Back to Journals » Drug Design, Development and Therapy » Volume 9
Role of sodium tungstate as a potential antiplatelet agent
Authors Fernández-Ruiz R, Pino M, Hurtado B, García de Frutos P, Caballo C, Escolar G, Gomis R, Diaz-Ricart M
Received 8 November 2014
Accepted for publication 21 January 2015
Published 26 May 2015 Volume 2015:9 Pages 2777—2786
DOI https://doi.org/10.2147/DDDT.S77221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Shu-Feng Zhou
Rebeca Fernández-Ruiz,1,2 Marc Pino,3 Begoña Hurtado,4 Pablo García de Frutos,4 Carolina Caballo,3 Ginés Escolar,3 Ramón Gomis,1,2,5 Maribel Diaz-Ricart3
1Diabetes and Obesity Research Laboratory, Institut d'Investigacions Biomediques August Pi i Sunyer (IDIBAPS), Rosellón, Barcelona, 2Centro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas, Barcelona, 3Hemotherapy–Hemostasis, Hospital Clínic, Universidad de Barcelona, IDIBAPS, Villarroel, Barcelona, 4Institutode Investigaciones Biomédicas de Barcelona, Consejo Superior de Investigaciones Científicas, Institut d’Investigacions Biomediques August Pi i Sunyer, Rosellón, Barcelona, 5Hospital Clinic, Universitat de Barcelona, Villarroel, Barcelona, Spain
Purpose: Platelet inhibition is a key strategy in the management of atherothrombosis. However, the large variability in response to current strategies leads to the search for alternative inhibitors. The antiplatelet effect of the inorganic salt sodium tungstate (Na2O4W), a protein tyrosine phosphatase 1B (PTP1B) inhibitor, has been investigated in this study.
Methods: Wild-type (WT) and PTP1B knockout (PTP1B-/-) mice were treated for 1 week with Na2O4W to study platelet function with the platelet function analyzer PFA-100, a cone-and-plate analyzer, a flat perfusion chamber, and thrombus formation in vivo. Human blood aliquots were incubated with Na2O4W for 1 hour to measure platelet function using the PFA-100 and the annular perfusion chamber. Aggregometry and thromboelastometry were also performed.
Results: In WT mice, Na2O4W treatment prolonged closure times in the PFA-100 and decreased the surface covered (%SC) by platelets on collagen. Thrombi formed in a thrombosis mice model were smaller in animals treated with Na2O4W (4.6±0.7 mg vs 8.9±0.7 mg; P<0.001). Results with Na2O4W were similar to those in untreated PTP1B-/- mice (5.0±0.3 mg). Treatment of the PTP1B-/- mice with Na2O4W modified only slightly this response. In human blood, a dose-dependent effect was observed. At 200 µM, closure times in the PFA-100 were prolonged. On denuded vessels, %SC and thrombi formation (%T) decreased with Na2O4W. Neither the aggregating response nor the viscoelastic clot properties were affected.
Conclusion: Na2O4W decreases consistently the hemostatic capacity of platelets, inhibiting their adhesive and cohesive properties under flow conditions in mice and in human blood, resulting in smaller thrombi. Although Na2O4W may be acting on platelet PTP1B, other potential targets should not be disregarded.
Keywords: sodium tungstate, protein tyrosine phosphatase 1B, platelet adhesion, antiplatelet agents
Introduction
Atherothrombosis remains the leading cause of morbidity and mortality in Western society. Platelets play a key role in hemostasis, but they are also responsible for the pathologic thrombus formation underlying the clinical manifestations of acute atherothrombotic vascular disease.1 Therefore, modulation of platelet activation is a main goal for the development of pharmacological strategies to prevent the occurrence of cardiovascular accidents. Platelet activation occurs through multiple pathways and current agents do not interfere with all of them.
In patients suffering from acute coronary syndromes or undergoing percutaneous coronary intervention, oral antiplatelet treatment is routinely administered to inhibit platelet-mediated thrombus formation and the subsequent vessel occlusion. While currently available oral antiplatelet agents such as aspirin and P2Y12 adenosine 5′-diphosphate (ADP) receptor antagonists reduce the incidence of ischemic events, the residual risk for morbidity and mortality remains substantially elevated.2,3 Interindividual response variability to aspirin and, especially, to clopidogrel makes it difficult to find the appropriate balance between the risk of thrombosis and bleeding in association with the treatment with these compounds.4–7 Therefore, there is continuous search for new alternatives providing more rapid and consistent platelet inhibition.
Sodium tungstate (Na2O4W) has been demonstrated to be effective in normalizing blood glucose levels and in decreasing the body weight gain and adiposity in animal models of diabetes8 and obesity. Na2O4W has been shown to increase the expression of the uncoupling protein 1 (UCP1) and 3 (UCP3) genes,8,9 implicated in the control of adaptive thermogenesis, the production of reactive oxygen species by mitochondria, the regulation of ATP synthesis, and the regulation of fatty acid oxidation. Na2O4W was patented as an antiobesity agent. In three different phase I studies in healthy volunteers, Na2O4W demonstrated a very wide tolerance window for a 6-week period. In a phase II study in obese patients under treatment with Na2O4W for 6 weeks, the antiobesity effect was low although positive.10 Tungstate shares molecular similarities with vanadate (VO43−), both being salts of trace transition metals in biological systems, with insulin-mimetic effects.11 Vanadate and Na2O4W are phosphate analogs and they act as protein tyrosine phosphatase (PTP) inhibitors. Because vanadate has been shown to inhibit PTP1B, it would be plausible to think that Na2O4W could also exhibit a similar effect.12 PTP1B plays a major role in the activation of platelets13 and is required for normal platelet thrombus formation in living mice.14 In this regard, we have indirect in vitro evidence of a potential effect of Na2O4W on platelet function.
The aim of the present study was to investigate the potential effect of Na2O4W as an antiplatelet agent using different approaches aimed to evaluate the adhesive and cohesive properties of platelets. Primary hemostasis was evaluated in wild-type (WT) and PTP1B knockout mice, after a week of treatment with Na2WO4 in drinking water. In vitro studies were also carried out in human blood samples treated with Na2O4W.
Materials and methods
Experimental design
Studies were designed to evaluate the effect of Na2WO4 on platelet function. WT and PTP1B knockout (PTP1B−/−) mice15,16 were treated for a week with Na2WO4 (2 g/L in drinking water). To analyze platelet function under flow conditions, four different approaches were applied: the platelet function analyzer PFA-100, the cone-and-plate analyzer (CPA), a small flat perfusion chamber, and the inferior vein cava (IVC) ligation model (an in vivo model that evaluates the pathophysiology of deep vein thrombosis in mice).
Studies on human blood were performed in vitro with either whole blood or platelet-rich plasma (PRP) incubated with Na2WO4. The effect of different concentrations of Na2WO4 (100 μM, 200 μM, and 400 μM, for 1 hour at 37°C) on platelet function was evaluated using the PFA-100 and the annular perfusion chamber. The concentration of 200 μM was then applied for aggregometry and thromboelastometry.
Studies on mice
All the experiments were performed in 8-week-old male mice littermates, of a mixed C57BL/6J×129 background. Mice were housed under standard conditions of light (12-hour light/dark cycles) and temperature (21°C). Animals were fed ad libitum with standard chow diet (type A04 from Panlab, Barcelona, Spain) and received 2 g/L Na2O4W for a week. All animal procedures were approved by the Animal Ethics/Research Committee of the University of Barcelona, and principles of laboratory animal care were followed.
Animals were anesthetized with an intraperitoneal injection of ketamine–xylazine, and intracardiac puncture was performed to obtain approximately 800 μL of blood anticoagulated with 110 mM trisodium citrate (1:10, vol/vol).
Hemostatic capacity in the PFA-100
The PFA-100 can be considered a substitute for the classic bleeding time test. The system monitors platelet interaction on membranes coated with collagen–ADP (Col–ADP) and collagen–epinephrine (Col–Epi).17 The system comprises a microprocessor-controlled instrument and a disposable test cartridge containing a biologically active membrane. The instrument aspirates a blood sample (citrated blood) under constant vacuum from the sample reservoir through a capillary and a microscopic aperture cut into the membrane. The membrane is coated with Col–Epi or Col–ADP. The presence of these biochemical stimuli, as well as the high shear rates generated under the standardized flow conditions, result in platelet attachment, activation, and aggregation, slowly building a stable platelet plug at the aperture. The time required to obtain full occlusion of the aperture is reported as the “closure time,” which is expressed in seconds.
CPA testing
The CPA was used as previously described.18 The device tests platelet adhesion and aggregation in citrated whole blood under arterial flow conditions (1,800/second for 2 minutes). Blood is in contact with the polystyrene surface of a well by using laminar flow. Three parameters were evaluated: the surface covered by platelets (expressed in percentage, %SC), the average size of the polystyrene-bound platelet clusters or platelet aggregates (AS, μm2), and the number of platelet groups (OB, n).
Experiments in flat perfusion devices
The interaction of mouse platelets with collagen-coated coverslips (100 μg per coverslip) was explored in a low-volume flat perfusion device. Citrated blood samples were perfused for 5 minutes at a shear rate of 1,200/second adjusted with a peristaltic pump (Renal Systems, Minneapolis, MN, USA).
Perfused surfaces were fixed (2.5% glutaraldehyde in 0.15 M phosphate-buffered saline [PBS], 4°C, 24 hours), stained with toluidine blue (0.02%), and platelet interactions evaluated by light microscopy. Morphometric analysis was performed using a computerized system (Image J), and platelet interactions were globally expressed as the percentage of the surface covered by platelets (%SC).
In vivo thrombosis model
IVC ligation model was used as previously described.19 Briefly, mice (n=8 for each group) were anesthetized with a 2% mixture of isoflurane–oxygen and placed in a supine position. After laparotomy, intestines were exteriorized and sterile saline was applied during the whole procedure to prevent drying. Posterior venous branches were cauterized and all visible side branches (usually one or two) were ligated. Then, gentle separation from aorta was performed and IVC was ligated using a 7-0 polypropylene suture immediately below the renal veins to obtain complete blood stasis. After surgery, peritoneum and skin were closed with a 6.0 mm-size monofilament suture. Mice were euthanized after 3 hours, and the vessel just below the renal veins and proximal to the confluence of the common iliac veins was excised. Mice showing any sign of tissue injury during surgery were excluded from further analysis.
Studies with human blood
Blood sampling
Blood was drawn from healthy volunteers who had not ingested drugs affecting platelet function in the previous 10 days. All donors gave their written informed consent to participate in the study. The study was performed in agreement with the Declaration of Helsinki and was approved by the Ethical Committee of the Hospital Clinic (Barcelona). Blood was anticoagulated with 110 mM trisodium citrate (1:10, vol/vol) and incubated with different concentrations of Na2WO4 (0 μM, 100 μM, 200 μM, and 400 μM) for 1 hour at 37°C.
Experiments in annular perfusion devices
Aorta segments of enzymatically denuded New Zealand rabbits were mounted inverted on the central plastic rod of the perfusion chamber.20 Citrated blood samples were recirculated for 10 minutes at shear rates of 800/second and 1,200/second adjusted with a peristaltic pump (Renal Systems). Perfused surfaces were rinsed with 0.15 M PBS, fixed (2.5% glutaraldehyde in 0.15 M PBS, 4°C, 24 hours), dehydrated with a raising ethanol gradient, embedded in JB-4, thin sectioned for light microscopy, and stained with methylene blue. Platelet interactions were evaluated with light microscopy and a software21 that quantifies platelet coverage – expressed as percentage (%SC) – and calculates interactions higher than 5 μm (%T).
Aggregation studies using turbidimetric techniques
PRP was obtained by centrifugation of whole blood (120 g, 15 minutes). PRP aliquots were incubated with Na2O4W by gentle stirring in a conventional aggregometer (Aggrecorder PA 3210 aggregometer; Menarini Diagnostic, Firenze, Italy). Platelets were activated with arachidonic acid (AA, 1.6 mM), ADP (4 μM and 2 μM), collagen (Col, 5 μg/mL), and ristocetin (R, 1 mg/mL) (10 minutes, 37°C) under stirring. Changes in turbidimetric patterns were registered and results expressed as percentage maximal aggregation.22
Thromboelastometry studies
Dynamic thrombelastography of whole-blood coagulation was explored using the EXTEM, INTEM, and FIBTEM tests in the rotational thromboelastometry analyzer (PentapharmGmbH, Munich, Germany).23 EXTEM uses tissue factor as activator and measures changes in the extrinsic pathway of coagulation, fibrinogen and fibrin polymerization, and platelet function. In the FIBTEM test, platelet function is eliminated with cytochalasin D, obtaining a fibrin clot. The INTEM test uses ellagic acid to measure changes in the intrinsic pathway of coagulation.
Clotting time, clot formation time, clot amplitude after 10 minutes (A10), maximum velocity (MaxV), and time to maximum clot formation velocity (MaxV-t) were evaluated. The clotting time and the clot formation time indicate the dynamics of clot formation. The clot amplitude gives information about clot strength and stability, largely dependent on fibrinogen and platelets, and MaxV and MaxV-t express the clot formation velocity.
Statistics
Results are expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed with raw data using Student’s t-tests for paired and unpaired samples. The results were considered significant at P<0.05.
Results
Effect of sodium tungstate on mouse platelets
Platelet function in the PFA-100
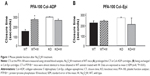
In WT mice (n=8), closure times were 148±17.4 seconds and 211±18 seconds when using the Col–ADP and Col–Epi cartridges, respectively. Closure times in the Col–Epi cartridges were already prolonged in untreated animals and no significant additional effect of Na2O4W was detected (227±10 seconds). In Col–ADP cartridges, Na2O4W treatment significantly prolonged the closure time up to 272.8±12.4 seconds (P<0.01) (Figure 1).
In PTP1B−/− mice (n=8), closure times in untreated animals were 292.9±4.5 seconds and 256±10 seconds when using the Col–ADP and Col–Epi cartridges, respectively. In Na2O4W-treated mice, closure times did not significantly differ either in the Col–ADP cartridge (258.6±13.3 seconds) or in the Col–Epi cartridge (196.6±61 seconds) groups (Figure 1).
Platelet interaction in the CPA
In WT animals (n=8), SC (%), AS (μm2), and OB (n) in the absence of Na2O4W were 1.40%±0.39%, 32.9±1.82 μm2, and 47.9±11.7, respectively. After treatment, both SC and OB increased significantly to 4.5%±1.4% and 783.1±229.4, respectively (P<0.05); AS decreased significantly to 22.2±0.8 μm2 (P<0.05) (Figure 2). The increased SC may be due to the higher number of single platelets (OB) that are unable to aggregate among them in the presence of Na2O4W but that may adhere onto the plastic surface, probably through unspecific mechanisms.
In untreated PTP1B−/− mice (n=8), SC, AS, and OB were 1.75%±0.79%, 32.1±2.8 μm2, and 100.1±25.28, respectively. After treatment with Na2O4W, SC and AS decreased slightly to 0.64%±0.14% and 26.13±02.1 μm2 (P<0.01), respectively, and OB increased moderately to 146.5±33.87 (Figure 2).
Platelet interaction with collagen under flow conditions
The %SC on collagen-coated surfaces perfused for 5 minutes with blood from WT mice was 44.39%±3.43% (mean ± SEM, n=5). When adhesion studies were performed with the blood of Na2O4W-treated animals, %SC was reduced to 30.72%±1.26%, this decrease being statistically significant (P<0.01) (Figure 3).
With blood from PTP1B−/− mice, no changes were observed after Na2O4W treatment. The %SC was 13.86%±0.35% and 16.23%±0.74% before and after treatment with Na2O4W, respectively (Figure 3).
In vivo thrombosis model
Because the previous experiments showed that Na2O4W decreases the hemostatic capacity of platelets, we wanted to explore the effect of this compound in vivo. Mice treated with Na2O4W showed decreased thrombi weights after 3 hours in the IVC stasis model (4.6±0.7 mg in treated mice vs 8.9±0.7 mg in nontreated mice; P<0.001; Figure 4). Moreover, we also studied thrombus formation in the PTP1B−/− mice and analyzed whether these differences could be potentiated by Na2O4W. PTP1B−/− mice showed smaller thrombi than WT mice (5.0±0.3 mg in nontreated PTP1B−/− mice vs 8.9±0.7 mg in nontreated WT mice; P<0.001), reflecting a defect in platelet function in these mice, as previously described.14 Na2O4W reduced thrombi weight to a significantly greater extent in these animals (2.8±0.4 mg in treated PTP1B−/− mice vs 5.0±0.3 mg in nontreated PTP1B−/− mice; P<0.01), indicating that the mechanisms by which Na2O4W affects hemostasis would be not only through PTP1B. Therefore, other potential targets should not be disregarded.
Effect of sodium tungstate on human platelets
Platelet function in the PFA-100
Closure times in the absence of Na2O4W were 149.1±6.1 seconds (mean ± SEM, n=10) and 99.9±4.3 seconds in Col–Epi and Col–ADP cartridges, respectively (Figure 5).
In the presence of 100 μM, 200 μM, and 400 μM of Na2O4W, closure times were prolonged in a concentration-dependent manner to 182.3±5.5 seconds (P<0.01), 203.6±17.9 seconds (P<0.01), and 198.3±7.8 seconds (P<0.001), respectively, in the Col–Epi cartridges (Figure 5). In the Col–ADP cartridges, only the highest concentration of Na2O4W used (400 μM) resulted in a significantly delayed closure time (175.7±36 seconds, P<0.005) (Figure 5).
Platelet interaction with subendothelial surfaces under flow conditions
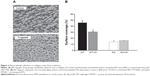
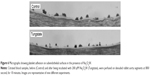
Perfusion assays were carried out with denuded vessel segments at moderate shear rates. In the absence of Na2O4W, morphometric analysis of the surfaces perfused at 800/second showed that %SC in control samples after 10 minutes of perfusion was 33.0%±1.0% (mean ± SEM, n=9). In the presence of 100 μM (n=4), 200 μM (n=9), and 400 μM (n=4) Na2O4W, %SC was significantly reduced to 30.7%±3.9%, 18.0%±2.4% (P<0.001), and 27.0%±2.7% (P<0.05). Aggregates of >5 μm (%T) were found covering 56.3%±4.4% of the SC in the absence of Na2O4W, while on those surfaces perfused with blood samples preincubated with 100 μM, 200 μM, and 400 μM of this compound, there was a significant decrease in the percentage of the surface covered by large thrombi, to 38.1%±12.7%, 32.1%±4.0% (P<0.005), and 38.2%±9.4%, respectively (Figure 5B). Representative micrographs are shown in Figure 6.
The concentration of 200 μM of Na2O4W was used to perform the remaining experiments with human blood. Using this concentration, similar results were obtained at a shear rate of 1,200/second (reductions of 63.8%±4.8% and 67.4%±3.6% in the %SC and %T, both P<0.05, respectively, were observed in the presence of Na2O4W).
Platelet aggregation using turbidimetric techniques
Samples of PRP before and after being incubated with 200 μM Na2O4W for 1 hour were activated with AA, ADP 4, ADP 2, Col, and R for 5 minutes. Results expressed as percentage of maximal aggregation (mean ± SEM, n=10) were 82.5%±3.3%, 86.4%±3.6%, 78.0%±8.1%, 91.5%±2.8%, and 71.1%±6.8%, respectively. In the presence of Na2O4W, percentage of maximal aggregation was 81.2%±7.0%, 82.8%±5.4%, 54.3%±9.0%, 84.7%±6.4%, and 72.7%±6.2%, respectively. Although results did not differ significantly, a decrease in the aggregating response to the lower concentration of ADP assayed was detected (P=0.06).
Thromboelastometric properties of blood clot
In the presence of 200 μM Na2O4W, no significant differences were observed in the parameters measured in the three different tests applied (Table 1).
Discussion
The majority of drugs developed as antiplatelet agents basically affect platelet membrane receptors and metabolic pathways and have a major impact on platelet function. Although most of them have shown clinical efficacy in reducing morbidity and mortality in patients with atherothrombotic disease, these agents are associated with a residual risk of thrombotic events, risk of bleeding, and high variability in the patients’ responses. The use of a simple molecule targeting signaling proteins such as PTPs, which regulate partial aspects of platelet function, could lead to a more manageable effect on platelet function. Our present in vivo and in vitro studies demonstrate that Na2O4W significantly inhibits platelet interaction with subendothelial surfaces under flow conditions, decreasing the adhesive and cohesive properties of platelets without interfering with the thromboelastometric properties of blood clot. These differences resulted in smaller thrombi in a deep vein thrombosis mouse model induced by blood stasis.
Thrombotic complications attributed to platelets require prior formation of a mural thrombus. The thrombus or embolized portions can be responsible for downstream ischemic complications. Several factors are known to participate in the regulation of thrombus formation. Platelet adhesion to the vessel wall increases with shear stress.24 Glycoprotein IIb-IIIa is expressed in an active conformation after platelets become exposed to damaged arterial surfaces under flow conditions.25,26 Thrombin generated through the activation of the coagulation system, and thromboxane A2 (TXA2) generated through arachidonic acid metabolism, are powerful activating agents, facilitating platelet deposition and growth of aggregates.27–29 In addition, platelets possess several receptors for ADP on their membrane surface and contain ADP and other vasoactive substances in their storage granules, which are released during platelet secretion,29 a step of critical importance in the regulation of platelet responses.30
Inhibition of platelet aggregation can be achieved by either the blockade of membrane receptors or by interfering with intracellular pathways. Inhibition of arachidonic acid metabolism by aspirin and blockade of the ADP receptors by thienopyridines are currently the more widely used antiplatelet strategies. In fact, current standard treatment for the prevention of thrombosis after percutaneous coronary intervention in acute coronary syndrome patients is dual antiplatelet therapy with aspirin and the thienopyridine clopidogrel.31–33 However, high platelet reactivity and genetic polymorphisms affect patient response to antiplatelet treatment.34–36 Although newly developed thienopyridine compounds, such as prasugrel, seem to be effective antiplatelet agents, they exhibit increased associated risk of bleeding and higher acquisition cost compared with clopidogrel. Thus, there is continuous search for newer agents with less variable antiplatelet activity and lower associated bleeding risk.
Na2O4W is a phosphatase inhibitor having antidiabetic properties,37,38 with an excellent therapeutic profile in both long- and short-term treatments.39,40 Administered orally, Na2O4W normalizes glycemia in mouse models of diabetes.40,41 It also increases the total amount and translocation of GLUT4 transporter in muscle42 and restores the glucose hepatic metabolism in streptozotocin-induced diabetic rats.11 In streptozotocin-treated neonatal rats, Na2O4W administration stimulates insulin secretion43 and regenerates β-cell population.41 Na2O4W also reduces weight gain and adiposity by increasing energy dissipation and fatty acid oxidation rate in an obese rat model.8 In a phase II study10 in obese patients treated with Na2O4W for 6 weeks, the antiobesity effect was positive although low, probably due to the short treatment duration and the lack of a hypocaloric diet used synergistically, among other limitations. Despite all the evidence generated, the precise molecular mechanisms of Na2O4W action are not yet defined. In the context of diabetes, a recent study in an animal model44 points to the effect of Na2O4W on different phosphatases, such as PTP1B and PP1, and also on G-proteins, all these being key elements in platelet function.
In our studies with Na2O4W-treated WT mice, hemostasis was significantly delayed, as evidenced by prolonged closure times at the PFA-100, using the COL–ADP cartridge, and lower rates of surface covered by platelets on perfused collagen surfaces. The PFA-100 assay was designed for the screening of primary hemostasis in humans, and not in other animal species, and some differences between the results with mice and human blood could be expected. The in vivo model of thrombosis in mice showed a consistent inhibitory effect of Na2O4W on thrombi formation. Interestingly, when blood samples were from PTP1B−/− mice, results were similar to those obtained with blood from WT mice in the presence of Na2O4W and no additional effect was observed when PTP1B−/− mice were treated. In experiments with human blood, a moderate and consistent effect decreasing both the adhesive and cohesive properties of platelets, when interacting with denuded vascular segments under flow conditions, was observed in the presence of Na2O4W. Patterns of interaction were more similar to those reported with the ADP receptor inhibitors than with aspirin. It is interesting to mention that the effect of Na2O4W on hemostasis seems to be directed toward platelet function because no action on the coagulation system could be observed. The effect of Na2O4W on the hemostatic properties of platelets was detected only when applying flow techniques using whole blood. Although the effect seems restricted to platelets, an in vivo model showed a marked decrease in venous thrombus formation, indicating that Na2O4W acted as an antithrombotic drug. Indeed, previous studies45,46 have shown that the IVC stasis model requires a proper platelet function through interaction with endothelial cell-derived von Willebrand factor.
The effects of Na2O4W on the adhesive and cohesive properties of platelets could be related to PTP1B, as derived from results in the mouse model. In fact, results with blood from the PTP1B−/− mice are quite coincident with the results with blood from WT animals treated with Na2O4W, and treatment of PTP1B−/− mice with Na2O4W did not modify this response. PTP1B is a tyrosine phosphatase that regulates platelet functions that are dependent on outside–in αIIbβ3 signaling,14 such as platelet spreading on fibrinogen and thrombus formation under flow conditions, by activation of c-Src, although it seems that it is not required for the agonist-induced activation of αIIbβ3.14 Despite this evidence, Na2O4W may be acting on other signaling mechanisms, which should be further explored.
Na2O4W is a compound with a reasonably delimited pharmacotoxicologic profile. It has satisfactorily passed three phase I clinical trials involving healthy volunteers without clinical, laboratory, or electrographic findings suggesting toxicity.10 Na2O4W has been shown to be effective in both normalizing blood glucose levels and decreasing the body weight gain and adiposity. Through the present study, we have generated evidence for the first time demonstrating the effect of Na2O4W modulating the adhesive and cohesive functions of platelets. Considering that the socioeconomic burden of atherothrombosis is still increasing in our society and that the current antiplatelet treatments are associated with thrombotic and/or bleeding risks, Na2O4W offers a potential antiplatelet strategy that should be further explored.
Acknowledgments
This work was supported by the Secretaría de Estado de Investigación, Desarrollo e Innovación, and the Ministerio de Economía y Competitividad of Spain (SAF2011-28214 and SAF2010-19527); the Red de Investigación Cardiovascular, Instituto de Salud Carlos III, of Spain (RD12/0042/0016); and the Government of Catalonia (2009 SGR 1426). BH is a recipient of a Juan de la Ciervas’ grant from the Instituto de Salud Carlos III (JCI-2011-10417). We are also grateful to Professor Angela M Valverde for kindly providing the mouse model.
Disclosure
The authors report no conflicts of interest in this work.
References
Jackson SP. Arterial thrombosis: insidious, unpredictable and deadly. Nat Med. 2011;17:1423–1436. | ||
Roth GJ, Stanford N, Majerus PW. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975;72:3073–3076. | ||
Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107:1591–1598. | ||
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345: 494–502. | ||
Wiviott SD, Braunwald E, McCabe CH, et al; TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. | ||
Garcia-Rodriguez LA, Hernandez-Diaz S, de Abajo FJ. Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br J Clin Pharmacol. 2001;52:563–571. | ||
Peters RJ, Mehta SR, Fox KA, et al; Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) Trial Investigators. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the clopidogrel in unstable angina to prevent recurrent events (CURE) study. Circulation. 2003;108:1682–1687. | ||
Claret M, Corominola H, Canals I, et al. Tungstate decreases weight gain and adiposity in obese rats through increased thermogenesis and lipid oxidation. Endocrinology. 2005;146:4362–4369. | ||
Barceló-Batllori S, Kalko SG, Esteban Y, Moreno S, Carmona MC, Gomis R. Integration of DIGE and bioinformatics analyses reveals a role of the antiobesity agent tungstate in redox and energy homeostasis pathways in brown adipose tissue. Mol Cell Proteomics. 2008;7:378–393. | ||
Hanzu F, Gomis R, Coves MJ, et al. Proof-of-concept trial on the efficacy of sodium tungstate in human obesity. Diabetes Obes Metab. 2010;12:1013–1018. | ||
Barbera A, Rodriguez-Gil JE, Guinovart JJ. Insulin-like actions of tungstate in diabetic rats. Normalization of hepatic glucose metabolism. J Biol Chem. 1994;269:20047–20053. | ||
Huyer G, Liu S, Kelly J, et al. Mechanism of inhibition of protein tyrosine phosphatases by vanadate and pervanadate. Biol Chem. 1997;272:843–851. | ||
Kuchay SM, Kim N, Grunz EA, Fay WP, Chishti AH. Double knockouts reveal that protein tyrosine phosphatase 1B is a physiological target of calpain-1 in platelets. Mol Cell Biol. 2007;27:6038–6052. | ||
Arias-Salgado EG, Haj F, Dubois C, et al. PTP1B is an essential positive regulator of platelet integrin signalling. J Cell Biol. 2005;170:837–845. | ||
Klaman LD, Boss O, Peroni OD, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. | ||
Gonzalez-Rodriguez A, Mas Gutierrez JA, Sanz-Gonzalez S, Ros M, Burks DJ, Valverde AM. Inhibition of PTP1B restores IRS1-mediated hepatic insulin signaling in IRS2-deficient mice. Diabetes. 2010;59:588–599. | ||
Kundu SK, Heilmann EJ, Sio R, Garcia C, Davidson RM, Ostgaard RA. Description of an in vitro platelet function analyzer-PFA-100. Semin Thromb Hemost. 1995;21:106–112. | ||
Shenkman B, Savion N, Dardik R, Tamarin I, Varon D. Testing of platelet deposition on polystyrene surface under flow conditions by the cone and platelet analyzer: role of platelet activation, fibrinogen and von Willebrand factor. Thromb Res. 2000;99:353–361. | ||
Diaz JA, Obi AT, Myers DD Jr, et al. Critical review of mouse models of venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:556–562. | ||
Galan AM, Lopez-Vilchez I, Diaz-Ricart M, et al. Serotonergic mechanisms enhance platelet-mediated thrombogenicity. Thromb Haemost. 2009;102:511–519. | ||
Escolar G, Bastida E, Castillo R, Ordinas A. Development of a computer program to analyze the parameters of platelet-vessel wall interaction. Haemostasis. 1986;16:8–14. | ||
Born GVR. Aggregation of blood platelets by adenosine-diphosphate and its reversal. Nature. 1962;194:927–929. | ||
Anderson L, Quasim I, Soutar R, Steven M, Macfie A, Korte W. An audit of red cell and blood product use after the institution of thromboelastometry in a cardiac intensive care unit. Transfus Med. 2006;16: 31–39. | ||
Weiss HJ, Turitto VT, Baumgartner HR. Platelet adhesion and thrombus formation on subendothelium in platelets deficient in glycoproteins IIb-IIIa, Ib, and storage granules. Blood. 1986;67:322–330. | ||
Lages B, Weiss HJ. Enhanced increases in cytosolic Ca2+ in ADP-stimulated platelets from patients with delta-storage pool deficiency: a possible indicator of interactions between granule-bound ADP and the membrane ADP receptor. Thromb Haemost. 1997;77:376–382. | ||
Born GV. Adenosine diphosphate as a mediator of platelet aggregation in vivo: an editorial view. Circulation. 1985;72:741–746. | ||
Inauen W, Baumgartner HR, Bombeli T, Haeberli A, Straub PW. Dose and shear rate-dependent effects of heparin on thrombogenesis induced by rabbit aorta subendothelium exposed to flowing human blood. Arteriosclerosis. 1990;10:607–615. | ||
Orvim U, Roald HE, Stephens RW, Roos N, Sakariassen KS. Tissue factor-induced coagulation triggers platelet thrombus formation as efficiently as fibrillar collagen at arterial blood flow conditions. Arterioscler Thromb. 1994;14:1976–1983. | ||
Salatti JA, Fenton J, Anton P, Sakariassen KS. Alpha-thrombin bound to extracellular endothelial matrix induces pronounced fibrin deposition and platelet thrombus growth in flowing non-anticoagulated human blood. Blood Coagul Fibrinolysis. 1994;5:561–566. | ||
White JG. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968;31:604–622. | ||
Kushner FG, Hand M, Smith SC Jr, et al. Focused updates: ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction and ACC/AHA/SCAI guidelines on percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2009;54:2205–2241. | ||
Antman EM, Hand M, Armstrong PW, et al. Focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2007;117:296–329. | ||
King SB 3rd, Smith SC Jr, Hirshfeld JW Jr, et al. Focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2007;117:261–295. | ||
Jernberg T, Payne CD, Winters KJ, et al. Prasugrel achieves greater inhibition of platelet aggregation and a lower rate of non-responders compared with clopidogrel in aspirin treated patients with stable coronary artery disease. Eur Heart J. 2006;27:1166–1173. | ||
Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. | ||
Bonello L, Tantry US, Marcucci R, et al; Working Group on High on-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–933. | ||
Egloff MP, Cohen PT, Reinemer P, Barford D. Crystal structure of the catalytic subunit of human protein phosphatase 1 and its complex with tungstate. J Mol Biol. 1995;254:942–959. | ||
Foster JD, Young SE, Brandt TD, Nordlie RC. Tungstate: a potent inhibitor of multifunctional glucose-6-phosphatase. Arch Biochem Biophys. 1998;354:125–132. | ||
Domingo JL. Vanadium and tungsten derivatives as antidiabeticagents: a review of their toxic effects. Biol Trace Elem Res. 2002;88:97–112. | ||
Barberà A, Gomis RR, Prats N, et al. Tungstate is an effective antidiabetic agent in streptozotocin-induced diabetic rats: a long-term study. Diabetologia. 2001;44:507–513. | ||
Barbera A, Fernandez-Alvarez J, Truc A, Gomis R, Guinovart JJ. Effects of tungstate in neonatally streptozotocin-induced diabetic rats: mechanism leading to normalization of glycaemia. Diabetologia. 1997;40:143–149. | ||
Giron MD, Caballero JJ, Vargas AM, Suarez MD, Guinovart JJ, Salto R. Modulation of glucose transporters in rat diaphragm by sodium tungstate. FEBS Lett. 2003;542:84–88. | ||
Rodriguez-Gallardo J, Silvestre RA, Egido EM, Marco J. Effects of sodium tungstate on insulin and glucagon secretion in the perfused rat pancreas. Eur J Pharmacol. 2000;402:199–204. | ||
Zafra D, Nocito L, Domínguez J, Guinovart JJ. Sodium tungstate activates glycogen synthesis through a non-canonical mechanism involving G-proteins. FEBS Lett. 2013;587:291–296. | ||
Brill A, Fuchs TA, Chauhan AK, et al. von Willebrand factor-mediated platelet adhesion is critical for deep vein thrombosis in mouse models. Blood. 2011;117:1400–1407. | ||
von Brühl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.