Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Role of the Bronchodilator Test Defined by the Forced Vital Capacity in Chronic Obstructive Pulmonary Disease Phenotyping
Authors Zhang X, Wu Z , Liu Y , Jiang S
Received 7 March 2020
Accepted for publication 6 May 2020
Published 28 May 2020 Volume 2020:15 Pages 1199—1206
DOI https://doi.org/10.2147/COPD.S252902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chunxue Bai
Xiaoling Zhang,1,* Zhenchao Wu,2– 4,* Yi Liu,1,2,4 Shujuan Jiang1,4
1Department of Pulmonary and Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong University, Jinan 250021, Shandong, People’s Republic of China; 2Department of Pulmonary and Critical Care Medicine, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan 250000, Shandong, People’s Republic of China; 3Department of Pulmonary and Critical Care Medicine, The Third Affiliated Hospital of Shandong First Medical University, Jinan 250000, Shandong, People’s Republic of China; 4Shandong Key Laboratory of Infectious Respiratory Disease, Jinan 250000, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shujuan Jiang Email [email protected]
Purpose: In clinical practice, some chronic obstructive pulmonary disease (COPD) patients experienced a remarkable increase in forced vital capacity (FVC) after bronchodilator administration, whereas forced expiratory volume in the first second (FEV1) remains substantially unchanged. We assume this may relate to airway inflammatory type. We aim to analyze the clinical characteristics and explore the usefulness of the bronchodilator test, especially FVC, in this new COPD phenotype.
Patients and Methods: A total of 346 COPD patients with exacerbation who underwent bronchodilator tests, fractional exhaled nitric oxide (FeNO) measurements and blood eosinophil counts were analyzed. The characteristics, FeNO levels, and blood eosinophil counts were compared between patients with and without significant bronchodilator responsiveness in terms of FVC.
Results: Patients with significant FVC responsiveness displayed poorer lung function and higher FeNO levels compared with those without considerable FVC responsiveness (Z= − 5.042 to − 0.375, p=0.000– 0.022). There is a discernible linear relationship between FeNO levels and FVC responsiveness to bronchodilator use (r=0.251, P=0.001). The application of bronchodilator responsiveness of FVC for detecting high FeNO levels in COPD patients exhibited relatively high sensitivity (61.8%) and specificity (86.7%).
Conclusion: We demonstrated that COPD patients with significant FVC responsiveness had higher FeNO levels than non-responders and established a simple method for detecting high FeNO values. FVC responders may be identified as a separate group of COPD patients.
Keywords: COPD, pulmonary function test, bronchodilator responsiveness, forced vital capacity, fractional exhaled nitric oxide
Introduction
Chronic obstructive pulmonary disease (COPD) is a disease characterized by persistent airflow obstruction, which is not fully reversible and is usually progressive and is associated with elevated chronic inflammatory responses to noxious particles or gases in the airways.1 The prevalence of COPD is skyrocketing, and it is expected to be the world’s third most fatal disease by 2030.2
COPD is heterogeneous in nature. COPD patients with similar forced expiratory volume in the first second (FEV1) display different clinical manifestations, quality of life, exacerbation frequencies, and responses to treatment.3 Nowadays, COPD phenotyping is increasingly becoming a topic of interest to more and more physicians aiming at classifying patients into distinct clinical subgroups to initiate appropriate individualized care which may lead to a better prognosis and quality of life for patients.4,5
The bronchodilator test plays a crucial role in the management of COPD, eg, the diagnosis of COPD is confirmed in cases with FEV1/FVC ratios below 0.7 after bronchodilator administration. Flow response after bronchodilator inhalation is universally and frequently used as an indicator for assessing responsiveness in general practice. However, in moderate to severe COPD cases, flow response may be poor, yet the response in terms of volume can be relatively favorable, which is probably due to a reduction in hyperinflation.6–9 It appears that patients with significant FVC responsiveness are apt to progress to more severe COPD stages.
Airway hyperresponsiveness is associated with airway inflammatory, especially Th2 cell-induced eosinophilic inflammation. Recent research had proved eosinophilic inflammation dominated COPD is an important COPD phenotype. Its clinical characteristics are airway responsiveness (the change of FEV1), higher eosinophil counts, and higher FeNO concentrations. Although some studies were focused on the role of the change of FEV1 value after bronchodilator inhalation, but there are rarely studies about the role of the change of FVC value in COPD, and we are not clear about the relationship between the change and FeNO or eosinophil.
On these grounds, this study’s objectives were as follows: 1) to compare the characteristics of COPD patients classified as FVC responders and non-responders during the bronchodilator test and 2) to evaluate the benefits of the bronchodilator test in terms of FVC in COPD phenotype.
Patients and Methods
Patients
A total of 346 patients with COPD exacerbation were sequentially enrolled from June 2017 to August 2019. All patients were outpatients from Respiratory Clinic of Shandong Provincial Hospital. They were definitely diagnosed with COPD by spirometry based on the Global initiative for chronic obstructive lung disease (GOLD 2017) criteria.1 COPD exacerbations are defined as an acute worsening of respiratory symptoms that result in additional therapy.1 COPD patients simultaneously diagnosed with lung cancer, interstitial pulmonary disease, asthma, bronchiectasis, history of pulmonary resection, or significant comorbidities other than COPD that might influence the ability to perform pulmonary function tests, fractional exhaled nitric oxide (FeNO) measurements and blood eosinophil counts were excluded.
Observational Indicators
Basic medical information: gender, age, height and weight, smoking history were recorded. Smoking history: patients who still smoking without quitting smoking were defined current smokers; patients who formerly had smoking history, but have quitted smoking more than 3 months were defined ex-smokers.
Exhaled nitric oxide measurements (FeNO): FeNO levels were assessed according to the ATS/ERS guidelines using a NO analyzer (SV-eCO-01, Sunvon, China).10 The subjects were informed to deeply inhale NO-free air and immediately fully exhale via a mouthpiece at a constant flow rate (50 mL/s) for 10 s. Every FeNO measurement was acquired before the reversibility tests in order to prevent spirometry from influencing the FeNO levels.
Pulmonary function test (PFT): The bronchodilator tests were conducted according to the American Thoracic Society (ATS) and the European Respiratory Society (ERS) guidelines.11–13 Patients received good technique-conducting and tested by a unified and qualified equipment (Master Screen Body, Jaeger, Germany). The second time FVC maneuver was performed, similar to the first one, 15 minutes after the administration of 400 μg of albuterol (Ventolin, GlaxoSmithKline) via a metered-dose inhaler. Changes in the FEV1 were expressed as 1) An absolute change relative to the baseline (ΔFEV1) and 2) A percentage change relative to the baseline (ΔFEV1%baseline). Changes in the FVC were expressed in a similar manner. Predicted values of spirometry were calculated from the reference equations published by Zheng and Zhong.14 The bronchodilator test was considered significant if an increase in either FEV1 or FVC≥12% was observed and ≥200 mL as an absolute value compared with the baseline.15
Blood Eosinophil counts (EOS): Blood eosinophil and total white blood cell counts were obtained by blood routine test using an Automated Cell Counter (Sysmex, Kobe, Japan).
Before the above tests, both physician and examination operator should inquire the patients smoking history and verify the patients had no medication history within 3 days as bronchodilators, corticosteroids. All three tests were performed on the same day. Otherwise, patients were excluded from this study.
Study Design
Based on a previous study which is about Exhaled nitric oxide, systemic inflammation, and the spirometry response to inhaled fluticasone propionate in severe COPD patients.16 We assumed a population mean of pre-bronchodilator FVC is 2.61L and sample mean is 2.44L. The study was powered (alpha of 0.05 and beta of 0.20) for pre-bronchodilator FVC and the change in FVC after the administration of 400 μg of albuterol to detect this difference with a level of confidence of 95%. This resulted in a sample size calculation of 169 patients, allowing for 20% of consented subjects to either fail spirometry screening.
All of the 346 COPD patients were divided into two groups according to the FVC changes in response to albuterol administration including: (I) positive FVC group (PFVC): Patients with ΔFVC≥200 mL and ΔFVC%baseline≥12%; (II) negative FVC group (NFVC): referring to the rest of the patients without significant responsiveness in terms of FVC. Next, patients with ΔFEV1≥200 mL and ΔFEV1%baseline≥12% were excluded and the remaining 180 patients then were separated into two groups in a similar way: (I) pure positive FVC group (PPFVC): composed of patients with ΔFVC≥200 mL and ΔFVC%baseline≥12% without significant responsiveness with respect to FEV1; (II) negative FVC-negative FEV1 group (NNFVC): comprised of patients without significant responsiveness neither in terms of FVC nor FEV1.
The study was approved by the Shandong Provincial Hospital Affiliated to Shandong University Ethics Committee (No. 2016–23). This action did not produce any potential harm to patients. This operation was strictly obeyed with the Helsinki Declaration. All patients have signed an informed consent. All patient information was kept confidential.
Statistical Analysis
The data were analyzed using the SPSS 22.0 software (SPSS Inc., Chicago, IL, USA). Qualitative variables were expressed as proportions and numbers, whereas quantitative variables were expressed as median (interquartile range, IQR) (non-normal distribution). Comparisons between PFVC and NFVC, PPFVC and NNFVC were investigated using the Mann–Whitney U-test or χ2-square test where appropriate. The Spearman correlation was used to evaluate the relationships between the FeNO concentration and bronchodilator responsiveness vis-a-vis FVC and FEV1. Receiver operating characteristic curves were generated for selected cutoff values of bronchodilator responsiveness in terms of FVC to detect high FeNO values. P<0.05 was considered statistically significant.
Results
Baseline Characteristics of COPD Patients with or Without Significant Responsiveness in Terms of FVC
All the 346 patients with COPD were recruited in this study, the mean age was 59.3±10.5 years, 232 (67.1%) were males and 114 (32.9%) were females. The mean age, sex, and body mass index (BMI) were similar within each subgroup. Patients with significant responsiveness related to the FVC had lower baseline FVC values, FEV1 values, FEV1/FVC ratios, forced expiratory flows at 75% of the FVC (MEF75), forced expiratory flows at 50% of the FVC (MEF50), forced expiratory flows at 25% of the FVC (MEF25), maximal mid-expiratory flows (MMEF), and higher baseline residual volume/total lung capacity (RV/TLC) ratios, compared with who were without significant FVC responsiveness (Z= −5.042 to -0.375, p=0.000–0.008) (Table 1).
 |
Table 1 The Clinical Characteristics of COPD Patients |
FeNO Levels and Blood Eosinophil Counts of Patients with or Without Significant Responsiveness in Terms of FVC
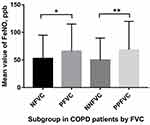
A significant difference in FeNO values was observed between the subgroups (Figure 1). For the entire cohort, FeNO levels in patients with significant FVC responsiveness were significantly higher than the NFVC group (Z=−2.284, p=0.022); Similar tendency on parameters was observed in the PPFVC and NNFVC groups (Z=−2.588, p=0.010) (Table 1).
There is no significant discrepancy observed regarding the blood eosinophil counts between the subgroups (Table 1).
Cut-off Values of FVC Responsiveness Used to Detect High FeNO Levels
For the entire sample (NFVC and PFVC groups), positive correlation was depicted between FeNO and ΔFVC (r=0.216, P=0.000), ΔFVC%baseline (r=0.154, P=0.004) (Figure 2). The correlation between FeNO and ΔFEV1 (r=0.071, P=0.186) or ΔFEV1%baseline (r=0.040, P=0.459) were not significant. Excluded the patients with significant responsiveness in terms of FEV1, concerning the NNFVC and PPFVC groups, linear relationships between FeNO levels and ΔFVC (r=0.251, P=0.001), ΔFVC%baseline (r=0.190, P=0.011) (Figure 2) were equally noticed. Meanwhile, the relationships between FeNO and ΔFEV1 (r=−0.002, P=0.980) or ΔFEV1%baseline (r=−0.047, P=0.535) were also no difference.
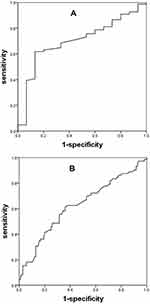
Regarding the NNFVC and PPFVC groups population, the area under the receiver operating characteristic curve (AUC) for detecting FeNO levels ≥25 parts per billion (ppb) with ΔFVC was calculated as 0.701 (p=0.010), the AUC for detecting FeNO levels ≥50ppb with ΔFVC was determined to be 0.623 (p=0.005) (Figure 3). The ΔFVC cutoff values with the best sensitivity and specificity were selected according to the Youden index: 0.165 L of ΔFVC with a sensitivity of 61.8% and a specificity of 86.7% for detecting FeNO levels ≥25 ppb, 0.205 L of ΔFVC with a sensitivity of 61.1% and a specificity of 63.9% for detecting FeNO levels ≥50 ppb.
Discussion
This study’s principal finding can be stated as follows: 1) FeNO concentrations in COPD patients were significantly and positively correlated with ΔFVC; 2) The application of bronchodilator responsiveness in terms of FVC to detect high FeNO levels in COPD cases is a comparatively simple method with relatively high sensitivity and specificity; 3) FVC responders exhibited poorer baseline lung functions than non-responders. Chen et al reported that RV/TLC% predictions were positively correlated with ΔFVC (r=0.386) and ΔFVC%baseline (r=0.495), using of FVC bronchodilator responsiveness in detecting hyperinflation in COPD patients demonstrated high sensitivities and specificities.8 As showed in this study, RV/TLC values were elevated and were found to be higher in FVC responders than non-responders. This may be partly accounted for by the higher degree of hyperinflation, which airway obstruction made, in FVC responders.
COPD patients on the mild side of the severity spectrum differ from those on the severe side regarding the relation between their FEV1 and FVC responses to bronchodilator inhalation. Bronchodilator response of advanced lung function parameters depends on COPD severity.17 In more severe cases of COPD patients, FVC remarkably rises after bronchodilator administration whereas FEV1 remains substantially constant,7 isolated volume response to bronchodilators is a characteristic of severe emphysema in COPD patients.6,9,18 In other words, FVC responders may suffer from more severe airway hyperresponsiveness, airway obstruction, and emphysema. It is well known that COPD is an inflammatory disease affecting both the airways and the lungs, which induces the release of numerous proinflammatory cytokines and mediators. This cascade of events might include the synthesis of induced nitric oxide synthase (iNOS) by macrophages, epithelium, and vascular smooth muscles in the airway.19 We speculated that FVC responders with more severe airway hyperresponsiveness, airway obstruction, and emphysema sustained more severe airway inflammation or airway eosinophilic inflammation which in turn elevated their FeNO levels, considering that excessive inflammation plays an important pathophysiologic role in the progress of COPD. The magnitude of change in the FVC after bronchodilator administration may to some extent reflect the degree of the undergoing inflammatory process in COPD. Another possible explanation is the decreased pulmonary diffusing capacity of NO due to irreversible structural defects caused by emphysema.20,21
Airway inflammation is a consistent feature of COPD. Despite the fact that neutrophilic infiltration is the most common inflammatory phenotype in COPD, eosinophilic infiltration does exist in a subset of COPD patients, even after the careful exclusion of patients with any features of asthma.22 Singh et al revealed that 37% of COPD patients have eosinophilic airway inflammation.23 One possible explanatory hypothesis is that smoking and other mechanisms that recruit neutrophils into the airway mucosa in COPD also cause a certain degree of eosinophil influx.22 Furthermore, these patients exhibit the greater response to corticosteroid therapy.22,24 Eosinophilic COPD appears to be a distinct patient subgroup with an increased corticosteroid response and better prognoses.23
Identifying COPD patients with an eosinophilic inflammation may be of great help in COPD management. A positive correlation between FeNO levels and eosinophilia has been confirmed in induced sputum and bronchoalveolar lavage.25 Additionally, it has been considered a marker for eosinophilic airway inflammation, especially in asthma.25 For COPD, similar outcomes have been demonstrated in previous researches.26,27 But considered convenience and comfort for patients, a safe and simple test to reflex the inflammatory type and level is necessary. So, the research about the relationship between FVC and FeNO had a practical meaning. Soter et al proved that FeNO values were reliable surrogate markers of eosinophilic inflammation in COPD patients. There was a significant positive correlation between the percentage of sputum eosinophils and FeNO concentrations both during exacerbations (r=0.593, p<0.001) and hospital discharge (r=0.337, p=0.044).26 FeNO had a positive relationship with COPD severity and eosinophilic inflammation level.28,29 Moreover, COPD patients with higher levels of FeNO had a better responsiveness to corticosteroid therapy.27,30 In summary, FeNO measurements in COPD patients may help to improve the classification of patients into the aforementioned subclinical categories and promote more personalized treatment regimens.31
Frankly, there some limitations in this study. FeNO levels in our data seem higher than in previous studies.32 One explanation for this disparity may be the variations in population samples. As high as 52.3% (181/346) of patients in this study had peripheral blood eosinophil counts ≥2%. Due to this study’s shortage of clinical data, it is possible that its cohort included some patients with the so-called “Asthma–COPD overlap” phenotype.33 It may be one reason for higher FeNO concentrations and higher eosinophil counts percent. One thing to note, there is no difference between NFVC and PFVC (or NNFVC and PPFVC) group in EOS indicators. We think COPD is airway inflammatory disease, it would display the lesion locally first and significant, compared with general lesion, so the change of FVC or FeNO would be more evident than peripheral blood eosinophil counts. In the next research we will cover this limitation and observe the relationship between airway eosinophilic inflammation level and airway obstruction and iNOS. Besides, FeNO measurements can be affected by a number of factors, including different smoking histories, inhaled corticosteroid use, diet, different methodologies for measurement of exhaled NO levels, anthropometric variables, and so on.34 All the above factors might induce variability and limit the comparison between studies. We will pay more attention on the COPD rat model to detect the iNOS level, eosinophil counts in airway epithelium and smooth muscle, test the lung function of COPD rat model, study the mechanism of role of iNOS in airway hyperresponsiveness, airway obstruction, and emphysema in further research.
Conclusion
In conclusion, as far as we know this is the first time that linear relationships were demonstrated between FeNO levels and FVC responsiveness to bronchodilators. Since FeNO is useful for identifying eosinophilic inflammation responding better to corticosteroid therapy, these data may have pertinent implications for the management of COPD patients. Considering the relationship between ΔFVC and emphysema, FVC responders may be identified as a different group of COPD patients. The usefulness of the bronchodilator test with respect to the FVC in the clinical management of COPD should be evaluated.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi:10.1183/13993003.00164-2019
2. Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442. doi:10.1371/journal.pmed.0030442
3. Vestbo J, Anderson W, Coxson HO, et al. Evaluation of COPD longitudinally to identify predictive surrogate end-points (ECLIPSE). Eur Respir J. 2008;31(4):869–873. doi:10.1183/09031936.00111707
4. Han MK, Agusti A, Calverley PM, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. Am J Respir Crit Care Med. 2010;182(5):598–604. doi:10.1164/rccm.200912-1843CC
5. Burgel PR, Paillasseur JL, Caillaud D, et al. Clinical COPD phenotypes: a novel approach using principal component and cluster analyses. Eur Respir J. 2010;36(3):531–539. doi:10.1183/09031936.00175109
6. O’Donnell DE, Forkert L, Webb KA. Evaluation of bronchodilator responses in patients with “irreversible” emphysema. Eur Respir J. 2001;18(6):914–920. doi:10.1183/09031936.01.00216501
7. Schermer T, Heijdra Y, Zadel S, et al. Flow and volume responses after routine salbutamol reversibility testing in mild to very severe COPD. Respir Med. 2007;101(6):1355–1362. doi:10.1016/j.rmed.2006.09.024
8. Chen C, Jian W, Gao Y, Xie Y, Song Y, Zheng J. Early COPD patients with lung hyperinflation associated with poorer lung function but better bronchodilator responsiveness. Int J Chron Obstruct Pulmon Dis. 2016;11:2519–2526. doi:10.2147/copd.S110021
9. Quanjer PH, Ruppel GL, Langhammer A, et al. Bronchodilator response in FVC is larger and more relevant than in FEV1 in severe airflow obstruction. Chest. 2017;151(5):1088–1098. doi:10.1016/j.chest.2016.12.017
10. Exhaled NO. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912–930. doi:10.1164/rccm.200406-710ST
11. Miller MR, Hankinson JA, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi:10.1183/09031936.05.00034805
12. Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi:10.1183/09031936.05.00035005
13. Macintyre N, Crapo RO, Viegi G, et al. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26(4):720–735. doi:10.1183/09031936.05.00034905
14. Zheng J, Zhong N. Normative values of pulmonary function testing in Chinese adults. Chin Med J (Engl). 2002;115(1):50–54.
15. Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi:10.1183/09031936.05.00035205
16. Kunisaki KM, Rice KL, Janoff EN, Rector TS, Niewoehner DE. Exhaled nitric oxide, systemic inflammation, and the spirometric response to inhaled fluticasone propionate in severe chronic obstructive pulmonary disease: a prospective study. Ther Adv Respir Dis. 2008;2(2):55–64. doi:10.1177/1753465808088902
17. Jarenback L, Eriksson G, Peterson S, Ankerst J, Bjermer L, Tufvesson E. Bronchodilator response of advanced lung function parameters depending on COPD severity. Int J Chron Obstruct Pulmon Dis. 2016;11:2939–2950. doi:10.2147/copd.S111573
18. Cerveri I, Pellegrino R, Dore R, et al. Mechanisms for isolated volume response to a bronchodilator in patients with COPD. J Appl Physiol (1985). 2000;88(6):1989–1995. doi:10.1152/jappl.2000.88.6.1989
19. Brindicci C, Kharitonov SA, Ito M, et al. Nitric oxide synthase isoenzyme expression and activity in peripheral lung tissue of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(1):21–30. doi:10.1164/rccm.200904-0493OC
20. Yang X, Tang X, Cao Y, et al. The bronchiectasis in COPD-OSA overlap syndrome patients. Int J Chron Obstruct Pulmon Dis. 2020;15:605–611. doi:10.2147/copd.S243429
21. Lu Z, Huang W, Wang L, Xu N, Ding Q, Cao C. Exhaled nitric oxide in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:2695–2705. doi:10.2147/copd.S165780
22. Brightling CE, Monteiro W, Ward R, et al. Sputum eosinophilia and short-term response to prednisolone in chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2000;356(9240):1480–1485. doi:10.1016/s0140-6736(00)02872-5
23. Singh D, Kolsum U, Brightling CE, Locantore N, Agusti A, Tal-Singer R. Eosinophilic inflammation in COPD: prevalence and clinical characteristics. Eur Respir J. 2014;44(6):1697–1700. doi:10.1183/09031936.00162414
24. Leigh R, Pizzichini MM, Morris MM, Maltais F, Hargreave FE, Pizzichini E. Stable COPD: predicting benefit from high-dose inhaled corticosteroid treatment. Eur Respir J. 2006;27(5):964–971. doi:10.1183/09031936.06.00072105
25. Dweik RA, Boggs PB, Erzurum SC, et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184(5):602–615. doi:10.1164/rccm.9120-11ST
26. Soter S, Barta I, Antus B. Predicting sputum eosinophilia in exacerbations of COPD using exhaled nitric oxide. Inflammation. 2013;36(5):1178–1185. doi:10.1007/s10753-013-9653-8
27. Gao J, Zhang M, Zhou L, et al. Correlation between fractional exhaled nitric oxide and sputum eosinophilia in exacerbations of COPD. Int J Chron Obstruct Pulmon Dis. 2017;12:1287–1293. doi:10.2147/copd.S134998
28. Lazar Z, Kelemen A, Galffy G, Losonczy G, Horvath I, Bikov A. Central and peripheral airway nitric oxide in patients with stable and exacerbated chronic obstructive pulmonary disease. J Breath Res. 2018;12(3):036017. doi:10.1088/1752-7163/aac10a
29. Tang B, Huang D, Wang J, Luo LL, Li QG. Relationship of blood eosinophils with fractional exhaled nitric oxide and pulmonary function parameters in chronic obstructive pulmonary disease (COPD) exacerbation. Med Sci Monit. 2020;26:e921182. doi:10.12659/msm.921182
30. Dummer JF, Epton MJ, Cowan JO, et al. Predicting corticosteroid response in chronic obstructive pulmonary disease using exhaled nitric oxide. Am J Respir Crit Care Med. 2009;180(9):846–852. doi:10.1164/rccm.200905-0685OC
31. Alcazar-Navarrete B, Romero-Palacios PJ, Ruiz-Sancho A, Ruiz-Rodriguez O. Diagnostic performance of the measurement of nitric oxide in exhaled air in the diagnosis of COPD phenotypes. Nitric Oxide. 2016;54:67–72. doi:10.1016/j.niox.2016.02.003
32. Alcazar-Navarrete B, Ruiz Rodriguez O, Conde Baena P, Romero Palacios PJ, Agusti A. Persistently elevated exhaled nitric oxide fraction is associated with increased risk of exacerbation in COPD. Eur Respir J. 2018;51(1):1701457. doi:10.1183/13993003.01457-2017
33. Takayama Y, Ohnishi H, Ogasawara F, Oyama K, Kubota T, Yokoyama A. Clinical utility of fractional exhaled nitric oxide and blood eosinophils counts in the diagnosis of asthma-COPD overlap. Int J Chron Obstruct Pulmon Dis. 2018;13:2525–2532. doi:10.2147/copd.S167600
34. Dressel H, de la Motte D, Reichert J, et al. Exhaled nitric oxide: independent effects of atopy, smoking, respiratory tract infection, gender and height. Respir Med. 2008;102(7):962–969. doi:10.1016/j.rmed.2008.02.012
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.