Back to Journals » Clinical Ophthalmology » Volume 15
Retrospective Analysis of Retinal Imaging in COVID-19 Positive Patients at a Tertiary Eye Care Center
Authors Patel NS, Moon JY, Katz R , Wai KM, Sobrin L, Vavvas DG, Miller JB
Received 3 March 2021
Accepted for publication 14 April 2021
Published 3 September 2021 Volume 2021:15 Pages 3727—3731
DOI https://doi.org/10.2147/OPTH.S309346
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Neal S Patel,1,* Jade Y Moon,1,* Raviv Katz,1 Karen M Wai,1 Lucia Sobrin,2 Demetrios G Vavvas,2 John B Miller1,2
1Harvard Retinal Imaging Lab, Mass Eye and Ear, Boston, MA, USA; 2Retina Service, Mass Eye and Ear, Boston, MA, USA
*These authors contributed equally to this work
Correspondence: John B Miller
Retina Service, Mass Eye and Ear, Department of Ophthalmology, Harvard Medical School, Harvard Retinal Imaging Lab, 243 Charles St, Boston, MA, 02114, USA
Tel +617 573-3750
Fax +617 573-3698
Email [email protected]
Purpose: Since the start of the COVID-19 pandemic, numerous authors have published data demonstrating retinal changes found in patients with COVID-19. However, others have debated the significance of these findings and the effects of COVID-19 on the retina remain uncertain. This study aims to better understand retinal findings in patients with COVID-19.
Patients and Methods: A retrospective review of patients with a history of a positive COVID-19 polymerase chain reaction test was performed between March 1st, 2020 and October 31st, 2020. Patients were included if they presented within 90 days of their first positive COVID-19 test and underwent color fundus photography and/or OCT of the macula. All images were reviewed by two independent graders who assessed the presence of retinal heme, cotton wool spots, vascular sheathing, and disc edema, as well as hyper-reflective changes, intra-retinal fluid, and sub-retinal fluid on OCT.
Results: A total of 119 eyes from 61 patients were included. Among 83 eyes which underwent OCT of the macula, inner retinal hyper-reflective changes were seen in 16.9% (n=14), outer retinal hyper-reflective changes in 18.1% (n=15), intra-retinal fluid in 28.9% (n=24), and sub-retinal fluid in 14.5% (n=12). Among 48 eyes which underwent color fundus photography, retinal hemorrhage was seen in 27.1% (n=13), optic disc edema in 2.1% (n=1), and cotton wool spots in none of the eyes. Sub-analysis of 70 eyes from 41 patients with no alternative retinal pathology to potentially explain the above findings revealed none of the above exam findings on OCT of the macula (n=35), fundus photography (n=28), or documented exam (n=66).
Conclusion: While a number of patients seen after COVID-19 infection demonstrated retinal findings, all could be explained by pre-existing retinal conditions. In a sub-group of eyes without pre-existing retinal disease, we did not identify any retinal findings that could be associated with COVID-19.
Keywords: COVID-19, SARS-CoV-2, retina, optical coherence tomography, fundus photography
Introduction
Severe Acute Respiratory Syndrome-Coronavirus-2 (SARS-CoV-2) is a novel coronavirus that was first detected in Wuhan, China at the end of 2019.1 The virus has since quickly spread to the rest of the world, and Coronavirus Disease-2019 (COVID-19) was declared a pandemic by the World Health Organization on March 11, 2020. Although COVID-19 mainly affects the lower respiratory tract causing acute respiratory distress syndrome (ARDS), damage to other organ systems has been reported, including gastrointestinal, cardiovascular, and neurological manifestations.2–4 Currently, not much is known about the ocular involvement of COVID-19. However, ocular involvement of Orthocoronaviridae, a subfamily of coronavirus that SARS-CoV-2 belongs to, is well established in animal models.5 In murine and feline species, Orthocoronaviridae strains have been associated with uveitis, retinitis, and optic neuritis. The first evidence of ocular involvement of coronaviruses in humans arose in the early 21st century during the SARS outbreak caused by SARS-CoV-1, as physicians noted the possibility of transmission through tear samples.6,7
With this knowledge in mind, the possibility of ocular involvement of SARS-CoV-2 has gained significant interest.8 Marinho et al suggested that on OCT (optical coherence tomography) imaging of the retina in 9 patients, there may be manifestations of the disease such as hyper-reflective lesions at the level of the ganglion cell and inner plexiform layers.8 However, Vavvas et al and others have raised concerns about the interpretation of these OCT findings.9 In 43 patients with COVID-19 pneumonia, Pirraglia et al did not find retinal manifestations except in one patient, who was later diagnosed with probable fungal retinitis.10 At the same time, Invernizzi et al compared 54 patients with a COVID-19 diagnosis to 133 control patients, and demonstrated that along with increased rates of retinal hemorrhages and cotton wool spots, the mean arterial diameter and mean venous diameter were higher in retinal imaging from patients with COVID-19 compared to control patients.11 Despite continued interest in the retinal manifestations of COVID-19, there has been no consensus established on this subject. We aim to contribute to the ongoing need for further study of this topic.
Herein, we present a retrospective observational study of SARS-CoV-2 PCR+ patients who presented to ophthalmology clinics at Mass Eye and Ear (Boston, MA, USA) between March 1, 2020 and October 31, 2020. The main objective of our study was to identify any retinal findings that could be associated with COVID-19.
Patients and Methods
Patient Selection
A retrospective cross-sectional study was conducted on patients seen at a single tertiary eye care center in Boston, MA, USA. All appointments and examinations were performed with appropriate precautions and in accordance with institutional guidelines on COVID-19. A chart review of the electronic medical record was performed on all patients who presented to ophthalmology clinics between March 1st, 2020 and October 31st, 2020. Patients were included if they had a history of a positive SARS-CoV-2 polymerase chain reaction (PCR) test and underwent retinal imaging including color fundus photography or OCT of the macula. If a patient had multiple visits, data from the first clinic visit after their first positive SARS-CoV-2 test was used. Patients whose first positive PCR test result for SARS-CoV-2 was collected greater than 90 days prior to their clinic appointment were excluded from the study. In order to evaluate the maximum number of eyes, patients with concomitant retinal diseases were not excluded from the study.
Review of Retinal Findings
In all patient charts that were reviewed, OCT images of the macula were taken with Heidelberg Spectral Domain OCT (Spectralis®, Heidelberg, Germany). These OCT images were all volumetric scans of the macula composed of a minimum of 73 B-scans (120 microns between B-scans at maximum). These images were reviewed specifically for presence of inner retinal hyper-reflective changes, outer retinal hyper-reflective changes, intra-retinal fluid, and sub-retinal fluid. Wide-field color fundus photography was taken on an Optos (Optos, Marlborough, MA, USA) imaging device. Disc photography was performed on a Topcon (Topcon TRC-50DX, Topcon Corporation, Tokyo, Japan) fundus camera. These images were reviewed for presence of retinal hemorrhages, optic disc edema, and cotton wool spots. All images were interpreted by two independent graders. In addition to the review of retinal imaging, a chart review was also performed to assess documented retinal exam findings from the clinic visit on the same date as retinal images was taken. All reviews were performed once with the entire cohort and repeated with the subgroup with no pre-existing retinopathy or underlying disease.
Statistical Analysis
Normal distribution was analyzed by the Shapiro–Wilk Test. Normally distributed continuous variables were reported as the mean values and standard deviations (SD). Continuous variables that were not normally distributed were reported as the median values and interquartile ranges (IQR). Categorical variables were reported as counts and percentages.
Ethics Approval
Institutional review board approval was obtained from Mass General Brigham for the Protection of Human Subjects (#2020P000988). The authors certify that this work is HIPAA compliant and adhered to the tenets of the Declaration of Helsinki.
Results
Demographic Information
During the study period, 158 patients with a history of a positive SARS-CoV-2 PCR test presented to ophthalmology clinics at Mass Eye and Ear. Of these, 109 patients underwent color fundus photography or OCT of the macula. Forty-seven patients were excluded as their most recent positive SARS-CoV-2 PCR test was collected more than 90 days prior to their appointment. Ultimately, 119 eyes of 61 patients were included in the study. Three eyes from three patients were not included due to media opacities preventing interpretable retinal imaging (one eye with vitreous hemorrhage attributed to an established history of proliferative diabetic retinopathy, one eye with significant corneal edema, and one eye with an intraocular gas bubble from recent retinal surgery). The demographic and clinical characteristics of the cohort can be found in Table 1. The mean number of days between the first positive SARS-CoV-2 PCR test and ophthalmic examination was 60.4. The minimum number of days was 4 days and the maximum 90 days.
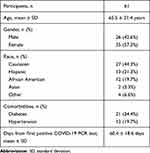 |
Table 1 Demographics and Clinical Characteristics |
Retinal Findings
In our cohort of 119 eyes, 83 eyes underwent OCT of the macula, 42 eyes underwent wide-field color fundus photography, 6 eyes underwent color disc photography, and 109 eyes had a documented retinal exam on chart review. In eyes that underwent OCT of the macula (n=83), median central macular thickness was noted to be 264 microns (IQR 54.75). Inner retinal hyper-reflective changes were seen in 14 eyes (16.9%), outer retinal hyper-reflective changes were seen in 15 eyes (18.1%), intra-retinal fluid was seen in 24 eyes (28.9%), and sub-retinal fluid was seen in 12 eyes (14.5%). In eyes that underwent wide-field color fundus photography (n=42), retinal hemorrhage(s) were seen in 12 eyes (28.6%) and optic disc edema was seen in one eye (2.4%). In eyes that underwent color disc photography (n=16), retinal hemorrhage(s) were seen in one eye (16.7%). A full list of findings from images and exams are summarized in Table 2.
 |
Table 2 Retinal Imaging and Exam Findings |
A sub-analysis of 70 eyes from 41 patients with no pre-existing retinopathy or underlying disease to explain possible new findings was performed. Please see Table 3 for a list of given diagnoses to explain abnormal retinal findings leading to exclusion from this sub-group. Careful review of these eyes identified no abnormalities on OCT of the macula (n=35), wide-field color fundus photography (n=25), color disc photography (n=3), or documented retinal exam (n=66). In this sub-group, median central macular thickness was noted to be 255 microns (IQR 43) in the 35 eyes that underwent OCT of the macula.
 |
Table 3 Concomitant Ocular Disease in Patients with Retinal Pathology |
Discussion
In this study, we did not identify any retinal findings directly associated with COVID-19 infection. All retinal findings noted in patients with COVID-19 at our institution could be explained by the patients’ pre-existing conditions. Importantly, in a subset of eyes with no pre-existing conditions (many of which were contralateral “healthy” eyes in patients with monocular conditions such as rhegmatogenous retinal detachment or retinal vein occlusion), no findings that could be associated with COVID-19 were seen.
Marinho et al were among the first to publish results suggesting retinal findings that could be associated with COVID-19, namely hyper-reflective changes in the inner retina.8 However a number of other authors have questioned these findings, raising the possibility that these hyper-reflective changes can be attributed to normal retinal structures, namely retinal blood vessels.9,12–16 While similar appearing hyper-reflective structures were seen in this review of OCT imaging, all could be attributed to retinal blood vessels when correlated to simultaneous near infrared reflectance registration images of the retina. This finding further calls into question the retinal manifestations of COVID-19 that were proposed early in the pandemic.
Studies by Caporossi et al, Pereira et al, and Pirraglia et al included retinal examinations of patients with severe COVID-19, many of whom were at the time admitted in the intensive care unit (ICU).10,17,18 While the first two groups reported evidence of microvascular disease such as retinal hemorrhages, cotton wool spots, retinal microaneurysms, and tortuous retinal vessels, Pirraglia et al did not see any of these changes in their cohort. Our study also did not show any evidence of these changes in setting of a COVID-19 infection. It remains unclear if these microvascular changes can be attributed directly to COVID-19 or if they are a consequence of the generalized state of hemodynamic instability and heightened inflammatory response seen in many patients who require care in the ICU or are on positive pressure ventilation. Of note, such retinal findings have been previously demonstrated in ICU patients independent of infection with SARS-CoV-2.19 While our study did not stratify participants by the severity of their COVID-19 illness due to the retrospective design of the study and limited access to data outside our own hospital system, it is likely skewed towards including patients with milder infections or those who have at the least overcome severe illness by the time they were seen in clinic. This may also help to explain why such retinal findings were not seen in our cohort.
In one study examining retinal thickness and volume in patients with COVID-19, Abrishami et al found a trend towards increased retinal thickness in patients with history of COVID-19, though their comparison to age-matched controls did not reach statistical significance.20 More recently, Abrishami et al have further reported changes in retinal microvasculature in patients who have recovered from COVID-19. Specifically, they noted decreased vessel density in the superficial and deep capillary plexus of the foveal and parafoveal zone on OCT angiography of patients with history of COVID-19 infection.21 While data from our study cannot be directly compared to this group, future measurements of retinal parameters may be an objective and quantifiable target for further investigation.
There were some important limitations to our analysis of the potential retinal findings from COVID-19. First, this was a retrospective study. As a result, retinal imaging was limited to studies that were necessary for each patient’s clinical needs, which may have inflated the overall rate of positive findings. This may have been further exacerbated by the clinical mindset of most providers during the COVID-19 pandemic, in which clinic appointments were limited to patients with urgent ophthalmic issues in the early stages of the pandemic. This was in line with guidelines published by the American Academy of Ophthalmology (AAO) on March 18th, 2020, which advised eye care providers to cease “providing any treatment other than urgent or emergent care immediately.”22 Yet, many more routine visits resumed within a few months of the first shutdowns minimizing the impact of this potential limitation. Further, the mean number of days from a positive SARS CoV-2 PCR to imaging was 60.4 days. There may be acute changes that were not captured due to the delay in imaging.
Conclusion
The retina is a uniquely accessible and informative window into the inner workings of human physiology. Examination of the retina has the potential to deepen our understanding of the vasculotropic and neurotropic characteristics of the now ubiquitous SARS-CoV-2 virus. In this study at a large tertiary care center in a pandemic hotspot, we found no retinal changes associated with a history of COVID-19 infection. Given the variation in the existing literature, additional study and greater longitudinal follow-up is required to better understand the effects of COVID-19 on the retina.
Disclosure
Dr John B Miller reports personal fees from Zeiss, Heidelberg, Genentech, Allergan, Alcon, and Sunovion, outside the submitted work. The authors report no other conflicts of interest in this work. De-identified data can be provided by reasonable request, delivered to the corresponding author.
References
1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi:10.1056/nejmoa2001017
2. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
3. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(7):819–824. doi:10.1001/jamacardio.2020.1096
4. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi:10.1001/jamaneurol.2020.1127
5. Seah I, Agrawal R. Can the coronavirus disease 2019 (COVID-19) affect the eyes? A review of coronaviruses and ocular implications in humans and animals. Ocul Immunol Inflamm. 2020;28(3):391–395. doi:10.1080/09273948.2020.1738501
6. Chan WM, Yuen KSC, Fan DSP, Lam DSC, Chan PKS, Sung JJY. Tears and conjunctival scrapings for coronavirus in patients with SARS. Br J Ophthalmol. 2004;88(7):968–969. doi:10.1136/bjo.2003.039461
7. Loon S-C, Teoh SCB, Oon LLE, et al. The severe acute respiratory syndrome coronavirus in tears. Br J Ophthalmol. 2004;88(7):861. doi:10.1136/bjo.2003.035931
8. Marinho PM, Marcos AAA, Romano AC, Nascimento H, Belfort JR. Retinal findings in patients with COVID-19. Lancet. 2020;395(10237):1610. doi:10.1016/S0140-6736(20)31014-X
9. Vavvas DG, Sarraf D, Sadda SR, et al. Concerns about the interpretation of OCT and fundus findings in COVID-19 patients in recent Lancet publication. Eye. 2020;34(12):2153–2154. doi:10.1038/s41433-020-1084-9
10. Pirraglia MP, Ceccarelli G, Cerini A, et al. Retinal involvement and ocular findings in COVID-19 pneumonia patients. Sci Rep. 2020;10(1):17419. doi:10.1038/s41598-020-74446-6
11. Invernizzi A, Torre A, Parrulli S, et al. Retinal findings in patients with COVID-19: results from the SERPICO-19 study. EClinicalMedicine. 2020;27:100550. doi:10.1016/j.eclinm.2020.100550
12. Collison FT, Carroll J. Seeking clarity on retinal findings in patients with COVID-19. Lancet. 2020;396(10254):e38. doi:10.1016/S0140-6736(20)31917-6
13. Duh EJ. Seeking clarity on retinal findings in patients with COVID-19. Lancet. 2020;396(10254):e39. doi:10.1016/S0140-6736(20)31913-9
14. Ouyang P, Zhang X, Peng Y, Jiang B. Seeking clarity on retinal findings in patients with COVID-19. Lancet. 2020;396(10254):e35. doi:10.1016/S0140-6736(20)31921-8
15. Venkatesh P. Seeking clarity on retinal findings in patients with COVID-19. Lancet. 2020;396(10254):e36. doi:10.1016/S0140-6736(20)31922-X
16. Brandão-de-resende C, Diniz-Filho A, Vasconcelos-Santos DV. Seeking clarity on retinal findings in patients with COVID-19. Lancet. 2020;396(10254):e37. doi:10.1016/S0140-6736(20)31918-8
17. Caporossi T, Bacherini D, Tartaro R, VIrgili G, Peris A, Giansanti F. Retinal findings in patients affected by COVID 19 intubated in an intensive care unit. Acta Ophthalmol. 2020. doi:10.1111/aos.14734
18. Pereira LA, Soares LCM, Nascimento PA, et al. Retinal findings in hospitalised patients with severe COVID-19. Br J Ophthalmol. 2020:
19. Erikson K, Liisanantti JH, Hautala N, et al. Retinal arterial blood flow and retinal changes in patients with sepsis: preliminary study using fluorescein angiography. Crit Care. 2017;21(1):86. doi:10.1186/s13054-017-1676-3
20. Abrishami M, Tohidinezhad F, Emamverdian Z, et al. Macular thickness and volume assessed by spectral-domain optical coherence tomography in patients with coronavirus disease 2019: a case-control study; 2020.
21. Abrishami M, Emamverdian Z, Shoeibi N, et al. Optical coherence tomography angiography analysis of the retina in patients recovered from COVID-19: a case-control study. Can J Ophthalmol. 2021;56(1):24–30. doi:10.1016/j.jcjo.2020.11.006
22. Recommendations for urgent and nonurgent patient care. Available from: https://www.aao.org/headline/new-recommendations-urgent-nonurgent-patient-care.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
