Back to Journals » Clinical Ophthalmology » Volume 13
Risk factors for ocular surface damage in Mexican patients with dry eye disease: a population-based study
Authors Rodriguez-Garcia A, Loya-Garcia D , Hernandez-Quintela E, Navas A
Received 13 October 2018
Accepted for publication 22 November 2018
Published 21 December 2018 Volume 2019:13 Pages 53—62
DOI https://doi.org/10.2147/OPTH.S190803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Alejandro Rodriguez-Garcia,1 Denise Loya-Garcia,1 Everardo Hernandez-Quintela,2 Alejandro Navas3
1Tecnologico de Monterrey, Medical School and Health Sciences, Cornea and External Diseases Service, Institute of Ophthalmology and Visual Sciences, Monterrey, Mexico; 2Cornea and Refractive Surgery Service, Asociacion para Evitar la Ceguera en Mexico, I.A.P. Universidad Nacional Autonoma de Mexico, Ciudad de Mexico, Mexico; 3Cornea and Refractive Surgery Service, Instituto de Oftalmologia Conde de Valenciana, I.A.P. Universidad Nacional Autonoma de Mexico, Ciudad de Mexico, Mexico
Purpose: To analyze potential risk factors for ocular surface damage in a representative population of Mexican patients with dry eye disease (DED).
Patients and methods: A prospective and cross-sectional population-based epidemiologic cohort study was conducted through a survey of patients with symptoms, signs, known pre-existing diagnosis, and clinical conditions related to DED. Fluorescein staining, tear break-up time (TBUT), and Oxford lissamine green staining were performed on both eyes of patients enrolled in the study.
Results: A total of 2,725 surveys including 1,543 (56.6%) women and 1,182 (43.3%) men were analyzed. Most common pre-existing diagnosis included dry eye (58%), chronic blepharitis (17%), and ocular allergy (15%). More than 70% of patients had a positive fluorescein test, and this prevalence increased proportionally to the number of reasons for consultation. The same was true for gender (P<0.001) and age (P<0.0001), with women showing a strong correlation with age (R2=0.93912, P=0.001). The association between positive fluorescein staining and diagnosis was significant for dry eye (P<0.0001), Sjögren’s syndrome (P<0.0001), and glaucoma (P<0.05). No significant association between TBUT and age or gender was found, but the shorter the TBUT, the larger the prevalence of fluorescein staining. Reduced TBUT was seen more frequently in patients with dry eye (57%), ocular allergy (16%), and chronic blepharitis (15%). Most patients (39%) with Oxford grades III and IV were older, complained of red eye (51.0%), foreign body sensation (47.0%), burning (46.0%), and were using eye drops (67%) and systemic medications (47%).
Conclusion: The Mexican profile of patients with significant ocular surface damage related to DED includes women at older ages, complaining of red eye, foreign body, and burning sensation. Diagnoses of dry eye, Sjögren’s syndrome, and glaucoma were also risk factors for significant ocular surface damage, along with long-term use of preserved eyes drops and systemic medications.
Keywords: ocular surface disease, lacrimal dysfunction, tear break-up time, fluorescein staining, Oxford scale
Introduction
Dry eye disease (DED) represents a significant burden of public health with a prevalence higher than many systemic severe disorders like diabetes mellitus and heart disease.1–3 Multiple studies have demonstrated that DED symptoms have a significant impact on patient’s vision-related quality of life.4–6 Severe DED symptoms and signs have been correlated with difficulties in physical, social, and mental functioning, limiting the patient’s capacity to perform daily tasks such as watching TV, reading, driving, and carrying out their work, as well as inducing anxiety and depression.5,7,8
Epidemiologic studies have shown that the prevalence of dry eye symptoms with or without signs ranges from 5% to 50%, whereas when only signs are considered for diagnosis, the prevalence rates are generally higher, reaching up to 75% in certain populations.9,10 Many different factors influence the wide variation in DED frequency, including geographic, climate, and demographic aspects, but equally important is the lack of clear standardization of disease definition and classification nomenclature.9,11–13
DED has been recently redefined by the Dry Eye Disease Workshop (DEWS-2017) as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”14 Ocular inflammation and damage to the ocular surface, particularly the corneal and conjunctival epithelium, are the common denominators of the disease.15,16
The purpose of the present report is to analyze the prevalence of risk factors for ocular surface damage related to DED in a population-representative cohort from Mexico.
Patients and methods
This is a prospective and cross-sectional population-based epidemiologic cohort study. A survey was designed to evaluate the risk factors for ocular surface damage related to DED in the general population. The study was performed by a representative number of ophthalmologists from large cities of Mexico on all eligible patients visiting their clinics in a single given week. All patients who volunteered to participate in the study read and signed an informed consent approved by the Ethics and Research Committee of the GRoup of Evaluation on Conservatives in Ophthalmology (El GRECO) and the study complied with the tenets of the Declaration of Helsinki.
The survey was only applied to patients who had a pre-existing diagnosis related to DED or whenever symptoms and signs associated with diminished production or increased evaporation of tears were present. Demographic data and reason for consultation, consisting of red eye, burning, ocular pain, foreign body sensation, and blurred vision, were cumulatively collected from every patient. A selection of pre-existing diagnosis and ocular conditions related to dry eye was used to identify patients with potential ocular surface damage through specific close-ended and open questions. If the interviewer recorded one or more of these conditions, then a clinical evaluation of ocular surface damage including fluorescein staining, tear break-up time (TBUT), and the Oxford lissamine green staining scheme was performed in both patient’s eyes.17,18 All ophthalmologists who participated in the study received specific instructions of how the ocular surface evaluation should be performed to standardize the clinical parameters measured.
Only complete survey forms were included for analysis. Data obtained from every patient were collected in Excel spreadsheets (Windows® version, 2017, Windows update 4011165 for Microsoft Excel 2017; Microsoft Corporation, Redmond, WA, USA) and analyzed using R-Statistics version 3.4.3 for Windows (GNU-Free Software Foundation, Boston, MA, USA). Different types of statistical analysis were applied: descriptive statistics was performed to demographic variables (media, standard deviation, and range). Graphic analysis (bars, distribution pies, and frequency histograms) was also performed. Finally, the calculation of the estimations and contrasts of hypothesis was based on the distribution of comparative proportions using the chi-squared test for discrete variables and the Student’s t-test for comparison of mean differences of unequal variances. All hypothesis contrasts were considered significant with a P-value <0.05 and with a reliability of 95%. The risk factor for ocular surface damage related to DED was obtained by a multivariate logistic regression model using the presence of DED as the dependent variable.
Results
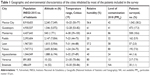
Fifty-five ophthalmologists from ten different states randomly distributed all over the country performed a dry eye poll among the eligible patients. The majority of patients surveyed (90.6%) lived in seven of the most inhabited cities of the country: Mexico City, Guadalajara, Monterrey, Puebla, Leon, Toluca, and Aguascalientes (Table 1). More than half of the population included for study lives at very high altitude: Mexico City (2,240 m above sea level), Toluca (2,605 m), and Puebla (2,147 m). Only the cities of Veracruz and Ensenada are located at sea level. The geographic, climate, and environmental characteristics of these cities are presented in Table 1.
Almost one-third of the patients analyzed (31.0%) worked at offices spending most of their time reading and using digital screens. The other two-thirds of the patients worked part-time in the office or at stores and mostly outside in a great variety of jobs like city workers, drivers, sellers, construction, etc.
From a total of 3,014 interviews performed within a 4-month period, only 2,725 complete surveys were considered for analysis. The population studied comprised 1,543 (56.6%) women and 1,182 (43.3%) men with an age range distribution as given in Table 2.
  | Table 2 Distribution of the population studied according to gender and age range |
The most common pre-existing diagnosis recorded was dry eye in 1,717 (63.0%) patients, followed by chronic blepharitis in 489 (17.9%) patients, and ocular allergy in 457 (16.7%) patients (Table 3).
A total of 1,135 (41.6%) patients were already on topical treatment with at least one unspecified eye drop, and 572 (20.9%) patients were on at least one systemic medication at the time the survey was applied. Also, one-third of patients (31.0%) referred to use an electronic device with a digital screen (desk-top, lap-top, tablet, or cell phone) on a daily basis (Table 3).
When comparing the proportion of patients with a positive corneal fluorescein staining and the pre-existing diagnosis, there was a significant association with the diagnosis of dry eye (P=0.0001), Sjögren’s syndrome (P=0.0001), and glaucoma (P=0.04). Patients with Sjögren’s syndrome had a 1.34 (OR, 95% CI =1.27–1.42) higher risk for developing ocular surface damage, while the risk factor was 1.33 (OR, 95% CI =1.26–1.41) for patients with dry eye and 1.19 (OR, 95% CI =1.12–1.26) for patients with glaucoma (Figure 1).
Around one-third of all respondents complained of typical dry eye symptoms including red eye, burning, foreign body sensation, and blurred vision (Figure 2). Of all patients who experienced at least one dry eye-related symptom, ≥70% had a positive fluorescein test with similar proportions according to symptomatology for all patients analyzed (Figure 2).
  | Figure 2 Prevalence of dry eye symptoms and its correlation with a positive fluorescein test in at least one eye. |
Moreover, the prevalence of a positive corneal fluorescein staining increased proportionally to the number of reasons for consultation recorded for each patient, from 66% with one reason to 92% with ≥4 reasons, being statistically significant for patients with four (P=0.036) and more than four reasons for consultation (P<0.009) (Figure 3).
  | Figure 3 Proportion of a positive fluorescein test according to the number of reasons for consultation. |
The same was true for both gender (P<0.001) and age (P<0.0001) as shown in Figure 4. Of note was the increased proportion of women who showed a positive fluorescein staining with age (R2=0.93912, P=0.001).
  | Figure 4 Proportions of a positive fluorescein test according to gender and its correlation with age. |
Regarding TBUT, no significant association was found between reduced TBUT and age or gender in our population, but the shorter the TBUT, the larger the prevalence of corneal fluorescein staining. This association was also true for higher Oxford Scale scores (Figure 5).
Around 29% of patients complaining of typical dry eye symptoms like red eye, foreign body sensation, pain, burning, and blurred vision also had shorter TBUTs (<10 seconds). On the other hand, larger proportions of reduced TBUT were recorded for patients with a pre-existing diagnosis of dry eye (57%), ocular allergy (16%), and chronic blepharitis (15%).
The distribution of patients with ocular surface damage according to the Oxford score is presented in Table 4.
The largest proportion of patients (45.0%) had grade I staining, while 22.0% of them had grade II staining. The great majority of patients with grades III and IV in the Oxford scale were women (64%), and most of these patients (39%) belonged to the oldest age group (>65 years old) and complained of red eye (51.0%), foreign body sensation (47.0%), and burning (46.0%). In addition, patients in these grades were using eye drops (67%), systemic medications (47%), and had a previous diagnosis of dry eye (63%) at the time of the analysis (Figure 6).
A total of 1,135 (41.6%) patients were already using eye drops at the time of the survey; 88% used preserved drops and only 12% used unpreserved eye drops. As many as 80% of patients with Oxford grade IV staining score were on lubricant eye drops, 63% of them with preserved formulations and only 12% with preservative-free lubricant eye drops. Overall, there was a higher proportion of patients with positive corneal fluorescein staining using preserved eye drops (P=0.002). Also, there was a significantly higher rate of ocular surface damage in patients using preserved eye drops for more extended periods of time (P=0.046) (Figure 7).
  | Figure 7 Comparison of positive corneal fluorescein staining between patients previously treated with preserved and preservative-free eye drops over time. |
The great majority of patients, between 70% and 93%, using systemic medications showed positive fluorescein staining (P=0.0024). Patients reported using mainly hypoglycemic and anti-hypertensive drugs (data not shown).
Finally, 76% of patients with a history of cataract surgery and 77% with refractive surgery had a positive corneal fluorescein staining (P=0.754).
Discussion
Studies searching for risk factors for ocular surface damage related to DED are important because they provide specific information with regard to the epidemiologic features of a given population based on geographic, environmental, demographic, socio-economic, and cultural aspects.2,19,20 The population analyzed in the present study is a representative sample of different geographic regions from Mexico, a country with multivariate climates and socio-economic and cultural characteristics. The majority of patients in the present study live and work part- or full-time outside in densely populated urban areas like Mexico City, Guadalajara, and Monterrey, which are also ranked among the ten most polluted cities in the country with high particulate matter-10 μm (PM10) indexes (Table 1). Several studies have demonstrated that environmental factors influence the prevalence and play an important role in the pathogenesis of DED.21 In fact, a whole new concept of environmental dry eye disease has gained recognition as a new clinical subtype of dry eye.22 High air pollution from automobile exhausts and industrial emissions in densely populated cities produce ocular surface damage increasing the incidence of ocular discomfort and tear film instability.23,24 Moreover, most of the urban areas where our study was performed are located at a very high altitude (>1,500 m above sea level), and it has been shown that high-altitude exposure contributes significantly to tear film instability and TBUT reduction.25–27 On the other hand, at least one-third of our patients work in closed environments like offices, health care facilities, and poorly ventilated confinements with high variations in airflow and humidity. Also, many of them expend most of their time in front of a computer or other digital screen displays and are exposed to other internal toxic elements like high carbon dioxide concentrations, air conditioning, carpeting, and other furniture materials which may contribute to their dry eye symptoms and ocular surface damage.28–30
Like in other DED population-based reports, around one-third of responders in the present study complained of dry eye symptoms.11,19,31 Also, more than 70% of those evaluated had a positive fluorescein test, and this prevalence increased proportionally to the number of reasons for consultation recorded for each patient. This finding implies that more than two-thirds of the population surveyed, complaining of one or more symptoms of dry eye, had some degree of ocular surface damage. Moreover, superficial punctate keratitis was found in 68% of these patients, expressed as significantly positive corneal fluorescein staining in at least one eye, with a higher proportion in women at older ages (Figures 3 and 4). This significant ocular surface damage reported in our patients is similar to what previous epidemiologic reports on DED have encountered.9,11,13 Additionally, the most commonly reported diagnoses in the present study, namely, dry eye, chronic blepharitis, and ocular allergy, are also among the most common causes of DED reported worldwide.16,32,33 Not surprisingly, dry eye, Sjögren’s syndrome, and glaucoma were the three diagnoses recorded from our patients that showed a higher correlation with significant corneal fluorescein staining (Figure 1). Sjögren’s syndrome, one of the most severe forms of DED, has been shown to cause significant damage to the ocular surface, reflected by higher Sjögren’s International Collaborative Clinical Alliance (SICCA) (≥5 points) and Oxford (grades III–IV) scores.17,34,35 On the other hand, glaucoma and topical anti-glaucoma therapy have more recently been mentioned as frequent and serious causes of ocular surface disease.36–38 The number of anti-glaucoma medications and the frequency of drops administered per day are known to increase the ocular surface damage in these patients.36,39,40
In regard to treatment, the great majority of patients with severe ocular surface damage (Oxford grade IV staining score) were already on long-term lubricant eye drops at the time of the survey; 63% of them were using preserved formulations as opposed to only 16% who were using preservative-free drops. Knowing the potential and cumulative toxic effect of detergent preservatives like benzalkonium chloride present in many topical eye formulations, it was not surprising to find a significantly higher rate of ocular surface damage in patients using preserved lubricant eye drops for longer periods of time41 (Figure 7). Hypoglycemic and anti-hypertension systemic medications were also found to have a significant correlation with ocular surface damage related to DED. In general, different types of systemic drugs may exacerbate dry eye signs and symptoms, particularly anti-histamines, anti-depressants, anti-anxiolytics, and diuretic drugs.42
Conclusion
Patients with DED are exposed to many different factors that contribute in different ways to their signs and symptoms. In the present study, geographic and adverse environmental factors may have a significant contribution to the high prevalence of ocular surface damage that was found.
The present report shows that the profile of Mexican patients with significant ocular surface damage related to DED includes older age women complaining of red eye, foreign body sensation, and burning. Previous or known diagnoses of dry eye, Sjögren’s syndrome, or glaucoma were also risk factors for developing significant ocular surface damage, along with long-term use of topical treatment with preserved eyes drops and systemic medications.
The limitations of population-based studies evaluating DED and its consequences are based on differences in ethnic, geographic, cultural, and socio-economic aspects, and also on the methodology and clinical characteristics included in the study. Therefore, comparative analysis is difficult and limited. In the present report, we did not explore all risk factors for DED due to the nature of the survey and the way it was designed. For those reasons, detailed and extensive screening was not possible. However, the data obtained in the present survey are of value for the identification of potential risk factors for significant ocular surface damage related to dry eye in Mexican patients.
Acknowledgments
The authors acknowledge the participation of the members of the El GRECO study group for the patient recruitment and collection of data, and Jose Delgado Bracho for the statistical analysis. This survey was funded by Théa Laboratories, Mexico.
Disclosure
The authors report no conflicts of interest in this work.
References
Centers for Disease Control and Prevention (CDC). National Diabetes Statistics Report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services; 2017. | ||
Galor A, Feuer W, Lee DJ, et al. Prevalence and risk factors of dry eye syndrome in a United States veterans affairs population. Am J Ophthalmol. 2011;152(3):377–384. | ||
Schiffman RM, Walt JG, Jacobsen G, Doyle JJ, Lebovics G, Sumner W. Utility assessment among patients with dry eye disease. Ophthalmology. 2003;110(7):1412–1419. | ||
Le Q, Zhou X, Ge L, Wu L, Hong J, Xu J. Impact of dry eye syndrome on vision-related quality of life in a non-clinic-based general population. BMC Ophthalmol. 2012;12(1):22. | ||
Miljanović B, Dana R, Sullivan DA, Schaumberg DA. Impact of dry eye syndrome on vision-related quality of life. Am J Ophthalmol. 2007;143(3):409–415. | ||
Mertzanis P, Abetz L, Rajagopalan K, et al. The relative burden of dry eye in patients’ lives: comparisons to a U.S. normative sample. Invest Ophthalmol Vis Sci. 2005;46(1):46–50. | ||
Rajagopalan K, Abetz L, Mertzanis P, et al. Comparing the discriminative validity of two generic and one disease-specific health-related quality of life measures in a sample of patients with dry eye. Value Health. 2005;8(2):168–174. | ||
Li M, Gong L, Sun X, Chapin WJ. Anxiety and depression in patients with dry eye syndrome. Curr Eye Res. 2011;36(1):1–7. | ||
Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II epidemiology report. Ocul Surf. 2017;15(3):334–365. | ||
McCarty CA, Bansal AK, Livingston PM, et al. The epidemiology of dry eye in Melbourne, Australia, Ophthalmology. 1998;105(6):1114–1119. | ||
Moss SE, Klein R, Klein BE. Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol. 2000;118(9):1264–1268. | ||
Schein OD, Muñoz B, Tielsch JM, Bandeen-Roche K, West S. Prevalence of dry eye among the elderly. Am J Ophthalmol. 1997;124(6):723–728. | ||
Schaumberg DA, Sullivan DA, Buring JE, Dana MR. Prevalence of dry eye syndrome among US women. Am J Ophthalmol. 2003;136(2):318–326. | ||
Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15(3):276–283. | ||
Uchino M, Yokoi N, Uchino Y, et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: the Osaka study. Am J Ophthalmol. 2013;156(4):759–766. | ||
Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report. Ocul Surf. 2017;15(3):438–510. | ||
Bron AJ, Evans VE, Smith JA. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea. 2003;22(7):640–650. | ||
McGinnigle S, Naroo SA, Eperjesi F. Evaluation of dry eye. Surv Ophthalmol. 2012;57(4):293–316. | ||
Lee AJ, Lee J, Saw SM, et al. Prevalence and risk factors associated with dry eye symptoms: a population based study in Indonesia. Br J Ophthalmol. 2002;86(12):1347–1351. | ||
Vehof J, Kozareva D, Hysi PG, Hammond CJ. Prevalence and risk factors of dry eye disease in a British female cohort. Br J Ophthalmol. 2014;98(12):1712–1717. | ||
Saxena R, Srivastava S, Trivedi D, Anand E, Joshi S, Gupta SK. Impact of environmental pollution on the eye. Acta Ophthalmol Scand. 2003;81(5):491–494. | ||
Alves M, Novaes P, Morraye MA, Reinach PS, Rocha EM. Is dry eye an environmental disease? Arq Bras Oftalmol. 2014;77(3):193–200. | ||
Novaes P, do Nascimento Saldiva PH, Kara-José N, et al. Ambient levels of air pollution induce goblet-cell hyperplasia in human conjunctival epithelium. Environ Health Perspect. 2007;115(12):1753–1756. | ||
Moen BE, Norbäck D, Wieslander G, et al. Can air pollution affect tear film stability? A cross-sectional study in the aftermath of an explosion accident. BMC Public Health. 2011;11:235. | ||
Gupta N, Prasad I, Himashree G, D’Souza P. Prevalence of dry eye at high altitude: a case controlled comparative study. High Alt Med Biol. 2008;9(4):327–334. | ||
Willmann G, Schatz A, Fischer MD, et al. Exposure to high altitude alters tear film osmolarity and breakup time. High Alt Med Biol. 2014;15(2):203–207. | ||
Lu P, Chen X, Liu X, et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea. 2008;27(5):545–551. | ||
Tsai DH, Lin JS, Chan CC. Office workers’ sick building syndrome and indoor carbon dioxide concentrations. J Occup Environ Hyg. 2012;9(5):345–351. | ||
Wolkoff P, Kärcher T, Mayer H. Problems of the “outer eyes” in the office environment: an ergophthalmologic approach. J Occup Environ Med. 2012;54(5):621–631. | ||
Skyberg K, Skulberg KR, Eduard W, Skåret E, Levy F, Kjuus H. Symptoms prevalence among office employees and associations to building characteristics. Indoor Air. 2003;13(3):246–252. | ||
Simpson TL, Situ P, Jones LW, Fonn D. Dry eye symptoms assessed by four questionnaires. Optom Vis Sci. 2008;85(8):E692–E699. | ||
Tong L, Zhou L, Beuerman RW, Zhao SZ, Li XR. Association of tear proteins with Meibomian gland disease and dry eye symptoms. Br J Ophthalmol. 2011;95(6):848–852. | ||
Hom MM, Nguyen AL, Bielory L. Allergic conjunctivitis and dry eye syndrome. Ann Allergy Asthma Immunol. 2012;108(3):163–166. | ||
Whitcher JP, Shiboski CH, Shiboski SC, et al. A simplified quantitative method for assessing keratoconjunctivitis sicca from the Sjögren’s Syndrome International Registry. Am J Ophthalmol. 2010;149(3):405–415. | ||
Shiboski SC, Shiboski CH, Criswell LA, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance Cohort. Arthritis Care Res. 2012;64(4):475–487. | ||
Leung EW, Medeiros FA, Weinreb RN. Prevalence of ocular surface disease in glaucoma patients. J Glaucoma. 2008;17(5):350–355. | ||
Skalicky SE, Goldberg I, Mccluskey P. Ocular surface disease and quality of life in patients with glaucoma. Am J Ophthalmol. 2012;153(1):1–9. | ||
Garcia-Feijoo J, Sampaolesi JR. A multicenter evaluation of ocular surface disease prevalence in patients with glaucoma. Clin Ophthalmol. 2012;6:441–446. | ||
Ghosh S, O’Hare F, Lamoureux E, Vajpayee RB, Crowston JG. Prevalence of signs and symptoms of ocular surface disease in individuals treated and not treated with glaucoma medication. Clin Exp Ophthalmol. 2012;40(7):675–681. | ||
Mathews PM, Ramulu PY, Friedman DS, Utine CA, Akpek EK. Evaluation of ocular surface disease in patients with glaucoma. Ophthalmology. 2013;120(11):2241–2248. | ||
Baudouin C, Renard J-P, Nordmann J-P. Prevalence and risk factors for ocular surface disease among patients treated over the long term for glaucoma or ocular hypertension. Eur J Ophthalmol. Epub 2012 Jun 11. | ||
Fraunfelder FT, Sciubba JJ, Mathers WD. The role of medications in causing dry eye. J Ophthalmol. 2012;2012(3):1–8. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.