Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Retinal Neovascularization in Two Patients with Incontinentia Pigmenti
Authors Dwiyana RF , Banjarnahor ID , Diana IA, Gondokaryono SP , Effendi RMRA , Feriza V
Received 23 February 2022
Accepted for publication 20 April 2022
Published 29 April 2022 Volume 2022:15 Pages 803—808
DOI https://doi.org/10.2147/CCID.S363179
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Reiva Farah Dwiyana, Ivan Daniel Banjarnahor, Inne Arline Diana, Srie Prihianti Gondokaryono, Raden Mohamad Rendy Ariezal Effendi, Vina Feriza
Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran-Dr.Hasan Sadikin Hospital, Bandung, Indonesia
Correspondence: Reiva Farah Dwiyana, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Bandung, Indonesia, Tel +62811247474, Email [email protected]
Abstract: Incontinentia pigmenti (IP) is a rare genodermatosis, inherited in an X-linked dominant pattern, making it generally found among women. Among several characteristics of IP are four phases of skin manifestation that tend to follow Blaschko’s lines, in addition to abnormalities of the eye, central nervous system (CNS), and teeth. Ocular involvement in IP patients can occur since birth, which can be classified into retinal or non-retinal disorders. Retinal disorders can result in detachment, which is a major ocular threat for IP patients. This article reports two IP cases with overlapped phases of skin disorders in baby girls with ocular manifestations since early life. Clinical signs and additional examination of the skin and eyes are utilized to make the diagnosis. All the features of the histopathological examination supported the diagnosis of IP, and ocular exams revealed abnormalities in the form of retinal neovascularization (RN). Although RN may resolve spontaneously, patients should be monitored for the development of other eye disorders such as visual impairment.
Keywords: genodermatosis, incontinentia pigmenti, ocular manifestation, retina
Introduction
Incontinentia pigmenti (IP) or Bloch-Sulzberger,1–3 is a very rare X-linked dominant disorder, with an incidence of 1.2 per 100.000 births.4,5 Ninety-eight percent of cases are found in women.3,6 This disorder mainly occurs following a mutation in the inhibitor of kappa β kinase gamma (IKBKG) gene,1–3 formerly known as nuclear factor-κβ (NF-κβ) essential modulator (NEMO),4,6 located on the Xq28 segment of the X chromosome.1,2,4,7–9
Skin abnormalities are the first and main clinical manifestations found in IP,2 which could appear at birth or immediately after, especially in the first two weeks of life.3,8 The dermatological abnormalities of IP characteristically follows Blaschko’s lines.4,8 There are four phases of skin disorders in IP,2,3 which includes the vesicular phase, verrucose phase, hyperpigmentation phase, and the hypopigmentation/atrophy phase.3,4 Landy and Donnai in 1993 made diagnostic criteria consisting of major (clinical features typical of IP) and minor criteria for IP10 and then in 2014, Minic et al11 modified it by adding IKBKG gene mutations into the diagnostic criteria.
Approximately 80% of IP cases present with extracutaneous manifestations,7 including anomalies of the teeth (30–92%), eyes (36–77%), CNS (28–66%), hair (13–35%), and nails (7–40%).7,9 CNS and ocular manifestations can remain undetected until infancy or early childhood, leading to severe functional nervous system abnormalities such as convulsion, mental retardation,3,12 and muscle spasticity,3 as well as visual impairments ranging from mild to severe, even blindness.2,7,13,14
Ocular manifestations in IP patients may occur since birth7 in the form of retinal or non-retinal disorders.10,15 Retinal disorders include neovascularization, fibrosis, and retinal detachment, whereas non-retinal disorders include nystagmus, cataracts, microphthalmia, uveitis, conjunctival pigmentation and hypoplasia of the iris.10,15 The role of the NF-kB protein in protecting capillary endothelial cells from apoptosis can be used to explain the retinal findings. IKBKG gene mutations cause cell death, which leads to microvascular problems such as retinal ischemia and neovascularization.9 Early screening of ocular disorders are important, as neovascularization of the retina can leads to retinal detachment and blindness,13,14 however some cases may resolve spontaneously.3 This case report aims to present two rare cases of IP in baby girl with ocular manifestations in the form of RN.
Case Presentation
Case 1
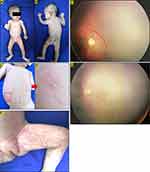
A two-month-old baby girl presented with a painless and non-itchy erythematous papules and vesicles on abdomen, groins, and both upper limbs (Figure 1A-D), as well as hyperpigmented macules arranged spindle-like in almost all parts of the body except the face, both palms and soles (Figure 1E). From history taking revealed that the skin lesions appeared since birth in the form of erythematous macules and papules, vesicles, pustules, also verrucous plaque on abdomen, back, both arms and legs. There was no family history with the same features. On physical examination, on almost all parts of the body except the scalp, face, palms and soles, there were hyperpigmented macules distributed linearly and swirled along Blaschko’s lines. Erythematous macules and vesicles were found on abdomen, groins, and both upper limbs. Based on these skin lesions, we diagnosed this patient was IP with vesicular phase overlapped with hyperpigmentation phase. Laboratory examination results showed eosinophilia (12%). No abnormalities were found in the EEG, whereas ophthalmological evaluation by the ophthalmologist showed RN on the peripheral side of the right eye (Figure 1F). Histopathological examination with routine hematoxylin eosin stains, did not support IP. There is no specific treatment for skin lesions, apart from moisturizers. No specific interventions was performed for the retinal disorder, but the patient was advised to undergo routine follow-up for further evaluation. On day 116 of evaluation, the skin lesions resolved, leaving hyperpigmented macular lesions, and the RN achieved spontaneous resolution (Figure 1G).
Case 2
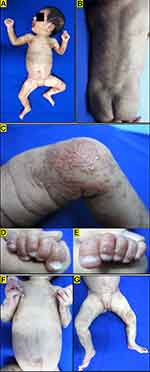
A two-month-old baby girl presented with painless and non-itchy erythematous papules as well as hyperpigmented macules and papules in almost all parts of the body except the face, both palms and feet (Figure 2A and B). From history taking revealed that the skin lesions appeared since birth in the form of erythematous papules and vesicles on both arms, which continuously increased in number and spread all over the body. Some of the erythematous papules and vesicles resolved and formed verrucous papules (Figure 2C-E) and also hyperpigmented macules (Figure 2F and G), whereas new lesions kept appearing. There was no family history with the same complaint. On physical examination, on almost all parts of the body except the face, both palms and feet, there were erythematous macules and papules, vesicles, verrucous papules and also hyperpigmented papules, distributed linearly and swirled along Blaschko’s lines. Linear skin lesions in the form of atrophic striae were found on the medial side of the upper right thigh. Laboratory examination results showed eosinophilia (27%). Based on the clinical features, we diagnosed this patient was IP with vesicular phase overlapped with hyperpigmentation and atrophy phases. No abnormalities were found in the EEG, whereas ophthalmological evaluation by the ophthalmologist showed RN on the peripheral side of the left eye. Histopathological examination with routine hematoxylin eosin stains indicated IP features. The patient was given with a moisturizer and 0.1% mometasone furoate cream to be applied on erythematous papules. No specific interventions were performed for the retinal disorder, but the patient was advised to undergo routine follow-up for further evaluation. On day 140 of evaluation, the skin lesions resolved, leaving hyperpigmented macular lesions, and the RN achieved spontaneous resolution. This patient suffered unrealized visual impairment (myopia and astigmatism) about 7 years later, and it might be related to retinal neovascularization in IP.
Discussion
The extracutaneous manifestations of IP can found in teeth, eyes, hair, nails and CNS.7,15 A total of 30–92% of patients have dental abnormalities (delayed tooth growth, anodontia, hypodontia, and deformities),15 35–70% of patients have eye involvement (strabismus, cataract, RN, optic atrophy, retinal pigment abnormalities),2,14,15 and 13–35% of patients have CNS involvement (seizures, motor and intellectual impairment).4 In addition, 28–66% of abnormalities in the hair (alopecia), 7–40% of nail abnormalities (ranging from mild pitting to onychodystrophy),15 and 11–30% of nipple aplasia can also be found.4,11,15 Typical skin manifestations of IP may help in early diagnosis of other organ involvement.3
Ocular abnormalities in IP patients is generally unilateral,4,17 and if bilateral involvement is observed, one eye tend to have milder manifestations.17 Clinical manifestations found in the eye may vary,14 and is generally classified into two groups, retinal and non-retinal abnormalities.10,13 Retinal abnormalities are caused by occlusive blood vessel conditions or ischemia, which is compensated by blood vessel proliferation.4,9,15 The impairment usually appears at birth or up to one year old,10 in the form of retinal blood vessel disorders, retinal fibrosis, or retinal detachment.10,15 Meanwhile, non-retinal disorders usually occur before the age of two.10 The most common non-retinal manifestations include strabismus (18–33%) and optic nerve atrophy (4%), whereas nystagmus, cataract, microphthalmia, uveitis, conjunctival pigmentation and hypoplasia of the iris are rarely found.10,15 Minic et al11 reported that retinal blood vessel disorders and retinal detachment are the most common ocular manifestations among IP patients. Untreated eye disorders may lead to retinal detachment and eventual blindness,16 whereas neovascularizations can remain stable and resolve spontaneously.7,17 There are currently no clear recommendations regarding the screening or follow-up examinations for eye disorders in patients with IP, but early and periodic eye evaluations are warranted to evaluate retinal involvement, therefore allowing early intervention if abnormalities are detected.3,4 A study by Holmstrom and Thoren17 recommended prompt retinal evaluation immediately after birth, followed by monthly evaluations up to the age of three to four months, quarterly evaluations up to the age of one year, biannual evaluations up to the age of three years, and yearly evaluations throughout the childhood period. Oranges et al18 conducted a randomized controlled trial in which preterm newborn IP patients with retinopathy of prematurity (ROP) that administered oral propranolol (0.25 or 0.5 mg/kg/6 hours) to preterm newborn IP patients with retinopathy of prematurity caused by ischemic processes and neovascularization. As a result, propranolol showed significantly decreased ROP progression in newborn patients.20
In all two patients, the diagnosis of IP was based on the clinical manifestations and histopathological examination results. In both cases, retinal neovascularization was discovered on the peripheral side of the eye, and the patient was recommended for periodic follow-up with no specific intervention. At the three to four-month follow-up, the retinal neovascularization resolved spontaneously without any treatment. However, in the second case, visual impairments were discovered after the patient reached school age, and it could not be determined whether they were caused by IP or not.
Conclusion
The prognosis of IP is generally good but accompanying extracutaneous manifestations of the central nervous system and/or the eyes can affect quality of life, therefore early screening and regular follow up is necessary.
Consent for Publication
The patient’s parents have signed the consent forms for the use of case details, images for publication, and for scientific purposes. Institutional approval from The Research Ethic Committee of Dr. Hasan Sadikin General Hospital Bandung, Indonesia has been obtained to publish the case details (approval number: LB.02.01/X.6.5/71/2022).
Acknowledgments
Authors would like to thank all staff of the Dermatology and Venereology Department, Faculty of Medicine Universitas Padjadjaran – Hasan Sadikin General Hospital Bandung and Division of Pediatric Ophthalmology, National Eye Center Cicendo Hospital Bandung.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Celia M, Fiona B. Mosaicism and linear lesions. In: Bolognia L, Schaffer J, Cerroni L, editors. Dermatology.
2. Nieman EL, Dorothy KG, et al. Ectodermal dysplasias. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, editors. Fitzpatrick’s Dermatology,
3. Paller AS, Mancini AJ. Disorders of pigmentation. In: Paller AS, Mancini AJ, editors. Hurwitz Clinical Pediatric Dermatology. Toronto: Elsevier; 2016:245–278.
4. Jones EA, Donai D. Incontinentia pigmenti. In: Hoeger P, Kinsler V, Yan A, editors. Harper’s Textbook of Pediatric Dermatology.
5. Mariath LM, Santa Maria FD, Poziomczyk CS, et al. Intrafamilial clinical variability in four families with incontinentia pigmenti. Am J Med Genet. 2018;176(11):2318–2324.
6. Itin P. Genodermatoses. In: Schachner LA, editor. Pediatric Dermatology. China: Mosby-Elsevier; 2011:391–393.
7. Peng J, Zhang Q, Long X, et al. Incontinentia pigmenti-associated ocular anomalies of paediatric incontinentia pigmenti patients in China. Acta Ophthalmol. 2019;97(3):265–272.
8. Wang R, Lara-Corrales I, Kannu P, et al. Unraveling incontinentia pigmenti: a comparison of phenotype and genotype variants. J Am Acad Dermatol. 2019;81(5):1142–1149.
9. Bryan J, Issa R, Bakall B, et al. Retinal Manifestations of Incontinentia Pigmenti: a Case Series of 14 Patients Highlighting the Importance of Intravenous Fluorescein Angiography and the Benefits of Early Laser Photocoagulation. J Vitreoretin Dis. 2021;5(1):60–65.
10. Berlin AL, Paller AS, Chan LS. Incontinentia pigmenti: a review and update on the molecular basis of pathophysiology. J Am Acad Dermatol. 2002;47(2):169–190.
11. Minić S, Trpinac D, Obradović M. Incontinentia pigmenti diagnostic criteria update. Clin Genet. 2014;85(6):536–542.
12. Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31(6):e45–e52.
13. Michel S, Reynaud C, Daruich A, et al. Early management of sight threatening retinopathy in incontinentia pigmenti. Orphanet J Rare Dis. 2020;15(1):1–5.
14. Bodemer C, Diociaiuti A, Hadj-Rabia S, et al. Multidisciplinary consensus recommendations from a European network for the diagnosis and practical management of patients with incontinentia pigmenti. J Eur Acad Dermatol Venereol. 2020;34(7):1415–1424.
15. Poziomczyk CS, Bonamigo RR, Santa Maria FD, et al. Clinical study of 20 patients with incontinentia pigmenti. Int J Dermatol. 2016;55(2):e87–e93.
16. O’Doherty M, Mc Creery K, Green AJ, Tuwir I, Brosnahan D. Incontinentia pigmenti ophthalmological observation of a series of cases and review of the literature. Br J Ophthalmol. 2011;95(1):11–16.
17. Holmström G, Thorén K. Ocular manifestations of incontinentia pigmenti. Acta Ophthalmol Scand. 2000;78(3):348–353.
18. Oranges T, El Hachem M, Filippeschi C, et al. The potential role of propranolol in incontinentia pigmenti. Dermatol Ther. 2021;34(1):e14737.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.