Back to Journals » Nanotechnology, Science and Applications » Volume 13
Role of Nanofluids in Drug Delivery and Biomedical Technology: Methods and Applications
Authors Sheikhpour M , Arabi M, Kasaeian A , Rokn Rabei A, Taherian Z
Received 28 April 2020
Accepted for publication 23 June 2020
Published 24 July 2020 Volume 2020:13 Pages 47—59
DOI https://doi.org/10.2147/NSA.S260374
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Mojgan Sheikhpour,1,2 Mohadeseh Arabi,3 Alibakhsh Kasaeian,4 Ali Rokn Rabei,4 Zahra Taherian5
1Department of Mycobacteriology and Pulmonary Research, Pasteur Institute of Iran, Tehran, Iran; 2Microbiology Research Center (MRC), Pasteur Institute of Iran, Tehran, Iran; 3School of Medicine, Tehran University of Medical Sciences, Tehran, Iran; 4Faculty of New Sciences and Technologies, University of Tehran, Tehran, Iran; 5Research Laboratory of Advanced Water and Wastewater Treatment Processes, Department of Applied Chemistry, Faculty of Chemistry, University of Tabriz, Tabriz, Iran
Correspondence: Mojgan Sheikhpour Tel +98-9122969712
Fax +98 21 64112285
Email [email protected]
Abstract: Recently, suspensions of several nanoparticles or nanocomposites have attained a vast field of application in biomedical research works in some specified conditions and clinical trials. These valuable suspensions, which allow the nanoparticles to disperse and act in homogenous and stable media, are named as nanofluids. Several studies have introduced the advantages of nanofluids in biomedical approaches in different fields. Few review articles have been reported for presenting an overview of the wide biomedical applications of nanofluids, such as diagnosis and therapy. The review is focused on nanosuspensions, as the nanofluids with solid particles. Major applications are focused on nanosuspension, which is the main type of nanofluids. So, concise content about major biomedical applications of nanofluids in drug delivery systems, imaging, and antibacterial activities is presented in this paper. For example, applying magnetic nanofluid systems is an important route for targeted drug delivery, hyperthermia, and differential diagnosis. Also, nanofluids could be used as a potential antibacterial agent to overcome antibiotic resistance. This study could be useful for presenting the novel and applicable methods for success in current medical practice.
Keywords: biomedical applications, nanofluid, diagnosis, therapy, drug delivery, antibiotic resistance
Introduction
Nanofluids are emulsions or suspensions of nanoparticles (NPs) in fluids, which could be used for straight heat transfer enhancement in many industrial applications, heat exchangers, transportation, electronics as well as biomedicine and food industry.1 There is an increasing interest in the use of nanofluids in nano biomedical technology; hence, their physicochemical properties undergo many changes in comparison with their solid forms. Recently, biological applications of nanofluids have been considered for several usages, such as drug delivery and antibacterial therapeutics.2 Also, many papers have been reported about combination systems using nanofluids, imaging and diagnosis, (eg drug delivery +imaging, high fever + imaging), or multimodal imaging (eg optical + MR imaging, PET + MR imaging).3 For the best and safe application of nanofluids, several key requirements have to be satisfied. These necessities are a good characterization of composition, size, crystallinity, and morphology. Besides, stability, non-agglomeration, and biocompatibility are essential properties.4 A great portion of the NPs disperses partially in water-based solutions. The dispersion stability mostly relies on the composition, structure, size, and morphology of the NPs.5 To attain a stable nanofluid, several procedures may be taken into consideration, which includes nanomaterial synthesis, physical stirring, ultrasonic homogenization, surface engineering, and using surfactant and other chemical additives.5 Also, the medical safety and legislation approval of these additives must be regarded. Most of the studies about nanofluids have been carried out on the material, surface science, and colloid theory. Hence, many properties of nano-based systems have been optimized. Although, the biomedical applications of nanofluids are far from simple in practice, and require further inspection.6–8
There are rare review articles regarding an exclusive consideration of the biomedical application of nanofluids. There are some papers which generally reviewed the vast uses of nanofluids in a broad range of fields. One of the areas, which is mentioned in such reviews, is the biomedical applications, in a concise and general form. Wong et al9 briefly mentioned some of the research works related to each subcategory of diverse applications of nanofluids. Wei et al10 only indicated the antibacterial activity, and drug delivery. Some authors have contented some paragraphs on the medical applications of nanofluids.11 Also, one could find another review paper, which did not describe the biomedical applications.12 According to the nanofluid definition, which was mentioned above, all of the nanoparticle-contained suspensions or nanosuspensions were considered, and the applications of such fluids in drug delivery and antibacterial and biomedical treatment are reviewed in this paper. In this review, the methods and applications of nanofluids in a diverse biomedical field are surveyed comprehensively.
Applications in Drug Delivery
The main purpose of the design of a drug delivery system is to release the drug in a controlled manner at the desired site. First studies in drug delivery systems were about drug release kinetics, pH and temperature sensitivity, polymers, nasal delivery, and oral drug delivery.13 The use of nanomaterial in drug delivery systems is one of the topics, which has been considered by many researchers in recent years to provide some benefits like being suitable for accurate delivery to the target cells, increased therapeutic properties and safety, decreased toxicity, and biocompatibility.14 Regarding the development of a nanofluid formulation for drug delivery, the system must afford drug loading and release characteristics, prolonged shelf life, and biocompatibility.15 One of the most important matters with nanofluidic drugs is the charge of NPs in the fluid. The surface charge of fundamental biological particles in blood is almost negative, and the inner walls of blood vessels are negatively charged. Thus, they repulse each other, and blood cells do not agglomerate in the vessels.16 Therefore, commonly, the therapeutic particles must be negatively charged to prevent aggregation.
Opsonization, the process by the opsonin proteins, is a pivotal mechanism that helps macrophages in defending against foreign particles such as bacteria and viruses. In the case of nano-drug delivery carriers, a reduction in the bioavailability of nano-colloidal drugs would be a problem.17 Considerable efforts have been devoted to dealing with these limitations, and several solutions have been presented. Polyethylene glycol (PEG) adsorption to nanoparticle is the most used method.17 Negatively charged albumin is another molecule that protects the NPs from opsonization.18 Several colloidal drug delivery systems (CDDS) of analgesic drugs have shown admissible efficacy in the preclinical and clinical studies.19,20
Magnetic Drug Delivery
Among different types of NPs, magnetic NPs have been noticed widely, as drug carriers. This issue is due to the controllability of these NPs, their small size, and surface property, which allow the carrier to be directed to the desired location via a magnetic field. In magnetic drug delivery, blood acts as the main fluid, while the magnetic NPs act as carriers of the drug. The drug-loaded magnetic NPs would be injected near the tumor, due to the intense and concentrated magnetic gradient; the tumor could absorb the drug. By using this method, the side effects of chemotherapy are reduced. Another advantage of this method is to use higher doses of anticancer drugs, without damaging the healthy side tissues.21–23
One of the most effective ways to treat cancer is the usage of magnetic NPs, placing a magnet near the tumor, and then injecting the drug into the closest vessels to the tumor site. The dynamics of these NPs may be influenced by the peristaltic motion of the waves, which are generated in the cone-shaped asymmetric channel walls. An analysis of the nanofluid flow, influenced by this type of movement, can greatly help the treatment of cancerous tissues.22 Superparamagnetic iron oxide NPs (SPIOs) is the most used magnetic nanoparticle for magnetic drug delivery in the clinical applications. New studies have discussed wide applications of SPIOs in cancer detection, screening, and treatment.24,25 Also, a study was done about green biosynthesis of superparamagnetic magnetite Fe3O4 NPs and their biomedical applications in targeted anticancer drug delivery system. Also, some examples of Fe3O4-based NPs, as an anticancer drug vehicle in treating different kinds of cancer cell lines using various anticancer drugs, have been reported in these recent years.26 Besides, in 2018, the enhanced photodynamic anticancer activities of multifunctional magnetic NPs (Fe3O4), conjugated with chlorin e6 and folic acid, in prostate and breast cancer cells were reported in a new investigation.27 The SPIOs are made of an iron oxide core and a shell with hydrophilic groups for facilitating solubility. Commonly, the shell could be a fatty acid, organic polymer, or a polysaccharide.28 In a study by Pattayil and Kunnoth29 in 2015, the drug (curcumin) was inserted into a modified cyclodextrin β cavity. Curcumin is one of the well-known medicines for treating cancer, and because of its low ability to dissolve in water, it is necessary to use a carrier to deliver it to the target tissue. The cyclodextrin β is a modified carrier suitable for hydrophilic drugs.30 In an interesting therapeutic dose study, Lim et al31 developed the pH‐sensitive drug‐delivering magnetic NPs (DMNPs) as theragnostic nanocarriers, which release doxorubicin (DOX) under the acidic conditions within cancer cells. They reported that the drug release kinetics of the DMNPs, combined with the NP accumulation pattern in the tumor cells, could be monitored by magnetic resonance imaging (MRI) to guide decisions regarding the optimization of drug dosing schedules. So, the reports of these valuable studies could be used to improve the efficacy of drugs and reduce their side effects in the smart drug delivery systems.32
Use of Microelectromechanical Bio-Systems
Regarding the application of microfluidic technology, researchers investigated how to transfer and control the flow. Microfluidic equipment generally has some components such as reservoirs, canals, pumps, valves, mixers, activators, filters, or heat exchangers for delivery with high precision. One of the applications of nano-drug delivery systems is to monitor the target cells’ responses to drug stimuli or to facilitate the drug development process.9,14,33-37 In this case, the NPs are in an aqueous solution, in which, the concentration of the particles and the solvent temperature are predetermined. As shown in Figure 1, these nanofluids are delivered to the living cells through the micro-channels. Among other applications of this microfluidic device, one can refer to the testing of pharmaceuticals and performing high-efficiency biomedical analyses.9,33-36 In Figure 1, there is a reservoir for storing the solution or cleaning fluid. The micro-channels could change the amount of fluid entering the test site by adjusting their resistance or internal pressure.
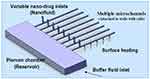 |
Figure 1 Nano delivery system with eight micro-channels Note: Reprinted from Publication International Journal of Heat and Mass Transfer, 51(23), Kleinstreuer C, Li J, Koo J, Microfluidics of nano-drug delivery, 5590–5597, Copyright (2008), with permission from Elsevier.38 |
The main goal is to achieve a uniform concentration of nanomedicine at the micro-channel output. The presence of appropriate heat flux from the lower microchannel wall ensures that the drug-fluid mixture is delivered to the live cells at an optimum temperature (37 °C). The amount of this heat flux is a function of the nanofluid velocity and the velocity of the cleaning fluid. The presence of this heat flux has also a positive effect on the drug’s concentration uniformity.38 In general, the concentration uniformity of the nano-drug is affected by some factors like the channel length, the NPs size, and the Reynolds number corresponding to the source of the nanofluid and the main microchannels.38
Nanocrystals and Nanoparticles
Nanocrystals are carrier-free drug NPs without any other jointed molecules. A nanocrystal nanofluid is a suspension of the poorly soluble drug (in most cases) in water, or another solvent stabilized with a surfactant or a stabilizer. The nanocrystal pharmaceutical products are available in the market; Rapamycin, Aprepitant, Fenofibrate, and Megestrol are some of the nanocrystal drugs. The main advantages of nanocrystals are their solubility enhancement, increased dissolution velocity, saturation solubility, user-friendliness, and high bioavailability.39 ZnO NPs and their combinations are the best in the nanobiotechnology and nanomedicine applications. The reason for this issue is the proper condition of electrons in the valence-shell of the NPs (with large bundles).40 The ZnO NPs, because of their biological properties (anti-bacterial, low toxicity, UV blocking), could be used in cancer treatment such as breast cancer.40 In some of the studies,41 the cytotoxic effects of zinc oxide NPs in hepatoma cells have been investigated. It was also shown in,41–43 that ZnO caused further damage to the rapid process of dividing cancerous cells.
In a study by Abdolmohammadi et al42 in 2017, the cytotoxic effects of two types of ZnO nanomaterials on the growth of MCF-7 cell lineage in breast cancer were investigated. The results of this study showed that, with increasing the concentrations of ZnO NPs in 48 and 72 hours of treatment, the survival rate of MCF-7 cell lines in breast cancer decreased. Dotan et al44 developed a nanofluid platform by conjugating multi-walled carbon nanotubes (CNTs) with either thyroid-specific antibody or thyroid-stimulating hormone. The nanofluid selectively targeted and killed the papillary thyroid cancer cells in vitro with minimal cytotoxic effect on the non-targeted cells.
Applications in Treatment
Hyperthermia Method
Hyperthermia is a method for treating cancer. The term hyperthermia is derived from the two Greek words, “hyper” and “thermia”, which mean rise and heat, respectively.45 The studies show that heating in the temperature range of 41 to 46 °C, for at least 20 to 60 minutes, stops the cancer cells’ growth.46,47 The hyperthermia method has some shortcomings like the heterogeneous distribution of temperature in the tumor mass and the inability to prevent overheating in deep-tissue tumors. Therefore, for responding to the need for an appropriate solution to overcome these problems, scientists have suggested magnetic NPs. With the help of magnetic NPs, the heat could be provided uniformly by this method, which is called as the MNFHT (magnetic nanofluid hyperthermia therapy). Also, the control of heat distribution and temperature is essential in this therapeutic approach.48,49
To express the distribution of energy, the SAR (Specific Absorption Rate) has been used, whose unit is W/m3 or W/g.35 This number indicates the power of magnetic NPs to eliminate cancerous cells, which is measured by numerous temperature sensors placed in the agarose gel. In a study by Salloum et al49 in 2007, nanofluids were injected into an agarose gel to study its movement in extracellular tissue. This research was carried out as in vitro, and agarose gel was used as a tissue-equivalent.50 The purpose of this study was to provide a method for controlled tumor heating; it was shown that, if the nanofluid distribution within the gel was spherical, this goal could be achieved. In tumors with unusual geometry, the multiple injection method has been proposed to achieve a uniform increase in the tumor temperature.49 According to the studies, the cubic geometry of NPs has more SAR than the other forms (spherical, rod, start, polyhedron, and cone).50
The SAR values are influenced by some factors such as the size and shape of the NPs, temperature, frequency of the magnetic field change, and the intensity of the magnetic field.46,50 One of the important factors in determining the efficiency of magnetic NPs, is their size. The small size of NPs has some advantages, including ultra-paramagnetic (ultra-magnetic) states of NPs, better dispersion, better heat distribution, prevention of agglomeration, and an increase of SAR. Despite these advantages, if the NP size is less than usual (2–5 nm), their performance would be reduced.50 In 2005, researchers reported that superparamagnetic NPs (10 to 40 nm in size) were suitable for clinical applications, due to generating significant heat and low-frequency variation.51
Magnetic Fluid Hyperthermia
Magnetic fluid hyperthermia is the selective exposure of heat to the desired tissue using magnetic NPs in the presence of an external magnetic field.52 In a study, conducted by Salloum et al53 on experimental lab mice in 2008, the rate of temperature increase in animal tissues during hyperthermia was investigated, by using magnetic NPs. In this research, two critical parameters in magnetic hyperthermia were mentioned, one was the rate of blood perfusion, and the other one was the amount of nanofluid, which was delivered to the tissue.53 Carbon nanotubes (CNTs) could be used under the influence of the magnetic field, to treat cancer cells. In the related research works, NPs are injected into the blood vessels near the tumor, and a magnet is placed near the tumor.54,55 A controlled drug delivery system has been developed, with doxorubicin-loaded magnetic PLGA, poly(lactic-co-glycolic acid), microsphere, for inhibition of tumor growth in breast cancer56(Figure 2).
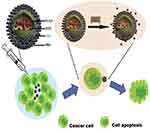 |
Figure 2 Depiction of magnetic PLGA hollow microsphere response in altering the magnetic field.Note: Reprinted from Publication Colloids and Surfaces B: Biointerfaces, 136, Fang K, Song L, Gu Z, et al, Magnetic field activated drug release system based on magnetic PLGA microspheres for chemo-thermal therapy, 712–720, Copyright (2015), with permission from Elsevier.56 |
Nano-Cryosurgery
The cryosurgery method is one of the most effective ways for complete treatment and control of tumor cells. In this method, liquid nitrogen or solid carbon dioxide is used. Creating an extremely cold condition causes the formation of ice crystals, and thus, the membrane of the desired cell could be broken due to a decrease in the amount of liquid water (dehydration).36,57 However, the use of this method has some problems such as inappropriate freezing, which may damage the healthy side tissues. The tumor tissue is loaded using highly-conductive NPs to improve the freezing capacity.35,36,57 Some researchers have investigated the toxicity of NPs and their effects on healthy tissues. By these investigations, they have found that Al2O3, Fe3O4, and Au NPs are biologically suitable, which could be widely used in medical applications.29,30,37,38 It has also been shown that Au NPs have the highest freezing efficiency among all types of utilized NPs, which produce a maximum ice-ball size. Besides increasing the freezing rate, the presence of NPs could also control the size and growth of ice crystals, which are formed during the freezing process. This property causes the destruction of undesired malignant tissues with minimum harm to the surrounding healthy tissues.36 The variation in kinds and dosages of NP solutions could play diverse roles in the freezing process and lead to different freezing efficacy. Therefore, the selection of NPs and optimization of the NP suspension dose would be important issues for future research works.58
Magnetic Nanofluids for Anemia Treatment
One of the preconditions for using magnetic iron oxide NPs in medical applications is their stability in the fluid interface.43 In the research work of Vazhnichaya et al59 in 2015, they obtained a nanofluid containing sustained magnetic NPs, which had anti-anemic properties. The stabilization of these NPs was accomplished by using PVP (polyvinyl pyrrolidine) and Mexidol (2-ethyl-6-methyl-3-hydroxy pyridine succinate). Then, they tested the material on experimental animals (109 male albino mice) in both reasonable conditions, after severe blood loss. The results of this study indicated that the nanofluid stimulated the production of red blood cells (RBCs), both hemoglobin and hematocrit, in healthy animals. When severe anemia occurs due to a blood loss, a nanofluid helps to recover this loss and increase the number of reticulocytes. This nanofluid stimulates erythropoiesis in the intact animals and activates the regeneratory reaction of erythron in anemia, due to blood loss.
Radiofrequency Ablation
The Radio Frequency Ablation (RFA) is a medical approach, in which the radio waves of short wavelengths (350 to 500 kHz) generate much heat in the desired tissue. In fact, in this method, an ablation electrode enters the tissue of the tumor, then the temperature increases because of the waves generated by the electrodes. The surrounding tissue, usually the tumor tissue, would be heated then, which leads to tissue destruction. If the given heat were sufficient enough, the tumor tissue would disappear within a few minutes.60,61 In a study by Wu et al,61 the application of iron NPs (iron NPs with carbon coating) was investigated by the RFA method. The results of the research showed that the use of nanofluid led to a 41% increase in thermal conductivity.61
Applications in Diagnosis
Imaging
One of the most interesting applications of nanomaterials in diagnostic medicine is their usage in magnetic resonance imaging (MRI). The use of magnetic NPs enhances the contrast and sensitivity in MRI (Figure 3). Super-paramagnetic NPs (SPMNPs) is one of the best choices for promoting contrast in MRI. These agents could consist of iron oxides, gold, and gadolinium NPs.62 The SPMNPs is composed of an iron oxide core and a polymeric matrix, which provide colloidal stability; the core could also contain Fe/Mn composite. Several injectable magnetic contrast agents have been proved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMEA). Gadolinium-based contrast agents are frequently used in MRI.63 Due to the gadolinium toxicity concerns, especially in patients with renal failure, its application confronts some limitations.62
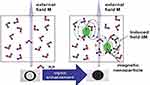 |
Figure 3 Magnetic contrast effect of magnetic nanoparticles in water, magnetic field results in darker magnetic resonance image.Note: Reprinted from Materials Science and Engineering: C, 33(8), Shokrollahi H, Contrast agents for MRI, 4485–4497, Copyright (2013), with permission from Elsevier.62 |
The MRI contrast agents, from the outlook of application, could be classified into three groups: the extracellular fluids (known as the intravenous contrast agents), the intravascular contrast agents (known as the blood pool agents), and the target-specific agents. Some of the MRI contrast agents are administered orally, and some formulations have been developed for intravenous injection.62,64 The safety and tolerance of the contrast media are important considerable factors. A good contrast agent must be biologically and chemically inert and fully eliminated from the body without any side effect and adverse reaction.64,65 Many of such contrast agents are available in the market, which is used consistently.66,67
The targeting of contrast agents leads to a high accumulation of agents at the target sites. By the targeted MRI, a high accumulation of contrast agents in the desired site leads to a specific diagnosis. The targeting agents usually attach to the tissue-specific antibodies on the target organ. Most of the target-specific contrast media have been exploited for liver imaging.68 Superparamagnetic iron oxide and liposome-based targeting contrast agents are commercially available.69 In this regard, Fe3O4O-dextran NPs (Fe3O4 with oxidized dextran coating) have been used to create contrast in MRI imaging. These NPs have important properties such as good stability, biocompatibility, bioconjugation, and low cytotoxicity.70 Huang et al71 showed the MRI images of cancer cells, which were added to Fe3O4O-dextran NPs (with antibody titers), at various concentrations. With an increase in the iron concentrations from 0.75 mM to 1.15 mM, the image of cancer cells was darker, indicating that the presence of NPs increased the contrast of MRI. Also, with an increase in the size of the nanoparticle core, at a constant concentration, the images were darkened from NP5 to NP15. The images were taken in two modes, with and without antibodies. These nanofluids are also suitable for their usage in drug delivery applications, magnetic hyperthermia, and high-efficiency magnetic bio-separation.
Antibacterial Applications
As a result of excessive use of the common antibacterial chemicals, resistant strains of pathogens emerge day by day. Hence, the development of new solutions toward the control of resistant pathogens seems to be necessary. Over the last few years, several organic and inorganic nanoparticle suspensions have shown antibacterial properties.72
Metal and Metal Oxide Nanofluids
Antibacterial agents play an essential role in the industry, including the disinfection of water, textile industries, medical applications, and food packaging.73–75 Among the various types of metal oxide NPs, zinc oxide (ZnO) has a significant ability to prevent bacterial growth in a wide range of bacteria.75,76 The antibacterial activity of ZnO nanofluids which are safe to human cells has mostly been studied among other nanofluids.77 Based on the morphological studies on the bacterial cell wall, treated with ZnO NPs, by SEM and FESEM, several aspects of ZnO toxicity have been revealed (these NPs cause damage to the bacterial cell wall). The creation of reactive oxygen species (ROS), and the release of antibacterial ions, mainly Zn2+, are other aspects of the ZnO toxicity.78 Using dispersants such as polyethylene glycol (PEG), deflocculants comprising sodium silicate, or sodium carbonate has improved the stability of ZnO nanofluids.78,79
Also, TiO2 and SiO2 NPs have similar antibacterial properties with ZnO2. However, the antibacterial properties of ZnO are higher than that of TiO2 and SiO2.75 Illumination with UV light leads to the production of reactive oxygen species (ROS) and more antibacterial effect. These three NPs are photosensitive, while TiO2 has been utilized in water disinfection systems.80 Also, MgO and Mg (OH) 2 nanomaterials have attracted much attention over the years, due to their applications in the field of pharmaceuticals.81 It has been reported that Mg(OH)2 nanomaterials have a significant antibacterial effect on E. coli, B. phytofirmans, and some other bacteria.82 Dong et al82 stated that the antibacterial mechanism of Mg(OH)2 NPs would be very different from those of metal-based compounds; accumulation of OH− ions in bacterial cells increases the pH level to 10, which results in the death of bacteria. Also, MgO nanoparticle is an effective bactericidal agent against the foodborne pathogens including E.coli and Salmonella Stanley.83 Lueng et al84 reported that the MgO nanofluid toxicity towards E. coli could occur in the absence of ROS production and oxidative stress. Therefore, they proposed a new theory on the MgO toxicity mechanism, comprising the damage of the cell membrane without lipid peroxidation.
The antibacterial activities of Cu and CuO NPs have been proved against a spectrum of gram-positive and gram-negative bacteria.85–88 The antibacterial activity depends on the size of NPs and the synthesis temperature of the nanoparticle. The smaller CuO NPs, the more antibacterial activity is achieved. The CuO NPs can path through the bacterial cell wall.88 It is imagined that these NPs bind to the cellular enzymes and block the vital activities of the cell.86,89 The CuO NPs have no significant cytotoxicity on the HeLa cell lines.86 Thus, it seems that CuO NPs cannot inter eukaryotic cells via the cytoplasmic membrane.
Silver and its compounds have been used for centuries for healing wounds and scalds, and disinfection of water. By the development of a new generation of antibiotics, the use of silver-based compounds has been limited. The advent of nanotechnology in recent decades has attracted new attention to the antibacterial use of nano-sized silver.90 Due to the cytotoxicity and environmental toxicity of silver nanomaterial, and its possible adverse effects, extensive research works have been conducted on the silver toxicity. Silver NPs have a board range of toxicity mechanisms, mainly the perturbation in mitochondrial function by altering mitochondrial membrane permeability. Moreover, silver NPs induce inflammatory responses due to the production of ROS.91 Because of the broad range of toxicity mechanisms of silver, the emergence of resistant strains seems to be implausible.92 In addition, gold NPs have been recognized as a biocompatible and relatively less cytotoxic nanomaterial with versatile applications. The antibacterial activity of gold NPs is not related to the production of ROS. According to Cui et al,93 the antibacterial activity of gold NPs is generally based on two mechanisms: inhibition of ATP synthesis by altering membrane potential and inhibition of tRNA binding to the ribosome.
According to the above explanations, in biomedical application, it should be borne in mind that metallic NPs have few shortcomings like biocompatibility issues, stability, and proper excretion from the body. For metal and metallic oxide NPs, the solubility problems are also important. The release of metal ions of dissolved NPs, has a relation with the toxicity that been observed. The biological characteristics of NPs have significant correlations with their nature and structures. Therefore, consideration of the changes in them such as surface modification and artificial control of size and shape, reduce their toxicity, and improve their biocompatibility.
Composite Nanoparticles
In other studies, to produce a combined magnetic nanoparticle with improved colloidal stability and appropriate antibacterial property, zinc oxide was combined with iron oxide. The anti-bacterial efficiency of these NPs were tested on two types of bacteria; Staphylococcus aureus and Escherichia coli.94,95 The results of these studies indicated that the anti-bacterial properties of this compound were proportional to the weight ratio of [Zn]/[Fe]; so that, when the ratio got higher, the antibacterial property increased.95 It was also shown that ZnO particles had the most antibacterial activity against E. coli. While the combination of zinc oxide and iron oxide, with a weight ratio of [Zn]/[Fe] greater than 1:1, had a great antibacterial effect on Staphylococcus aureus.95 In 2018, researchers evaluated the influence of a trimetallic Au/Pt/Ag-based nanofluid on different bacterial strains and showed that the trimetallic nanofluid had a better effect than both monometallic Au nanofluid and bimetallic Au/Pt nanofluid96 (Figure 4).
 |
Figure 4 The results of trimetallic Au/Pt/Ag-based nanofluid treatment for an enhanced antibacterial response; “A,” “B” and “C” in the agar plates correspond to Au, Au/Pt and Au/Pt/Ag nanofluid, respectively.Note: Reprinted from Materials Chemistry and Physics, 218, Yadav N, Jaiswal AK, Dey KK, et al., Trimetallic Au/Pt/Ag based nanofluid for enhanced antibacterial response, 10–17, Copyright (2018), with permission from Elsevier.96 |
Also, Fe3O4/oleic acid nanofluid has been proposed as a controlled release agent for various compounds.97 The role of Fe3O4/oleic acid for carrying cephalosporin antibiotics and the antibacterial activity of the nanofluid have been proved on S. aureus and E. coli cultures.87
Other Applications of Nanofluids
Nanofluids in Wound Dressing
Arising excessive infections in skin lesions is common during the treatment; therefore, the need for a proper dressing is much important and necessary. Anghel et al98 in 2013, investigated the use of iron nanofluid in wound dressing to prevent the colonization of Candida albicans and biofilm formation. The desired nanoparticle was a combination of active magnetic NPs such as iron oxide, and the dressing patches were immersed in this nanofluid. The final result of this study was that the combination of the unique features of iron oxide NPs and Satureja hortensis oil could result in providing a new product, which prevented the formation of biofilms and fungi on a variety of medical and clinical equipment surfaces.
Cryopreservation
He et al99 developed a quartz micro-capillary system for novel ultra-fast vitrification of mammalian cells. The results of their study indicate that vitrification at a low concentration of intracellular cryoprotectants would be a viable and effective approach for the cryopreservation of murine embryonic stem cells. The traditional vitrification methods usually use very high concentrations of cryoprotectants (commonly more than 4 M), which are often toxic. In this study, the concentration of cryoprotectant was 2M, the same as the concentration which is generally used for the traditional slow-freezing methods. Hence, this protocol had the advantages of the classical slow-freezing methods and fast cooling methods, simultaneously. This method was executed the vitrification of murine embryonic stem (ES) cells with a viability rate similar to the non-frozen control cells. The preservation of undifferentiated properties of the pluripotent murine post-vitrification ES cells was verified by the expression of three different proteins, including transcription factor Oct-4, membrane surface glycoprotein SSEA-1, and alkaline phosphatase. Figure 5 represents the immunofluorescence, and histochemical analysis of the pluripotent ES cells post vitrification.
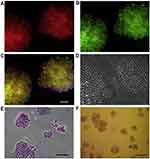 |
Figure 5 Fluorescence micrographs display high levels of staining for the surface glycoprotein SSEA-1 (A) and Oct-4 transcriptional activity, as denoted by GFP expression (B). (C) The merged view of the red and green channels indicates extensive co-expression of the two markers and DAPI nuclei staining (blue). Phase-contrast image shows cells with a high nuclei/cytoplasm ratio and compact colony formation typical of pluripotent ES cells (D). Histochemical staining shows the strong expression for alkaline phosphatase at 10× magnification (E), which was seen to be well distributed within each colony as observed at a lower 4× magnification (F).Note: Reprinted from Cryobiology, 56(3), He X, Park EYH, Fowler A, et al., Vitrification by ultra-fast cooling at a low concentration of cryoprotectants in a quartz micro-capillary: A study using murine embryonic stem cells, 223–232, Copyright (2008), with permission from Elsevier.99 |
Conclusion
Among the wide variety of applications of nanofluids, one could point out the usage of these fluids in medical and biomedical cases. In this study, the applications of nanosuspension nanofluids in drug delivery, medical treatment, disease diagnosis, antibacterial cases, wound dressing, and cryopreservation is surveyed. Based on these novel topics, the papers related to the mentioned case were studied and their contents and results were described and analyzed. This study aimed to establish a proper template for these special studies and making easy the future road map in case of the nanofluid applications in biomedical treatment, diagnosis, and antibacterial cases. Based on the conducted research works, a summary of concluding remarks are listed. Then, some suggestions are recommended for future works.
- In drug delivery, the main aim has been the prevention of aggregation and long-term stability. The reduction in the bioavailability of nano-colloidal drugs has been focused on by many researchers, and many studies have been carried out with this regard. Nanocrystals, CNTs, and gold, ZnO, and magnetic NPs have been used for their special properties. Magnetic nanoparticle-based hyperthermia has been used for heating the tumoral tissue, killing of tumor cells, or making them more sensitive to the effect of anti-cancer drugs and radiation. Nanofluids increase the destruction of tumor tissue via enhancing the thermal conductivity. For drug delivery, specifically for cancer cells, limited types of nanofluids have been tested. So, other types of magnetic nanofluids are recommended for obtaining possible valuable results per each type of disease and cancer.
- Nowadays, the application of nanofluids in targeted MRI, which is the most interesting method in imaging and diagnosis, has been increased significantly. The role of contrast agents is vital in the MRI, where some nanofluids such as Fe3O4-based fluids have been considered for this case. Trying other nanofluids could be useful for achieving the optimized contrasting behavior of different agents in different diagnosis cases.
- Photosensitive NPs such as ZnO, TiO2 and SiO2 have more antibacterial effect than other NPs; while the gold NPs have been recognized as a biocompatible and relatively less cytotoxic. Regarding the literature review and focus on the conducted research works, the following suggestions are recommended for future works:
- Finding new applications of nanofluids in biomedical and medical issues.
- Increasing the in vivo and in vitro testing trials on the nanofluids agents.
- Testing different types of nanofluids in drug delivery, treatment, diagnosis, and bacterial disinfection.
- Comparing different contrast agents for the diagnosis methods.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Wong KV, De Leon O. Applications of Nanofluids: Current and Future, in Nanotechnology and Energy. Jenny Stanford Publishing; 2017:105–132.
2. Lim E-K, Jang E, Lee K, et al. Delivery of cancer therapeutics using nanotechnology. Pharmaceutics. 2013;5(2):294–317. doi:10.3390/pharmaceutics5020294
3. Lim E-K, Kim T, Paik S, et al. Nanomaterials for theranostics: recent advances and future challenges. Chem Rev. 2015;115(1):327–394. doi:10.1021/cr300213b
4. Stankic S, Suman S, Haque F, et al. Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. J Nanobiotechnology. 2016;14(1):1–20.
5. Yu F, Chen Y, Liang X, et al. Dispersion stability of thermal nanofluids. Prog Nat Sci. 2017;27(5):531–542. doi:10.1016/j.pnsc.2017.08.010
6. Florence AT. “Targeting” nanoparticles: the constraints of physical laws and physical barriers. J Controlled Release. 2012;164(2):115–124. doi:10.1016/j.jconrel.2012.03.022
7. Eytan O, Elad D. Analysis of intra-uterine fluid motion induced by uterine contractions. Bull Math Biol. 1999;61(2):221–238. doi:10.1006/bulm.1998.0069
8. Mekheimer KS. Peristaltic flow of blood under effect of a magnetic field in a non-uniform channels. Appl Math Comput. 2004;153(3):763–777. doi:10.1016/S0096-3003(03)00672-6
9. Wong KV, De Leon O. Applications of nanofluids: current and future. Adv Mech Eng. 2010;2:519659. doi:10.1155/2010/519659
10. Yu W, Xie H. A review on nanofluids: preparation, stability mechanisms, and applications. J Nanomater. 2012;2012:17. doi:10.1155/2012/435873
11. Saidur R, Leong KY, Mohammed HA. A review on applications and challenges of nanofluids. Renewable Sustainable Energy Rev. 2011;15(3):1646–1668. doi:10.1016/j.rser.2010.11.035
12. Choi SUS. Nanofluids: from vision to reality through research. J Heat Transfer. 2009;131(3):
13. Park K. Controlled drug delivery systems: past forward and future back. J Controlled Release. 2014;190:3–8. doi:10.1016/j.jconrel.2014.03.054
14. Bañobre-López M, Teijeiro A, Rivas J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep Pract Oncol Radiother. 2013;18(6):397–400. doi:10.1016/j.rpor.2013.09.011
15. De Jong WH, Borm PJA. Drug delivery and nanoparticles: applicationsand hazards. Int J Nanomedicine. 2008;3(2):133–149. doi:10.2147/IJN.S596
16. Srinivasan S, Sawyer PN. Role of surface charge of the blood vessel wall, blood cells, and prosthetic materials in intravascular thrombosis. J Colloid Interface Sci. 1970;32(3):456–463. doi:10.1016/0021-9797(70)90131-1
17. Owens DE, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int J Pharm. 2006;307(1):93–102. doi:10.1016/j.ijpharm.2005.10.010
18. Roser M, Fischer D, Kissel T. Surface-modified biodegradable albumin nano- and microspheres. II: effect of surface charges on in vitro phagocytosis and biodistribution in rats. Eur J Pharm Biopharm. 1998;46(3):255–263. doi:10.1016/S0939-6411(98)00038-1
19. Puglia C, Tirendi GG, Bonina F. Emerging role of colloidal drug delivery systems (CDDS) in NSAID topical administration. Curr Med Chem. 2013;20(14):1847–1857. doi:10.2174/0929867311320140004
20. Gorfine SR, Onel E, Patou G, et al. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum. 2011;54(12):1552–1559. doi:10.1097/DCR.0b013e318232d4c1
21. Bárcena C, Sra AK, Gao J. Applications of Magnetic Nanoparticles in Biomedicine, in Nanoscale Magnetic Materials and Applications. Springer; 2009:591–626.
22. Kothandapani M, Prakash J. The peristaltic transport of Carreau nanofluids under effect of a magnetic field in a tapered asymmetric channel: application of the cancer therapy. J Mech Med Biol. 2015;15(03):1550030. doi:10.1142/S021951941550030X
23. Pankhurst QA, Connolly J, Jones SK, et al. Applications of magnetic nanoparticles in biomedicine. J Phys D: Appl Phys. 2003;36(13):R167. doi:10.1088/0022-3727/36/13/201
24. Katz E. Magnetic Nanoparticles. Multidisciplinary Digital Publishing Institute; 2020.
25. Hosu O, Tertis M, Cristea C. Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment. Magnetochemistry. 2019;5(4):55. doi:10.3390/magnetochemistry5040055
26. Yew YP, Shameli K, Miyake M, et al. Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug delivery system: A review. Arab J Chem. 2020;13(1):2287–2308. doi:10.1016/j.arabjc.2018.04.013
27. Choi K-H, Nam K, Cho G, et al. Enhanced photodynamic anticancer activities of multifunctional magnetic nanoparticles (Fe₃O₄) Conjugated with chlorin e6 and folic acid in prostate and breast cancer cells. Nanomaterials. 2018;8(9):722. doi:10.3390/nano8090722
28. Tietze R, Zaloga J, Unterweger H, et al. Magnetic nanoparticle-based drug delivery for cancer therapy. Biochem Biophys Res Commun. 2015;468(3):463–470. doi:10.1016/j.bbrc.2015.08.022
29. Jayaprabha KN, Joy PA. Citrate modified β-cyclodextrin functionalized magnetite nanoparticles: a biocompatible platform for hydrophobic drug delivery. RSC Adv. 2015;5(28):22117–22125. doi:10.1039/C4RA16044D
30. Banerjee SS, Chen D-H. Magnetic nanoparticles grafted with cyclodextrin for hydrophobic drug delivery. Chem Mater. 2007;19(25):6345–6349. doi:10.1021/cm702278u
31. Lim E-K, Huh Y-M, Yang J, et al. pH-triggered drug-releasing magnetic nanoparticles for cancer therapy guided by molecular imaging by MRI. Adv Mater. 2011;23(21):2436–2442. doi:10.1002/adma.201100351
32. Lim E-K, Chung BH, Chung SJ. Recent advances in pH-sensitive polymeric nanoparticles for smart drug delivery in cancer therapy. Curr Drug Targets. 2018;19(4):300–317. doi:10.2174/1389450117666160602202339
33. Meng E, Hoang T. Micro-and nano-fabricated implantable drug-delivery systems. Ther Deliv. 2012;3(12):1457–1467. doi:10.4155/tde.12.132
34. Bugliarello G, Sevilla J. Velocity distribution and other characteristics of steady and pulsatile blood flow in fine glass tubes. Biorheology. 1970;7(2):85–107. doi:10.3233/BIR-1970-7202
35. Wiedeman MP. Dimensions of blood vessels from distributing artery to collecting vein. Circ Res. 1963;12(4):375–378. doi:10.1161/01.RES.12.4.375
36. Singh S, Bhargava R. Simulation of phase transition during cryosurgical treatment of a tumor tissue loaded with nanoparticles using meshfree approach. J Heat Transfer. 2014;136(12):121101. doi:10.1115/1.4028730
37. Chua K, Chou S, Ho J. An analytical study on the thermal effects of cryosurgery on selective cell destruction. J Biomech. 2007;40(1):100–116. doi:10.1016/j.jbiomech.2005.11.005
38. Kleinstreuer C, Li J, Koo J. Microfluidics of nano-drug delivery. Int J Heat Mass Transf. 2008;51(23):5590–5597. doi:10.1016/j.ijheatmasstransfer.2008.04.043
39. Junghanns J-UAH, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomedicine. 2008;3(3):295–309. doi:10.2147/ijn.s595
40. Fakhroueian Z, Dehshiri AM, Katouzian F, et al. In vitro cytotoxic effects of modified zinc oxide quantum dots on breast cancer cell lines (MCF7), colon cancer cell lines (HT29) and various fungi. J Nanopart Res. 2014;16(7):2483. doi:10.1007/s11051-014-2483-2
41. Yuan L, Wang Y, Wang J, et al. Additive effect of zinc oxide nanoparticles and isoorientin on apoptosis in human hepatoma cell line. Toxicol Lett. 2014;225(2):294–304. doi:10.1016/j.toxlet.2013.12.015
42. Abdolmohammadi MH, Fallahian F, Fakhroueian Z, et al. Application of new ZnO nanoformulation and Ag/Fe/ZnO nanocomposites as water-based nanofluids to consider in vitro cytotoxic effects against MCF-7 breast cancer cells. Artif Cells Nanomed Biotechnol. 2017;45(8):1769–1777. doi:10.1080/21691401.2017.1290643
43. Tran N, Webster TJ. Magnetic nanoparticles: biomedical applications and challenges. J Mater Chem. 2010;20(40):8760–8767. doi:10.1039/c0jm00994f
44. Dotan I, Roche PJR, Paliouras M, et al. Engineering Multi-walled carbon nanotube therapeutic bionanofluids to selectively target papillary thyroid cancer cells. PLoS One. 2016;11(2):e0149723. doi:10.1371/journal.pone.0149723
45. Gas P, Essential Facts on the History of Hyperthermia and their Connections with Electromedicine. arXiv preprint arXiv:1710.00652, 2017.
46. Chiriac H, Petreus T, Carasevici E, et al. In vitro cytotoxicity of Fe–Cr–Nb–B magnetic nanoparticles under high frequency electromagnetic field. J Magn Magn Mater. 2015;380:13–19. doi:10.1016/j.jmmm.2014.10.015
47. Moroz P, Jones S, Gray B. Magnetically mediated hyperthermia: current status and future directions. Int J Hyperthermia. 2002;18(4):267–284. doi:10.1080/02656730110108785
48. Hedayatnasab Z, Abnisa F, Daud WMAW. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater Des. 2017;123:174–196. doi:10.1016/j.matdes.2017.03.036
49. Salloum M, Ma R, Zhu L. Controlling nanoparticle delivery in hyperthermia for cancer treatment: in vitro experimental study. in
50. Attaluri A, Ma R, Zhu L. Using microCT imaging technique to quantify heat generation distribution induced by magnetic nanoparticles for cancer treatments. J Heat Transfer. 2011;133(1):011003. doi:10.1115/1.4002225
51. Lv Y-G, Deng Z-S, Liu J. 3-D numerical study on the induced heating effects of embedded micro/nanoparticles on human body subject to external medical electromagnetic field. IEEE Trans Nanobioscience. 2005;4(4):284–294. doi:10.1109/TNB.2005.859549
52. Frazier N, Ghandehari H. Hyperthermia approaches for enhanced delivery of nanomedicines to solid tumors. Biotechnol Bioeng. 2015;112(10):1967–1983. doi:10.1002/bit.25653
53. Salloum M, Ma R, Zhu L. An in-vivo experimental study of temperature elevations in animal tissue during magnetic nanoparticle hyperthermia. Int J Hyperthermia. 2008;24(7):589–601. doi:10.1080/02656730802203377
54. Saleh H, Alali E, Ebaid A. Medical applications for the flow of carbon-nanotubes suspended nanofluids in the presence of convective condition using Laplace transform. J Assoc Arab Univ Basic Appl Sci. 2017;24(1):206–212. doi:10.1016/j.jaubas.2016.12.001
55. Ebaid A, Aly EH. Exact analytical solution of the peristaltic nanofluids flow in an asymmetric channel with flexible walls and slip condition: application to the cancer treatment. Comput Math Methods Med. 2013;2013:1–8. doi:10.1155/2013/825376
56. Fang K, Song L, Gu Z, et al. Magnetic field activated drug release system based on magnetic PLGA microspheres for chemo-thermal therapy. Colloids Surf B Biointerfaces. 2015;136:712–720. doi:10.1016/j.colsurfb.2015.10.014
57. Hou Y, Sun Z, Rao W, et al. Nanoparticle-mediated cryosurgery for tumor therapy. Nanomedicine. 2018;14(2):493–506. doi:10.1016/j.nano.2017.11.018
58. Liu J, Deng Z-S. Nano-Cryosurgery: advances and Challenges. J Nanosci Nanotechnol. 2009;9(8):4521–4542. doi:10.1166/jnn.2009.1264
59. Vazhnichaya YM, Mokliak YV, Zabozlaev AA. Effectiveness of Magnetite Nanoparticles Stabilized by 3-hydroxypyridine Derivative and Polyvinyl Pyrrolidone in Experimental Therapy of Acute Blood Loss. Современные технологии в медицине. 2015;7:84–91.
60. Friedman M, Mikityansky I, Kam A, et al. Radiofrequency ablation of cancer. Cardiovasc Intervent Radiol. 2004;27(5):427–434. doi:10.1007/s00270-004-0062-0
61. Wu Q, Zhang H, Chen M, et al. Preparation of carbon‐coated iron nanofluid and its application in radiofrequency ablation. J Biomed Mater Res B Appl Biomater. 2015;103(4):908–914. doi:10.1002/jbm.b.33275
62. Shokrollahi H. Contrast agents for MRI. Mater Sci Eng C. 2013;33(8):4485–4497. doi:10.1016/j.msec.2013.07.012
63. Estelrich J, Sánchez-Martín MJ, Busquets MA. Nanoparticles in magnetic resonance imaging: from simple to dual contrast agents. Int J Nanomedicine. 2015;10:1727–1741. doi:10.2147/IJN.S76501
64. Xiao Y-D, Paudel R, Liu J, et al. MRI contrast agents: classification and application. Int J Mol Med. 2016;38(5):1319–1326. doi:10.3892/ijmm.2016.2744
65. Shellock FG, Kanal E. Safety of magnetic resonance imaging contrast agents. J Magn Reson Imaging. 1999;10(3):477–484. doi:10.1002/(SICI)1522-2586(199909)10:3<477::AID-JMRI33>3.0.CO;2-E
66. Mornet S, Vasseur S, Grasset F, et al. Magnetic nanoparticle design for medical applications. Prog Solid State Chem. 2006;34(2):237–247. doi:10.1016/j.progsolidstchem.2005.11.010
67. Gao Z, Ma T, Zhao E, et al. Small is smarter: nano MRI contrast agents - advantages and recent achievements. Small. 2016;12(5):556–576. doi:10.1002/smll.201502309
68. Zhou Z, Lu ZR. Gadolinium‐based contrast agents for magnetic resonance cancer imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5(1):1–18. doi:10.1002/wnan.1198
69. Su HY, Chang-Qiang W, Dan-Yang L, et al. Self-assembled superparamagnetic nanoparticles as MRI contrast agents - A review. Chin Phys B. 2015;24(12):127506.
70. Wu M, Huang S. Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment. Mol clin oncol. 2017;7(5):738–746. doi:10.3892/mco.2017.1399
71. Huang S, Yan W, Hu G, et al. Facile and green synthesis of biocompatible and bioconjugatable magnetite nanofluids for high-resolution T 2 MRI contrast agents. J Phys Chem C. 2012;116(38):20558–20563. doi:10.1021/jp305211d
72. Beyth N, Houri-Haddad Y, Domb A, et al. Alternative antimicrobial approach: nano-antimicrobial materials. Evidence Based Complementary Altern Med. 2015;2015:1–16. doi:10.1155/2015/246012
73. Yamamoto O. Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorg Mater. 2001;3(7):643–646. doi:10.1016/S1466-6049(01)00197-0
74. Li Q, Mahendra S, Lyon DY, et al. Antimicrobial nanomaterials for water disinfection and microbial control: potential applications and implications. Water Res. 2008;42(18):4591–4602. doi:10.1016/j.watres.2008.08.015
75. Adams LK, Lyon DY, Alvarez PJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40(19):3527–3532. doi:10.1016/j.watres.2006.08.004
76. Jones N, Ray B, Ranjit KT, et al. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol Lett. 2008;279(1):71–76. doi:10.1111/j.1574-6968.2007.01012.x
77. Vandebriel RJ, De Jong WH. A review of mammalian toxicity of ZnO nanoparticles. Nanotechnol Sci Appl. 2012;5:61–71. doi:10.2147/NSA.S23932
78. Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano Micro Lett. 2015;7(3):219–242. doi:10.1007/s40820-015-0040-x
79. Zhang L, Jiang Y, Ding Y, et al. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J Nanopart Res. 2007;9(3):479–489. doi:10.1007/s11051-006-9150-1
80. Wei C, Lin WY, Zainal Z, et al. Bactericidal activity of TiO2 photocatalyst in aqueous media: toward a solar-assisted water disinfection system. Environ Sci Technol. 1994;28(5):934–938. doi:10.1021/es00054a027
81. Tong K, Song X, Xiao G, et al. Colloidal processing of mg(oh) 2 aqueous suspensions using sodium polyacrylate as dispersant. Ind Eng Chem Res. 2014;53(12):4755–4762. doi:10.1021/ie5002857
82. Dong C, Cairney J, Sun Q, et al. Investigation of Mg(OH)2 nanoparticles as an antibacterial agent. J Nanopart Res. 2010;12(6):2101–2109. doi:10.1007/s11051-009-9769-9
83. Jin T, He Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J Nanopart Res. 2011;13(12):6877–6885. doi:10.1007/s11051-011-0595-5
84. Leung YH, Ng AMC, Xu X, et al. Mechanisms of antibacterial activity of MgO: non-ROS mediated toxicity of MgO nanoparticles towards escherichia coli. Small. 2014;10(6):1171–1183. doi:10.1002/smll.201302434
85. Usman MS, El Zowalaty ME, Shameli K, et al. Synthesis, characterization, and antimicrobial properties of copper nanoparticles. Int J Nanomedicine. 2013;8:4467–4479. doi:10.2147/IJN.S50837
86. Mahapatra O, Bhagat M, Gopalakrishnan C, et al. Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J Exp Nanosci. 2008;3(3):185–193. doi:10.1080/17458080802395460
87. Grumezescu AM, Saviuc CChifiriuc MC, et al. Inhibitory activity of Fe(3) O(4)/oleic acid/usnic acid-core/shell/extra-shell nanofluid on S. aureus biofilm development. IEEE Trans Nanobioscience. 2011;10(4):269–274.
88. Thekkae Padil VV, Cernik M. Green synthesis of copper oxide nanoparticles using gum karaya as a biotemplate and their antibacterial application. Int J Nanomedicine. 2013;8:889–898. doi:10.2147/IJN.S40599
89. Akbar NS, Butt AW. Magnetic field effects for copper suspended nanofluid venture through a composite stenosed arteries with permeable wall. J Magn Magn Mater. 2015;381:285–291. doi:10.1016/j.jmmm.2014.12.084
90. Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. doi:10.1016/j.biotechadv.2008.09.002
91. Akter M, Sikder MT, Rahman MM, et al. A systematic review on silver nanoparticles-induced cytotoxicity: physicochemical properties and perspectives. J Adv Res. 2018;9:1–16. doi:10.1016/j.jare.2017.10.008
92. Chen X, Schluesener HJ. Nanosilver: a nanoproduct in medical application. Toxicol Lett. 2008;176(1):1–12. doi:10.1016/j.toxlet.2007.10.004
93. Cui Y, Zhao Y, Tian Y, et al. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33(7):2327–2333. doi:10.1016/j.biomaterials.2011.11.057
94. Vazhnichaya YM, Mokliak YV, Zabozlaev A. Effectiveness of magnetite nanoparticles stabilized by 3-hydroxypyridine derivative and polyvinyl pyrrolidone in experimental therapy of acute blood loss. Современные технологии в медицине. 2015;7(2 (eng)).
95. Gordon T, Perlstein B, Houbara O, et al. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Colloids Surf a Physicochem Eng Asp. 2011;374(1–3):1–8. doi:10.1016/j.colsurfa.2010.10.015
96. Yadav N, Jaiswal AK, Dey KK, et al. Trimetallic Au/Pt/Ag based nanofluid for enhanced antibacterial response. Mater Chem Phys. 2018;218:10–17. doi:10.1016/j.matchemphys.2018.07.016
97. Buteicǎ AS, et al. The anti-bacterial activity of magnetic nanofluid: fe3O4/oleic acid/cephalosporins core/shell/adsorption-shell proved on S. Aureus and E. Coli and possible applications as drug delivery systems. Digest J Nanomater Biostruct. 2010;5(4):927–932.
98. Anghel I, Grumezescu A, Holban A, et al. Biohybrid nanostructured iron oxide nanoparticles and Satureja hortensis to prevent fungal biofilm development. Int J Mol Sci. 2013;14(9):18110–18123. doi:10.3390/ijms140918110
99. He X, Park EYH, Fowler A, et al. Vitrification by ultra-fast cooling at a low concentration of cryoprotectants in a quartz micro-capillary: A study using murine embryonic stem cells. Cryobiology. 2008;56(3):223–232. doi:10.1016/j.cryobiol.2008.03.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
