Back to Journals » Medical Devices: Evidence and Research » Volume 15
Revision of Failed Sacroiliac Joint Posterior Interpositional Structural Allograft Stabilization with Lateral Porous Titanium Implants: A Multicenter Case Series
Authors Kranenburg A, Garcia-Diaz G, Cook JH, Thambuswamy M, James W, Stevens D, Bruggeman A, Chen Y, Capobianco R , Reckling WC, Siegal JD
Received 15 April 2022
Accepted for publication 12 July 2022
Published 20 July 2022 Volume 2022:15 Pages 229—239
DOI https://doi.org/10.2147/MDER.S369808
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Andy Kranenburg,1 Gabriel Garcia-Diaz,2 Judson H Cook,3 Michael Thambuswamy,4 Whitney James,5 David Stevens,6 Adam Bruggeman,7 Ying Chen,8 Robyn Capobianco,9 W Carlton Reckling,9 Joel D Siegal10
1Southern Oregon Orthopedics, Medford, OR, USA; 2OrthoSpine Advance Health, Inc, Merced, CA, USA; 3Central Texas Brain and Spine, Austin, TX, USA; 4Oklahoma Spine & Brain Institute, Tulsa, OK, USA; 5James Marco Health, Prescott, AZ, USA; 6Utah Spine Specialists, Bountiful, UT, USA; 7Texas Spine Care Center, San Antonio, TX, USA; 8OrthoNeuro Inc, New Albany, OH, USA; 9SI-BONE Inc, Santa Clara, CA, USA; 10Key Clinics LLC, Mayfield Heights, OH, USA
Correspondence: Andy Kranenburg, Tel +1 541-864-0263, Email [email protected]
Background: Distraction arthrodesis (DA) and stabilization of the sacroiliac (SI) joint by placing standalone structural allograft (SA) into the joint from a posterior trajectory has recently been introduced as a surgical procedure for chronic SI joint pain refractory to non-operative care.
Methods: Retrospective case series of patients with recurrent and/or persistent pain after placement of one or more interpositional/intraarticular standalone SAs between the ilium and sacrum using a posterior procedure to treat SI joint pain/dysfunction. Patients subsequently underwent surgical revision with porous titanium fusion implants using a lateral transfixing procedure. The demographic, clinical, and radiographic features of these cases are described.
Results: Data were available for 37 patients. The average (SD) age was 57 (13) years, 62% were female, and the average BMI was 31 (5.4). On average, two SA implants were placed per joint; 46% of cases were bilateral. At follow-up, two common themes were identified: lucencies around the implants and suboptimal implant position. None of the cases showed radiographic fusion of the SI joint prior to revision. One patient had an inflammatory reaction to the SA. All patients presented for revision due to either continued (49%) or recurrence (51%) of pain. In one revision case, the SA was forced ventrally, resulting in a sacral fracture, which was treated conservatively without sequelae.
Conclusions: The popularity of standalone SA for SI joint stabilization/fusion with a posterior procedure is increasing. This case series demonstrates that clinical failures from this procedure may require surgical revision. The proposed fusion strategy (DA) for these products is unproven in the SI joint, and, therefore, properly conducted prospective randomized clinical trials with long-term clinical and radiographic follow-up are important to establish the safety and efficacy of this approach. In the meantime, the placement of lateral titanium implants appears to be an effective revision strategy.
Keywords: distraction arthrodesis, structural allograft, sacroiliac joint, sacroiliac joint fusion, ligamentotaxis
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Deer has been published for this article.
Introduction
Pain from the sacroiliac joint (SI joint) is thought to be responsible for 15–30% of all chronic low back pain.1–5 SI joint pain reduces quality of life,6,7 and non-surgical treatments, while having some literature support,8–14 commonly fail to provide adequate long-term pain relief. Open fusion of the SI joint with posterior, lateral, or anterior approaches has been available for over 100 years15 but is no longer commonly performed due to its invasiveness, significant operative morbidity,16 long recovery periods, and lack of high-quality evidence indicating benefit.
Percutaneous screw fixation for chronic (non-traumatic) SI joint pain has been reported with successful results. In a small case series, 11/15 (73%) patients with isolated SI joint pain showed reasonable short-term responses; 3 (20%) required revision for screw loosening.17 Long-term loosening and recurrence of pain are common, and one series showed failure requiring revision within four years in 31% of cases.18 Subsequently, Luukkainen et al described the use of a fenestrated threaded cylindrical mesh cage filled with bone graft placed in a similar lateral to medial trajectory to achieve permanent stabilization/fusion of the SI joint.9 Placement of devices in this lateral-to-medial trajectory, where the device traverses three bone cortices for joint fixation can be referred to as joint “transfixation.”
The US Food and Drug Administration (FDA) has cleared over 30 metallic implants intended for SI joint fusion as medical devices through the 510k (substantial equivalence) pathway under the Center for Devices and Radiological Health (CRDH). These devices accomplish joint transfixation through placement in the lateral-to-medial trajectory as popularized by Routt et al.20,21 The entry point and trajectory may vary slightly from straight lateral to somewhat more posterolateral, but all these implants transfix the ilium and the SI joint in a consistent manner (Figure 1).
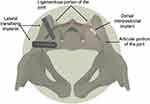 |
Figure 1 Pelvis shows two different SI joint fusion procedures: lateral transfixing implants on the left and dorsal interpositional standalone structural allograft on the right. |
Most published studies on the safety and efficacy of MIS SI joint fusion using devices placed in a lateral transfixing procedure involve triangular titanium porous implants (iFuse Implant System, SI-BONE). This implant provides immediate stability through 3 main mechanisms: 1) its anti-rotational triangular shape prevents motion, 2) each device has multiple points of fixation through three bony cortices, and 3) typically three devices are placed across the joint.22 Unlike smooth cylindrical screws, the implant provides permanent stabilization/fusion through bone ongrowth and ingrowth into the implants.23 Multiple prospective studies, including two randomized trials against non-surgical management,24,25 have demonstrated significant improvements in patient pain, disability, and objective physical function.26 The short-term outcomes observed at three months after treatment in these studies have shown to be durable out to 2 years. Long-term (2- and 5-year) prospective follow-up has further demonstrated the durability of outcomes and a high rate (88%) of radiographic fusion, defined as bridging bone across the SI joint and no failure at the device host bone interface.26–28
A new and significantly different procedure for SI joint stabilization/fusion has recently been popularized in the US.29 The procedure uses a dorsal trajectory (Figure 1) and involves placing one or more structural allografts (SA) into the ligamentous portion of the SI joint as a standalone procedure.30 Allografts are regulated by FDA as human cell and tissue products (HCT/P), and not as medical devices. The procedure is different from lateral transfixation in that it involves placement of standalone SA within the interosseous sacroiliac ligament through a dorsal trajectory. This is in contrast to transfixing devices that are placed across the intraarticular portion of the SI joint. A broach or other instrument is typically used to remove a portion of the supporting ligaments and the surrounding bone to create a channel into which a SA is placed. The goal of implant placement is to distract the joint, thereby increasing the tension of the supporting ligaments (posterior interosseous and posterior sacroiliac ligaments), a process called ligamentotaxis. It is proposed that ligamentotaxis may provide sufficient joint stability to allow for joint fusion through the SA. This fusion process has been termed distraction arthrodesis (DA). No study to date has reported on the radiographic outcomes of standalone SA in the SI joint.
This new procedure is different in several ways: 1) it uses a standalone structural allograft rather than a metallic implant, 2) it employs a different mechanism of action, distraction arthrodesis (DA) rather than transfixation, 3) addresses different anatomy (dorsal ligamentous joint versus the more ventral articular joint transfixed laterally), and 4) its safety, effectiveness, and durability have yet to be determined.
Clinical data on the effectiveness of these allograft products is limited to one retrospective study.29 Herein we report clinical failures of SI joint fusion with SAs that were subsequently treated with porous titanium implants. Our observations raise concerns about the safety and effectiveness of the use of standalone structural allograft for SI joint stabilization/fusion.
Methods
This multicenter retrospective case series was conducted at seven centers. Institutional review board approval was obtained (Dignity Health, 037649) and it was determined that the study met criteria for a waiver of consent. In accordance with IRB approval and under the Declaration of Helsinki, no consent was sought and no personally identifiable information was collected. Chart and billing records were reviewed by the treating physicians to identify patients who underwent SI joint stabilization/fusion with standalone SA products who subsequently underwent revision SI joint fusion with porous titanium implants. Demographic, clinical, and radiographic information were reviewed and collected in a shared electronic database. Deidentified radiographic images were reviewed.
Results
A total of 37 patients were identified. The average age at index procedure of SI joint stabilization/fusion using SA was 57 (SD 14, range 28–81). Sixty-two percent were female, and average BMI was 31 (5.4). About half (46%) of cases were bilateral. Three SAs were placed in 51% of cases, two implants were used in 30%, and 19% used a single implant. These represent six SA products (Cornerloc, Fusion Foundation Solutions, LLC; Transfasten, Captiva Spine; LinQ, PainTEQ; SiJoin, VGI Medical; SIFix, NuTech Spine, Inc.; PsiF, Omnia Medical). Concomitant use of demineralized bone matrix was reported in 79% of index cases.
Revision surgery occurred on average 25 months (SD 16, range 1–73) after initial placement of SA. Two patients had an additional SA placed posteriorly prior to undergoing surgical revision. All patients had confirmatory SI joint injections before proceeding with the lateral revision surgery. Indications for revision surgery were pain recurrence in 51%, failure to relieve pain in 49%; one patient had worsening pain associated with an adverse inflammatory reaction to the SA (see Case 1, Figure 2, presented below).
At the time of revision surgery, imaging studies across all patients showed universally that no radiographic fusion (incorporation of the SA with the host bone on both the iliac and sacral sides) occurred. Imaging showed that many SAs were in a suboptimal position, with locations partially outside of the joint space or even totally within the sacrum or ilium. Imaging available for review did not allow for a determination of initial SA malposition or if the implants changed position after initial placement (eg, migration and subsidence).
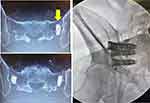
All patients underwent salvage SI joint fusion with porous titanium implants, either triangular in shape (iFuse Implant System, SI-BONE, Santa Clara, CA, USA) or a threaded implant (iFuse-TORQ, SI-BONE). Three devices were placed per treated side in all patients. Two adverse events were identified related to the iFuse procedure. In one case, the titanium implant pushed the SA through the ventral cortex of the sacrum. This patient was treated conservatively without sequelae. One patient experienced radicular pain in the S1 nerve root distribution due to symptomatic malposition of the cephalad implant with advancement of the TTI partially into the neuroforamen. The implant was removed, and a shorter implant was placed in the same position, with complete resolution of symptoms. Average pain relief at follow-up was 89% (SD 13%). One patient, who failed to report relief after SA placement, reported no pain relief or clinical improvement one year post-op from the lateral revision using triangular implants.
A brief description of six sample cases is presented below.
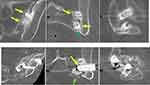
Case 1 (Figure 2). Patient underwent placement of 3 SAs (SiJoin, VGI Medical) in the right SI joint. Right leg pain occurred about 2 months later. At month 4 after the index procedure, the surgeon placed an additional SA (TransFasten, Captiva Spine) in the right SI joint. Several weeks after the second procedure, the patient experienced fever and chills. The surgeon suspected infection or inflammatory reaction. The patient was treated with antibiotics, and all allografts were removed approximately 3 months after the second procedure. At surgery, necrotic tissue was seen without purulence. Blood and tissue cultures were negative. There was significant bone destruction related to a presumed inflammatory reaction. Approximately 2 ½ years after initial SA placement, the patient underwent SI joint fusion with triangular titanium implants with resolution of pain.
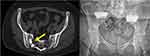
Case 2 (Figure 3). Patient underwent SA (Transfasten). One year later they reported pain in the back and hip. Approximately 2 years after initial SA placement, revision surgery was performed using triangular titanium implants. The third (caudal-most) implant pushed the previously placed SA ventrally, fracturing the anterior cortex of the sacrum. This resulted in fracture pain but no neurologic, vascular, or other sequelae. Pain was managed medically and completely resolved within 4 weeks. Subsequent imaging demonstrated complete fracture healing. Further, SI joint pain had improved by 80%.
Case 3 (Figure 4). Patient underwent placement of 2 SAs (CornerLoc) for left-sided SI joint pain. The pain persisted after surgery, continuing to worsen. Eleven months later a revision was performed using 2 triangular titanium and 1 threaded implant. The patient reported significant pain relief at the 3-week post-op visit.
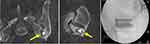 |
Figure 4 (Case 3): Yellow arrows point to placement of the SA into the ilium and not the joint space. Patient underwent successful revision using one threaded and two triangular porous devices. |
Case 4 (Figure 5). Patient initially had three SAs (SiJoin) placed. Patient continued to complain of pain and underwent multiple injections, medial branch blocks, and RF ablation to no avail. Nearly three years after continued non-operative management and development of contralateral pain, they underwent successful SI joint fusion using triangular titanium implants bilaterally with significant pain relief.
Case 5 (Figure 6). Patient underwent placement of two SAs (LinQ, PainTEQ) for left SI joint pain. After reporting no improvement, an additional SA was placed. Eleven months after the index procedure, the patient underwent surgical revision with 3 transfixing titanium implants, resulting in significant pain relief.
Case 6 (Figure 7). Patient underwent bilateral SA placement and never improved. Upon exam, patient had positive provocative maneuvers and received relief from intraarticular SIJ injections. CT scan showed SA had fractured. Patient underwent staged SIJ fusion surgery using titanium implants with complete resolution of symptoms.
Discussion
MIS surgical fusion of the SI joint with placement of metallic devices that transfix the joint is an accepted procedure for chronic SI joint pain/dysfunction. The available body of literature, developed over a decade, supports its efficacy when used in accordance with evidence-based guidelines and techniques.31,32 The use of SA as a treatment for chronic SI joint pain has gained in popularity. Our experience reported herein suggests caution should be used regarding this procedure.
In our current case series, we observed failed SI joint stabilization/fusion with standalone structural allograft products that were revised using titanium implants. Half were due to failure of the index procedure to provide improvement in pain and function, and half were revised due to a recurrence of pain. None showed radiographic fusion; all showed some evidence of pseudoarthrosis and poor implant position. One case illustrated the inherent risk of using a human cell and tissue product: inflammatory reaction. Currently, there are no prospective data available on the use of standalone SA for SI joint fixation/fusion.
The new and different procedure of distraction arthrodesis using standalone structural allografts has come with little to no evidence to support its effectiveness or safety. The retrospective case series illustrates surgical failures associated with the posterior procedure. The posterior technique may violate biologic and biomechanical principles of joint fusion surgery. The presumed mechanism of action utilizing ligamentotaxis/distraction arthrodesis has been disproven in the spine and SI joint.33,34 Moreover, since it is classified as an HCT/P and not a medical device, SA is not subject to the same adverse event and complaint reporting regulations that are required with medical devices. An additional concern is that companies promoting SA for a specific use (SI joint fusion) may be inconsistent with FDA regulations pertaining to HCT/Ps.35
SA is proposed to work via DA. DA is not a new concept in the literature; it has been used in the lumbar spine.33,36,37 It is accomplished by distracting the joint through placement of an implant between the bony surfaces. Joint distraction tensions the surrounding joint ligaments, leading to increased joint stability. Over time, fusion occurs via incorporation of the implant into host bone. Subsidence is not uncommon when used in the lumbar spine.33
Several biological and biomechanical requirements must be met for this technique to be effective. Proper preparation of the joint space is crucial such that there is direct contact between the graft and host cancellous bone. In the lumbar spine, this involves removal of surrounding disc material and the cartilaginous endplates. In the SI joint, this may involve removal of the avascular posterior interosseous ligament that typically surrounds the structural allograft. Careful preparation of the fusion bed is necessary to avoid joint subchondral bony fracture and graft subsidence. Removal of the soft tissue ligament insertion will improve graft incorporation. Adequate blood must be supplied to the area to provide the oxygen-rich environment necessary for osteoblast differentiation, and eventual allograft incorporation into the host bone. Finally, stability of the motion segment must be immediately obtained and maintained to allow these biological processes to occur.
Evidence demonstrating the efficacy of distraction arthrodesis of the SI joint is weak. Endres reported a series of patients treated with ligamentotaxis and distraction arthrodesis using a cylindrical threaded cage placed into the ligamentous SI joint.34 Improvements in pain and disability were modest. Fuchs et al described resection of posterior SIJ ligaments, removal of the interosseous ligaments, drilling open iliac and sacral cortical bone, and placement of a hollow tapered titanium screw up to 19mm in diameter followed by a large quantity of allograft or bone substitute.38 Improvements in pain and ODI were observed, along with a high rate of radiographic fusion when rh-BMP (off-label use) was used.38 Wise and Dall describe a similar procedure in which the posterior interosseous ligament was removed before placing a threaded fusion cage to distract the joint, with concomitant use of rh-BMP.39 Of 13 patients undergoing this procedure, moderate to large improvements in pain were observed, with a high rate of radiographic fusion. A recent meta-analysis suggests that implants placed with a dorsal procedure generally show poorer results compared to lateral transfixing devices.31
The use of standalone SA for SI joint fusion does not meet the above-described requirements for effective DA. First, an attempt to place SA intraarticularly using a dorsal trajectory would most likely not result in joint distraction as the joint articular surfaces are closely opposed, 1–2 mm, and the joint is not freely distensible. Further, there is no mechanism by which allografts could be placed intraarticularly without damage to subchondral bone of the sacrum/ilium unless the posterior interosseous ligament is removed, resulting in joint destabilization,40 the opposite of the desired effect. Moreover, SAs are typically placed into the ligamentous SI joint, which has poor vascularity. In the current case series, we noted that placement of the SA within the ligamentous portion of the joint was challenging, and malposition was common. The observed suboptimal device location in the current case series may reflect initial implant malposition and probably subsidence occurrence. Placing the SA in the ligamentous portion of the joint has several additional potential challenges. Over time, soft tissues loosen secondary to stress relaxation, which leads to loss of stability. Graft resorption occurs over time, leading to a loss of distraction (and thus stability). Continued joint motion may result in the graft not incorporating into the host bone and subsidence into the poor sacral alar bone observed in this older patient population. Rather, cells in the callous will differentiate into fibroblasts and fibrocartilage, which does not provide permanent joint immobilization. Indeed, observation of pseudoarthrosis was common in the current case series.
The biomechanics of lateral transfixation and dorsal interpositional SA have been studied. Lindsey et al demonstrated that porous triangular devices placed in a lateral transfixing procedure provided joint stability in a destabilized cadaver model, and this motion reduction was preserved after 5000 motion cycles.41 Sayed et al showed that in young (35-year-old) cadavers without osteoporosis or bony changes at the SI joint and intact SI joint ligaments, placement of SA significantly decreased motion at the SI joint.42 It is well known that ligament stiffness decreases with age, which alters the biomechanics of the pelvis.43–46 The mean age in a retrospective chart review study with the same authors and same SA manufacturer was 68 years old.29 Thus, the biomechanical results observed with SA may not be transferable to the broader population since the cadaver demographics are not reflective of the patient population.
The appeal of using SA is not unwarranted; allograft has several advantages along with challenges. Allografts can be processed into a wide range of physical forms (shape, size, strength, surface area, porosity) and create less artifact on imaging. The modulus of elasticity is similar to normal bone as opposed to the much harder materials of manufactured cages, resulting in less stress shielding.47 Lastly, they are less likely to be covered by biofilm48 commonly seen on metallic devices, thus more resistant to exogenous infection.49 However, they also come with significant drawbacks. Sterilization using irradiation decreases bone induction, formation, and union, compromising the intended graft incorporation and biomechanics, thereby reducing allograft strength by up to 50%.50–52 Islam et al found that gamma radiation sterilization severely impairs the high cycle fatigue life of SA.53 Hamer et al found a dose-dependent loss of strength after various irradiation levels alone and combined with deep-freezing.54 While overt immunologic reaction against allograft is rare, low-grade inflammation or GVH disease can be found around nearly all allografts. This can slow graft incorporation into the host bone.55 In the current case series, we observed one possible inflammatory response to SA.
Biomechanical studies of distraction arthrodesis and ligamentotaxis, coupled with poor clinical and radiographic outcomes, have resulted in the use of instrumentation with structural allograft in lumbar spinal fusion. There is a long history of SA in the lumbar spine.56 Clinical and radiographic results of DA using SA for lumbar interbody fusion have been reported as very poor (20%) to very good (90%).57 Higher level studies show consistently better clinical and radiographic results when interbody fusion is performed in conjunction with rigid instrumentation, typically pedicle screws and rods. In the late 1990s and early 2000s, DA using standalone threaded fusion cages showed early promise.36,37 Widespread early adoption was described in the lay press as “cage rage”. Subsequent reports and multiple studies showed significantly higher rates of cage subsidence, pseudoarthrosis, cage extrusion, as well as inferior clinical and radiographic results compared to cages backed up with rigid instrumentation.33,58–60 Biomechanical studies demonstrated that ligamentotaxis and distraction arthrodesis did not provide adequate stability to the motion segment whether stand-alone cages were placed from an anterior or a posterior approach.61–63 Pedicle screw instrumentation in conjunction with interbody structural allograft or cages provides significant additional stability to the motion segment, which is a critical factor for successful fusion.64 Current practice for lumbar interbody fusion is to supplement the interbody SA or cage(s) with rigid fixation.
The case series presented herein has strengths and weaknesses. The major strength of this case series is that it includes a large number of clinical failures with documented radiographic evidence showing both malposition and non-union. However, our data did not allow us to determine the rate of malposition due to suboptimal placement, migration, subsidence or iatrogenic mechanical displacement. Furthermore, our data do not allow us to determine the rate or timing of biomechanical failures or the rate of clinical failures.
Conclusions
In conclusion, a new procedure for SI joint stabilization/fusion involving placement of standalone structural allografts using a dorsal approach has demonstrated complications including persistent or recurrent post-operative pain, implant malposition, failure to achieve and/or maintain adequate joint stability through DA, structural failures, and lack of joint fusion. Infection or inflammatory reactions are also possible. The complication rate of the posterior SI joint posterior procedure using SA is unknown as no prospective clinical evidence has been published and the regulatory reporting requirements for SA-related adverse events are minimal for HCT/Ps. SA is being promoted for specific uses (SI joint fusion), in violation of FDA regulations. Given the minimally invasive nature of the dorsal interpositional/intraarticular procedure using standalone SA, randomized trials against sham should be performed. These trials should include radiographic endpoints that confirm joint fusion and long-term follow-up (at least 2 years) to confirm clinical durability. Until clinical efficacy is demonstrated and established, the posterior SI joint procedure using standalone SA may not be the treatment of choice for patients.
COI Statement
Two authors are employees of the titanium implant manufacturer.
Disclosure
AK reports consulting fees from SI-BONE; in addition, Dr Andy Kranenburg has a patent Torq implant with royalties paid to SI Bone. GD reports teaching honoraria from SI-BONE, Inc., JC has nothing to disclose, MT reports consulting fees from CoreLink, WJ reports consulting fees from Abbott Labs and Boston Scientific, DS has nothing to disclose, AB has contract for any teaching or speaking engagements on behalf of SI Bone, JS reports consulting fees from SI-BONE. RC and WR are employees of a device manufacturer.
References
1. Bernard TN, Kirkaldy-Willis WH. Recognizing specific characteristics of nonspecific low back pain. Clin Orthop Relat Res. 1987;(217):266–280. doi:10.1097/00003086-198704000-00029
2. Schwarzer AC, Aprill CN, Bogduk N. The sacroiliac joint in chronic low back pain. Spine. 1995;20(1):31–37. doi:10.1097/00007632-199501000-00007
3. Maigne JY, Aivaliklis A, Pfefer F. Results of sacroiliac joint double block and value of sacroiliac pain provocation tests in 54 patients with low back pain. Spine. 1996;21(16):1889–1892. doi:10.1097/00007632-199608150-00012
4. Irwin RW, Watson T, Minick RP, Ambrosius WT. Age, body mass index, and gender differences in sacroiliac joint pathology. Am J Phys Med Rehabil. 2007;86(1):37–44. doi:10.1097/PHM.0b013e31802b8554
5. Sembrano JN, Polly DW. How often is low back pain not coming from the back? Spine. 2009;34(1):E27–E32. doi:10.1097/BRS.0b013e31818b8882
6. Cher D, Polly D, Berven S. Sacroiliac Joint pain: burden of disease. Med Devices. 2014;7:73–81. doi:10.2147/MDER.S55197
7. Cher DJ, Reckling WC. Quality of life in preoperative patients with sacroiliac joint dysfunction is at least as depressed as in other lumbar spinal conditions. Med Devices. 2015;8:395–403. doi:10.2147/MDER.S92070
8. Luukkainen R, Nissilä M, Asikainen E, et al. Periarticular corticosteroid treatment of the sacroiliac joint in patients with seronegative spondylarthropathy. Clin Exp Rheumatol. 1999;17(1):88–90.
9. Luukkainen RK, Wennerstrand PV, Kautiainen HH, Sanila MT, Asikainen EL. Efficacy of periarticular corticosteroid treatment of the sacroiliac joint in non-spondylarthropathic patients with chronic low back pain in the region of the sacroiliac joint. Clin Exp Rheumatol. 2002;20(1):52–54.
10. Maugars Y, Mathis C, Berthelot JM, Charlier C, Prost A. Assessment of the efficacy of sacroiliac corticosteroid injections in spondylarthropathies: a double-blind study. Br J Rheumatol. 1996;35(8):767–770. doi:10.1093/rheumatology/35.8.767
11. Cohen SP, Hurley RW, Buckenmaier CC, Kurihara C, Morlando B, Dragovich A. Randomized placebo-controlled study evaluating lateral branch radiofrequency denervation for sacroiliac joint pain. Anesthesiology. 2008;109(2):279–288. doi:10.1097/ALN.0b013e31817f4c7c
12. Patel N, Gross A, Brown L, Gekht G. A randomized, placebo-controlled study to assess the efficacy of lateral branch neurotomy for chronic sacroiliac joint pain. Pain Med. 2012;13(3):383–398. doi:10.1111/j.1526-4637.2012.01328.x
13. Juch JNS, Maas ET, Ostelo RWJG, et al. Effect of radiofrequency denervation on pain intensity among patients with chronic low back pain: the mint randomized clinical trials. JAMA. 2017;318(1):68–81. doi:10.1001/jama.2017.7918
14. Mehta V, Poply K, Husband M, Anwar S, Langford R. The effects of radiofrequency neurotomy using a strip-lesioning device on patients with sacroiliac joint pain: results from a single-center, randomized, sham-controlled trial. Pain Physician. 2018;21(6):607–618. doi:10.36076/ppj.2018.6.607
15. Painter CF. Excision of the os innominatum. Arthrodesis of the sacro-iliac synchrondrosis. Boston Med Surg J. 1908;159(7):205–208. doi:10.1056/NEJM190808131590703
16. Buchowski JM, Kebaish KM, Sinkov V, Cohen DB, Sieber AN, Kostuik JP. Functional and radiographic outcome of sacroiliac arthrodesis for the disorders of the sacroiliac joint. Spine J. 2005;5(5):520–528. discussion 529. doi:10.1016/j.spinee.2005.02.022
17. Lippitt AB. Percutaneous fixation of the sacroiliac joint. In: Movement, Stability, and Low Back Pain : The Essential Role of the Pelvis. Churchill Livingstone; 1997:587–594.
18. Spain K, Holt T. Surgical revision after sacroiliac joint fixation or fusion. Int J Spine Surg. 2017;11:24–30. doi:10.14444/4005
19. Khurana A, Guha AR, Mohanty K, Ahuja S. Percutaneous fusion of the sacroiliac joint with hollow modular Anchorage screws: clinical and radiological outcome. J Bone Joint Surg Br. 2009;91(5):627–631. doi:10.1302/0301-620X.91B5.21519
20. Routt MLC, Simonian PT, Inaba J. Iliosacral screw fixation of the disrupted sacroiliac joint. Tech Orthop. 1995;9(4):300–314. doi:10.1097/00013611-199400940-00010
21. Routt MLC, Meier MC, Kregor PJ, Mayo KA. Percutaneous iliosacral screws with the patient supine technique. Operat Tech Orthop. 1993;3(1):35–45. doi:10.1016/S1048-6666(06)80007-8
22. Lindsey DP, Kiapour A, Yerby SA, Goel V. Biomechanical effect of number and spacing of sacroiliac joint stabilization implants: a finite element analysis. In:
23. MacBarb RF, Lindsey DP, Woods SA, Lalor PA, Gundanna MI, Yerby SA. Fortifying the bone-implant interface part 2: an in vivo evaluation of 3D-printed and TPS-coated triangular implants. Int J Spine Surg. 2017;11:16. doi:10.14444/4016
24. Polly DW, Swofford J, Whang PG, et al. Two-year outcomes from a randomized controlled trial of minimally invasive sacroiliac joint fusion vs. Non-surgical management for sacroiliac joint dysfunction. Int J Spine Surg. 2016;10:28. doi:10.14444/3028
25. Dengler J, Kools D, Pflugmacher R, et al. Randomized trial of sacroiliac joint arthrodesis compared with conservative management for chronic low back pain attributed to the sacroiliac joint. J Bone Joint Surg Am. 2019;101(5):400–411. doi:10.2106/JBJS.18.00022
26. Patel V, Kovalsky D, Meyer SC, et al. Prospective trial of sacroiliac joint fusion using 3D-printed triangular titanium implants: 24-month follow-up. Med Devices. 2021;14:211–216. doi:10.2147/MDER.S314828
27. Rudolf L, Capobianco R. Five-year clinical and radiographic outcomes after minimally invasive sacroiliac joint fusion using triangular implants. Open Orthop J. 2014;8(1):375–383. doi:10.2174/1874325001408010375
28. Whang PG, Darr E, Meyer SC, et al. Long-term prospective clinical and radiographic outcomes after minimally invasive lateral transiliac sacroiliac joint fusion using triangular titanium implants. Med Devices. 2019;12:411–422. doi:10.2147/MDER.S219862
29. Sayed D, Balter K, Pyles S, Lam CM, Multicenter Retrospective A. Analysis of the long-term efficacy and safety of a novel posterior sacroiliac fusion device. J Pain Res. 2021;14:3251–3258. doi:10.2147/JPR.S326827
30. Lee DW, Patterson DG, Sayed D. Review of current evidence for minimally invasive posterior sacroiliac joint fusion. Int J Spine Surg. 2021;15(3):514–524. doi:10.14444/8073
31. Lorio M, Kube R, Araghi A. International Society for the advancement of spine surgery policy 2020 update-minimally invasive surgical sacroiliac joint fusion (for chronic sacroiliac joint pain): coverage indications, limitations, and medical necessity. Int J Spine Surg. 2020;14(6):860–895. doi:10.14444/7156
32. NASS Coverage Policy. Recommendations: minimally invasive sacroiliac joint fusion; 2021. Available from: https://www.spine.org/Product-Details?productid=%7B9EAC3FC6-AF81-E611-851E-005056AF031E%7D.
33. Tempel ZJ, McDowell MM, Panczykowski DM, et al. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J Neurosurg Spine. 2018;28(1):50–56. doi:10.3171/2017.5.SPINE16427
34. Endres S, Ludwig E. Outcome of distraction interference arthrodesis of the sacroiliac joint for sacroiliac arthritis. Indian J Orthop. 2013;47(5):437–442. doi:10.4103/0019-5413.118197
35. Lorio MP. Editor’s introduction: update on current sacroiliac joint fusion procedures: implications for appropriate current procedural terminology medical coding. Int J Spine Surg. 2020;14(6):853–859. doi:10.14444/7136
36. Kuslich SD, Danielson G, Dowdle JD, et al. Four-year follow-up results of lumbar spine arthrodesis using the Bagby and kuslich lumbar fusion cage. Spine. 2000;25(20):2656–2662. doi:10.1097/00007632-200010150-00018
37. Kuslich SD, Ulstrom CL, Griffith SL, Ahern JW, Dowdle JD. The Bagby and kuslich method of lumbar interbody fusion. Spine. 1998;23(11):1267–1279. doi:10.1097/00007632-199806010-00019
38. Fuchs V, Ruhl B. Distraction arthrodesis of the sacroiliac joint: 2-year results of a descriptive prospective multi-center cohort study in 171 patients. Eur Spine J. 2017;27(1):194–204. doi:10.1007/s00586-017-5313-2
39. Wise CL, Dall BE. Minimally invasive sacroiliac arthrodesis: outcomes of a new technique. J Spinal Disord Tech. 2008;21(8):579–584. doi:10.1097/BSD.0b013e31815ecc4b
40. Bruna-Rosso C, Arnoux P-J, Bianco R-J, Godio-Raboutet Y, Fradet L, Aubin C-E. Finite element analysis of sacroiliac joint fixation under compression loads. Int J Spine Surg. 2016;10:16. doi:10.14444/3016
41. Lindsey D, Perez-Orribo L, Rodriquez-Martinez N, et al. Evaluation of a minimally invasive procedure for sacroiliac joint fusion – an in vitro biomechanical analysis of initial and cycled properties. Med Devices. 2014;2014(7):131–137. doi:10.2147/MDER.S63499
42. Sayed D, Amirdelfan K, Naidu RK, Raji OR, Falowski S. A cadaver-based biomechanical evaluation of a novel posterior approach to sacroiliac joint fusion: analysis of the fixation and center of the instantaneous axis of rotation. Med Devices. 2021;14:435–444. doi:10.2147/MDER.S347763
43. Ashby K, Yilmaz E, Mathkour M, et al. Ligaments stabilizing the sacrum and sacroiliac joint: a comprehensive review. Neurosurg Rev. 2021;45(1):357–364. doi:10.1007/s10143-021-01625-y
44. Noyes FR, Grood ES. The strength of the anterior cruciate ligament in humans and Rhesus monkeys. J Bone Joint Surg Am. 1976;58(8):1074–1082. doi:10.2106/00004623-197658080-00006
45. Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex. The effects of specimen age and orientation. Am J Sports Med. 1991;19(3):217–225. doi:10.1177/036354659101900303
46. Eichenseer PH, Sybert DR, Cotton JR. A finite element analysis of sacroiliac joint ligaments in response to different loading conditions. Spine. 2011;36(22):E1446–E1452. doi:10.1097/BRS.0b013e31820bc705
47. Kanayama M, Cunningham BW, Haggerty CJ, Abumi K, Kaneda K, McAfee PC. In vitro biomechanical investigation of the stability and stress-shielding effect of lumbar interbody fusion devices. J Neurosurg. 2000;93(2 Suppl):259–265. doi:10.3171/spi.2000.93.2.0259
48. Khatoon Z, McTiernan CD, Suuronen EJ, Mah TF, Alarcon EI. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon. 2018;4(12):e01067. doi:10.1016/j.heliyon.2018.e01067
49. McAfee PC. Interbody fusion cages in reconstructive operations on the spine. J Bone Joint Surg Am. 1999;81(6):859–880. doi:10.2106/00004623-199906000-00014
50. Dziedzic-Goclawska A, Ostrowski K, Stachowicz W, Michalik J, Grzesik W. Effect of radiation sterilization on the osteoinductive properties and the rate of remodeling of bone implants preserved by lyophilization and deep-freezing. Clin Orthop Relat Res. 1991;272:30–37.
51. Nguyen H, Morgan DAF, Forwood MR. Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2007;8(2):93–105. doi:10.1007/s10561-006-9020-1
52. Akkus O, Rimnac CM. Fracture resistance of gamma radiation sterilized cortical bone allografts. J Orthop Res. 2001;19(5):927–934. doi:10.1016/S0736-0266(01)00004-3
53. Islam A, Chapin K, Moore E, Ford J, Rimnac C, Akkus O. Gamma radiation sterilization reduces the high-cycle fatigue life of allograft bone. Clin Orthop Relat Res. 2016;474(3):827–835. doi:10.1007/s11999-015-4589-y
54. Hamer AJ, Strachan JR, Black MM, Ibbotson CJ, Stockley I, Elson RA. Biochemical properties of cortical allograft bone using a new method of bone strength measurement. A comparison of fresh, fresh-frozen and irradiated bone. J Bone Joint Surg Br. 1996;78(3):363–368. doi:10.1302/0301-620X.78B3.0780363
55. Siemionow K, Muschler G. Principles of bone fusion. In: The Spine. Elsevier, 2018:1085–1122.
56. Cloward RB. Gas-sterilized cadaver bone grafts for spinal fusion operations. A simplified bone bank. Spine. 1980;5(1):4–10. doi:10.1097/00007632-198001000-00002
57. Blumenthal SL, Baker J, Dossett A, Selby DK. The role of anterior lumbar fusion for internal disc disruption. Spine. 1988;13(5):566–569. doi:10.1097/00007632-198805000-00023
58. Button G, Gupta M, Barrett C, Cammack P, Benson D. Three- to six-year follow-up of stand-alone BAK cages implanted by a single surgeon. Spine J. 2005;5(2):155–160. doi:10.1016/j.spinee.2004.06.021
59. Mulholland RC. Cages: outcome and complications. Eur Spine J. 2000;9(Suppl 1):S110–S113. doi:10.1007/pl00008314
60. Pavlov PW, Spruit M, Havinga M, Anderson PG, van Limbeek J, Jacobs WC. Anterior lumbar interbody fusion with threaded fusion cages and autologous bone grafts. Eur Spine J. 2000;9(3):224–229. doi:10.1007/s005869900115
61. Oxland TR, Hoffer Z, Nydegger T, Rathonyi GC, Nolte LP. A comparative biomechanical investigation of anterior lumbar interbody cages: central and bilateral approaches*. J Bone Joint Surg Am. 2000;82(3):383–393. doi:10.2106/00004623-200003000-00009
62. Lund T, Oxland TR, Jost B, et al. Interbody cage stabilisation in the lumbar spine: biomechanical evaluation of cage design, posterior instrumentation and bone density. J Bone Joint Surg Br. 1998;80(2):351–359. doi:10.1302/0301-620x.80b2.7693
63. Dimar JR, Beck DJ, Glassman SD, Voor MJ, Wang M. Posterior lumbar interbody cages do not augment segmental biomechanical stability. Am J Orthop. 2001;30(8):636–639.
64. Wang ST, Goel VK, Fu CY, et al. Posterior instrumentation reduces differences in spine stability as a result of different cage orientations: an in vitro study. Spine. 2005;30(1):62–67. doi:10.1097/01.brs.0000150123.26869.48
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.