |
One-month postoperative satisfaction with their vision (Question 5) was the strongest predictor of the patient’s perception of consent quality, accounting for 46.7% of variance explained by the regression model. As an indication of this relationship, Figure 3 shows the proportion of patients who were satisfied/dissatisfied with their postoperative vision in the group of patients who felt properly consented for procedure, and those who believed the consenting process was inadequate. As much as 23.7% of “not consented properly” patients were dissatisfied with vision, whereas only 2.5% of patients were dissatisfied with vision in the group of patients who were happy with their consent process.
  | Figure 3 One-month postoperative satisfaction with vision according to patient’s perception of consent quality. |
The second strongest predictor in regression analysis was the postoperative difficulties patients experienced with various activities, such as night driving, tasks requiring close-up vision, and outdoor and sports activities (Questions 8–10), responsible for 25.4% of variance explained by the model. Figure 4 shows the percentage of patients who had a lot of difficulties or were unable to do the activities because of their vision. A significantly higher proportion of patients who were dissatisfied with the consent process experienced postoperative difficulty with any of these activities (Figure 4).
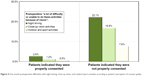  | Figure 4 One-month postoperative difficulties with night driving, close-up vision, and outdoor/sport activities according to patient’s perception of consent quality. |
Postoperative visual phenomena (12.2% variance explained) and postoperative dry eye difficulties (7.5% variance explained) were other significant predictors of satisfaction with consent. Figure 5 depicts the percentages of patients who had significant difficulties (scored 6 or 7 on a scale from 1 to 7) with postoperative optical side effects or dry eyes. Over 10% of patients who felt not consented properly for their procedure had significant difficulties with visual symptoms, whereas ≤2.5% patients had significant difficulties in the group of patients who were “consented properly.”
Satisfaction with surgeon’s care and the surgeon answering all patient’s questions (Questions 1 and 2, 8.2% of variance explained) was also a significant predictor in regression analysis. Of all the “consented properly” patients, 78.0% were very satisfied with surgeon’s care, whereas only 62.6% of patients were very satisfied within the group of “not consented properly” patients. A similar pattern was seen in Question 2: 76.2% of “consented properly” patients and 62.6% of “not consented properly” patients were very satisfied with their surgeon answering all their questions.
Meeting the surgeon PDOS or DOS had no effect on patient’s perception of adequate consenting process. A similar percentage of patients who felt consented properly (9.8%) and those who believed consenting process was not appropriate (10.7%) have met their surgeon PDOS (P=0.73).
Discussion
Patients undergoing private refractive surgery often have high expectations and some may find it difficult to accept outcomes that are not satisfactory. A robust consent process is therefore required to educate the patient and provide them with the information they need to make an informed decision whether to proceed. Literature agrees that better communication increases patient satisfaction and reduces the number of malpractice suits.21–23
Verbal communication with the treating physician alone, however, does not guarantee that the patient will be satisfied with the consenting process.14–18 A surgeon’s verbal discussion cannot be easily standardized, as it is extremely patient-directed.16,17 Some publications previously have highlighted the difficulties in performing consent by treating ophthalmologist in busy clinical practices.14–18 Ophthalmologist may need to consent several patients a day, which often results in a rote and mechanical discussion that may not fully educate the patient.15,16 If the preoperative consent discussion was performed only by the surgeon, without the help of other medical personnel or other audio/visual aides, a longer appointment time would be needed, which could result in a reduced availability of consultations and reduced affordability of the procedure. As a result, fewer patients may have access to the surgery.
Another concern, raised by several studies, is the amount of information patients can remember from a discussion with their surgeon.9–13 Guerin and O’Keeffe9 studied the recollection of information from the consenting process in 102 patients undergoing laser refractive surgery. DOS, only 2 patients remembered all five main risks outlined preoperatively, whereas 11 patients remembered no risks at all. Remaining patients only remembered some of the risks. Dhingra and colleagues10 questioned 82 patients undergoing elective phacoemulsification with intraocular lens implantation. All patients received standardized verbal and written information 2 weeks prior to surgery. Only one-third of the patients could correctly recollect the consent information DOS. Priluck et al11 questioned 100 patients undergoing a scleral buckling procedure about their informed consent discussion. As little as 23% of patients retained some information of the surgical risks, concluding that patients are likely to remember only the information that seems to be in favor of their decision to have surgery.
To overcome the problem of poor preoperative discussion recollection, several consenting approaches have been proposed, which mostly consist of combination of written, verbal, and audiovisual information.1–3,14–17 For example, Moseley et al15 evaluated the effect of presentation methods on understanding of cataract surgery and showed that subgroup of patients who watched educational video had significantly better understanding of the risks and benefits of the procedure. In a similar study, Wollinger et al16 used multimedia software to improve patients’ understanding of cataract surgery, in addition to verbal consent. This interactive tool presented cataract surgery using simple images and a clear spoken voice, allowing patients to process information in their own time. Patients who watched the video had significantly better understanding of cataract surgery than patients in control group. Authors attributed this to basic concepts of learning, where all senses have to be engaged in order to memorize presented information: they state that only 10%–15% of the read material, 25% of the heard material, and 40% of the seen images are kept in memory over the long term. This increases to 75% when all senses are used simultaneously.24 Numerous other studies from all areas of medicine confirmed that use of audiovisual tools can significantly enhance patients understanding of their medical condition or proposed surgical procedure.24–28
The informed consent process in our practice is very standardized, and the optometrist plays a key role. This includes discussing patient-specific potential risks, range of outcomes, and alternatives. In addition, the optometrist discusses the content of the educational video after the patient has viewed it as well as addressing any patient questions or concerns. The surgeon then reiterates all this information either DOS or PDOS, according to patient’s choice. There are specific declarations within the electronic medical records system that document these discussions which have been completed and signed by the optometrist, the surgeon, and the patient.
This study was conducted to determine whether the expectations of those patients who elected to meet the surgeon PDOS were better addressed. We specifically analyzed whether they felt they were properly consented and they were satisfied with the procedure and visual and ocular symptoms. This allowed us to evaluate the patient’s perspective of their consent after they experienced the outcome of their procedure. The multivariate regression analysis showed that meeting the surgeon PDOS or DOS had no effect on whether the patient felt they were properly consented. Meeting the surgeon also had no effect on satisfaction with visual outcomes, or postoperative visual acuity.
We also analyzed factors associated with their perception of consent. Perhaps not surprisingly, the patient’s satisfaction with the outcome of their procedure was the strongest predictor, accounting for 46.7% of variance explained by the regression model. Other significant predictors were also associated with quality of vision such as postoperative difficulties with night driving, close-up vision, sports or outdoor activities (25.4% explained variance), visual phenomena (12.2% explained variance), and ocular comfort (7.5% explained variance). Satisfaction with surgeon’s care and the surgeon answering all questions (regardless of whether patient has met the surgeon PDOS or DOS) was also a statistically significant factor in regression analysis, responsible for 8.2% of variance explained by the regression model.
A patient being disappointed with their outcome is strongly correlated to their belief that the consent process was inadequate. This agrees with literature, where the consent process is one of the most common causes of malpractice suits;4–8 however, it is rarely the primary cause of litigation in ophthalmology.18 It is the negative outcome that initiates litigation, with the lack of adequate consent used as a secondary factor in majority of ophthalmology malpractice cases.18
Despite a thorough preoperative explanation and repetition of information in the consent process, there will probably be some patients who will not retain,9–13 or even deny ever hearing, the information presented to them preoperatively.11 Some patients who are strongly determined to undergo refractive surgery might selectively process the information they hear, remembering only the positive facts, and unwilling to accept or pay attention to any adverse side effects.11,16,18 There is also a possibility that the patients were truly not sufficiently informed about the risks of the procedure. In our practice, the likelihood of negative outcome is presented to all patients in multiple ways, including written information, audiovisual tools, and a minimum of two verbal discussions (optometrist and surgeon). Additionally, patients sign a declaration after each consent step confirming that the information was presented to them. However, the intention of the study was not to understand why patients do not feel they were properly consented, but rather to analyze whether meeting the surgeon PDOS or DOS affected their perception of the consent 1 month after surgery, and this was not found to be a significant factor in the regression analysis.
As refractive surgery has become more popular, there is an increasing public awareness of the procedures.29–31 Prospective patients have access to nearly unlimited online information that can combine scientific facts with unfounded assertions, a process that has become known colloquially as “Dr Google.” Accordingly, patients need to be given precise information to help them with their decision process and ensure they have realistic expectations about the benefits and potential risks of the procedure. Our results show that a discussion with the treating physician on either DOS or PDOS has no effect on the patient’s perception of the quality of their consent, their postoperative satisfaction, or visual outcomes. The study outcome does not diminish the value of the surgeon in consenting process, but rather highlights that trained eye care specialists can perform an important supporting role in the consent process.
Our study had some limitations. The main limitation is its retrospective nature, and the selection process for DOS or PDOS consent, which was based on patient’s preference, rather than randomly assigned between the two groups. Although the questionnaire used in this study was previously found effective for reporting quality-of-life outcomes in a large number of refractive surgery patients,32–34 use of a validated quality-of-life instrument would be beneficial. Despite these limitations, the authors believe that having a large volume of patients who allowed detailed multivariate regression analysis significantly added to the value of this study.
Disclosure
Steven C Schallhorn, MD, is a Chairman of the Optical Express Medical Advisor Board, Zeiss Chief Medical Officer, and a consultant for Acufocus, and reports no other conflicts of interest in this work. None of the other authors have a financial or proprietary interest in the products and materials presented in this work.
References
Schenker Y, Fernandez A, Sudore R, Schillinger D. Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Med Decis Making. 2011;31(1):151–173. | ||
Abbott RL. Informed consent in refractive surgery. Curr Opin Ophthalmol. 1998;9(4):29–34. | ||
Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;28(1):CD001431. | ||
Mavroforou A, Michalodimitrakis E. Physicians’ liability in ophthalmology practice. Acta Ophthalmol Scand. 2003;81(4):321–325. | ||
Bhan A, Dave D, Vernon SA, et al. Risk management strategies following analysis of cataract negligence claims. Eye (Lond). 2005;19(3):264–268. | ||
Steven Bailey C, Bailey JA. Claims of alleged medical negligence in refractive surgery: causes and avoidance. Cont Lens Anterior Eye. 2007;30(2):144–147. | ||
Santos W, Solari HP, Ventura MP. [Litigation in ophthalmology: analysis of possible triggers]. Arq Bras Oftalmol. 2010;73(6):501–504. Portuguese. | ||
Ali N, Little BC. Causes of cataract surgery malpractice claims in England 1995–2008. Br J Ophthalmol. 2011;95(4):490–492. | ||
Guerin M, O’Keeffe M. Informed consent in refractive eye surgery: learning from patients and the courts. Ir Med J. 2012;105(8):282–283. | ||
Dhingra N, Clews S, Neugebauer M, Hubbard AD. What patient recall of the preoperative discussion before cataract surgery: results of a questionnaire survey. Eye (Lond). 2004;18:790–791. | ||
Priluck IA, Robertson DM, Buettner H. What patients recall of the preoperative discussion after retinal detachment surgery. Am J Ophthalmol. 1979;87(5):620–623. | ||
Pesudovs K, Luscombe CK, Coster DJ. Recall from informed consent counselling for cataract surgery. J Law Med. 2006;13(4):496–504. | ||
Scanlan D, Siddiqui F, Perry G, Hutnik CM. Informed consent for cataract surgery: what patients do and do not understand. J Cataract Refract Surg. 2003;29(10):1904–1912. | ||
Brown H, Ramchandani M, Gillow JT, Tsaloumas MD. Are patient information leaflets contributing to informed consent for cataract surgery? J Med Ethics. 2004;30(2):218–220. | ||
Moseley TH, Wiggins MN, O’Sullivan P. Effects of presentation method on the understanding of informed consent. Br J Ophthalmol. 2006;90(8):990–993. | ||
Wollinger C, Hirnschall N, Findl O. Computer-based tutorial to enhance the quality and efficiency of the informed-consent process for cataract surgery. J Cataract Refract Surg. 2012;38(4):655–659. | ||
Shukla AN, Daly MK, Legutko P. Informed consent for cataract surgery: patient understanding of verbal, written, and videotaped information. J Cataract Refract Surg. 2012;38(1):80–84. | ||
Kiss CG, Richter-Mueksch S, Stifter E, Diendorfer-Radner G, Velikay-Parel M, Radner W. Informed consent and decision making by cataract patients. Arch Ophthalmol. 2004;122(1):94–98. | ||
Davies M. Nurse practitioner-led consent in day case cataract surgery. Nurs Times. 2005;101(13):30–32. | ||
Lockey J. The provision of information for patients prior to cataract surgery. Br J Nurs. 2009;18(19):1207–1211. | ||
Beckman HB, Markakis KM, Suchman AL, Frankel RM. The doctor-patient relationship and malpractice. Lessons from plaintiff depositions. Arch Intern Med. 1994;154(12):1365–1370. | ||
Levinson W, Roter DL, Mullooly JP, Dull VT, Frankel RM. Physician-patient communication. The relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277(7):553–559. | ||
Shapiro RS, Simpson DE, Lawrence SL, Talsky AM, Sobocinski KA, Schiedermayer DL. A survey of sued and nonsued physicians and suing patients. Arch Intern Med. 1989;149(10):2190–2196. | ||
Heil H, Struck J. Multimediale Lernsystem als Ausbildungsintrumente [Multimedia learning systems as a training instrument]. In: Dette K, Haupt D, Polze C, editors. Multimedia Und Computeranwendungen in Der Lehre [Multimedia and computer applications in teaching]: 6. CIP-Kongress, Berlin, 6–8 Oktober 1992. Springer Verlag, Berlin, Germany. 1992:40–47. German. | ||
Wilson EAH, Park DC, Curt is LM, et al. Media and memory: the efficacy of video and print materials for promoting patient education about asthma. Patient Educ Couns. 2010;80(3):393–398. | ||
Murphy P, Chesson A, Walker L, Arnold C, Chesson L. Comparing the effectiveness of video and written material for improving knowledge among sleep disorders clinic patients with limited literacy skills. South Med J. 2000;93(3):297–304. | ||
Wang DS, Jani AB, Sesay M, et al. Video-based educational tool improves patient comprehension of common prostate health terminology. Cancer. 2015;121(5):733–740. | ||
Kandula NR, Nsiah-Kumi PA, Makoul G, et al. The relationship between health literacy and knowledge improvement after a multimedia type 2 diabetes education program. Patient Educ Couns. 2009;75(3):321–327. | ||
Alexander JK, Davidson RS. Managing expectations in refractive surgery. Int Ophthalmol Clin. 2016;56(2):1–17. | ||
Alió JL, Grzybowski A, Romaniuk D. Refractive lens exchange in modern practice: When and when not to do it? Eye Vis (Lond). 2014;1:10. | ||
Rana M, Shah S. Modern-day cataract surgery: can we match growing expectations? Br J Ophthalmol. 2014;98(10):1313–1314. | ||
Venter JA, Pelouskova M, Collins BM, Schallhorn SC, Hannan SJ. Visual outcomes and patient satisfaction in 9366 eyes using a refractive segmented multifocal intraocular lens. J Cataract Refract Surg. 2013;39(10):1477–1484. | ||
Brown MC, Schallhorn SC, Hettinger KA, Malady SE. Satisfaction of 13,655 patients with laser vision correction at 1 month after surgery. J Refract Surg. 2009;25(7 Suppl):S642–S646. | ||
Schallhorn SC, Venter JA, Hannan SJ, Hettinger KA, Teenan D. Effect of postoperative keratometry on quality of vision in the postoperative period after myopic wavefront-guided laser in situ keratomileusis. J Cataract Refract Surg. 2015;41(12):2715–2723. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





