Back to Journals » Clinical Ophthalmology » Volume 11
Resident-performed laser peripheral iridotomy in primary angle closure, primary angle closure suspects, and primary angle closure glaucoma
Authors Kam JP, Zepeda EM, Ding L, Wen JC
Received 6 August 2017
Accepted for publication 5 September 2017
Published 16 October 2017 Volume 2017:11 Pages 1871—1876
DOI https://doi.org/10.2147/OPTH.S148467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jason P Kam, Emily M Zepeda, Leona Ding, Joanne C Wen
Department of Ophthalmology, University of Washington, Seattle, WA, USA
Purpose: To investigate the power use and complication frequency of resident-performed laser peripheral iridotomy (LPI).
Methods: A retrospective analysis of 196 eyes from 103 patients who underwent neodymium: yttrium-aluminum-garnet laser iridotomy performed by resident physicians from January 1, 2010 through April 30, 2015 at a university-based county hospital was done. All patients were treated for primary angle closure, primary angle closure suspects, and primary angle closure glaucoma. Data were collected on pre- and post-laser intraocular pressure (IOP), ethnicity, laser parameters and complications. Mean power use and frequency of complications were evaluated. Complications included elevated post-laser IOP at 30–45 minutes (≥8 mmHg), hyphema, aborted procedures, and lasering non-iris structures. The number of repeated LPI procedures, was also recorded.
Results: Mean total power used for all residents was 78.2±68.7 mJ per eye. Power use by first-year trainees was significantly higher than second- and third-year trainees (103.5±75.5 mJ versus 73.7±73.8 mJ and 67.2±56.4 mJ, respectively, p=0.011). Complications included hyphema or microhyphema in 17.9% (35/196), IOP spikes in 5.1% (10/196), aborted procedures in 1.1% (3/196) and lasering non-iris structures in 0.5% (1/196). LPI was repeated in 22.4% of cases (44/196) with higher incidence of repeat LPI among non-Caucasian compared to the Caucasian subjects (p=0.02). Complication rates did not differ with increased training (p=0.16).
Conclusion: Total power used for LPI decreased with increased resident training, while the complication rate did not differ significantly among resident classes. Complication rates were comparable to rates reported in the literature for attending-performed LPIs.
Keywords: laser, iridotomy, resident, complications, power, energy
Introduction
Laser peripheral iridotomy (LPI) has been widely used and accepted as a treatment for all forms of angle closure glaucoma in which there is a component of pupillary block and is used as a prophylactic treatment for angle closure suspects.1,2 During ophthalmology residency training, the Accreditation Council for Graduate Medical Education (ACGME) currently recommends that all residents perform a minimum of 5 LPI procedures prior to graduation.3 Although LPIs are generally considered safe, complications are known to occur. Complications include transient blurred vision, intraocular pressure (IOP) rise, dysphotopsia, hyphema, closure of the iridotomy and damage to other tissues.1,2,4 While a number of studies have reported on the typical power use and complication rates among LPIs performed by attending and practicing ophthalmologists,4–8 none have reported on the power use and complication rates among resident-performed LPIs. The purpose of this study was to evaluate resident-performed LPIs and compare power use and complication rates to published data for attending-performed LPIs.
Materials and methods
This retrospective study was approved by the Institutional Review Board of the University of Washington. This study met the criteria for a Waiver of Consent and followed the policies of the University of Washington Medicine for data confidentiality. All patients between 18 and 99 years of age who had received an LPI by any resident physician from January 1, 2010 to April 30, 2015 at the Harborview Medical Center in Seattle, WA, were identified using procedure and ACGME case log books. Exclusion criteria were attending physician participation, inadequate information in the chart review, no follow-up after treatment, prior intraocular surgery and prior LPI treatment with argon laser. Patients were categorized based on 2015 American Academy of Ophthalmology Primary Angle Closure Preferred Practice Pattern9 and defined as follows: primary angle closure suspect (PACS) (≤180° iridotrabecular contact [ITC], normal IOP and no optic nerve damage); primary angle closure (PAC) (≥180° ITC with peripheral anterior synechiae [PAS] or elevated IOP, but no optic neuropathy); and primary angle closure glaucoma (PACG) (≥180° ITC with PAS, elevated IOP and optic neuropathy).
All laser procedures were performed under the supervision of an attending physician. The attending physician did not physically perform any portion of the laser procedure in the included patient population. Typically, patients received topical drops of tetracaine 0.5%, pilocarpine 1% and brimonidine tartrate 0.2% in the operative eye. Depending on physician preference, acetazolamide 250 mg or 500 mg was also given as a pretreatment. All iridotomies were performed using an Abraham lens (Ocular Abraham Iridectomy YAG laser lens; Ocular Instruments, Bellevue, WA, USA) in conjunction with hydroxypropyl methylcellulose as a coupling agent to focus the laser. The LPI procedures were performed using a neodymium:yttrium-aluminum-garnet (Nd:YAG) laser (Visulas Yag II; Carl Zeiss Meditec, Jena, Germany). No drops were administered after the treatment. Post-laser IOP was checked 30–45 minutes after the laser procedure using a Goldmann Applanation or a Tono-Pen AVIA Applanation Tonometer (Reichert, Depew, NY, USA). Data were collected on baseline demographics, indication for LPI, documented laser parameters (total mJ used, mJ per shot and/or total number of shots), pre- and post-procedure IOP, complications and need for repeat procedure. Complications included elevated IOP at 30–45 minutes after the LPI (≥8 mmHg increase in IOP compared to pre-laser IOP), hyphema of any amount, aborted procedures and lasering non-iris structures. Clinical data were gathered at baseline (the initial visit), immediately after procedure, at 1 week and 1 month after laser.
The mean power use among the 3 groups was analyzed using one-way analysis of variance. The ad hoc tests were performed with Bonferroni adjustment. Results are expressed as mean ± standard deviation (SD). Complication rates between the 3 types of glaucoma or the 3 resident classes were analyzed by chi-square test or Fisher’s exact test. Spearman’s correlation was performed to measure association between IOP spikes and energy use. Statistical analysis was conducted using Statistical Package for the Social Sciences (SPSS) Version 23.0 (IBM Corporation, Armonk, NY, USA) with p<0.05 considered statistically significant.
Results
A total of 221 eyes from 110 patients underwent Nd:YAG laser iridotomy by resident physicians over the 5-year study period. Of these, 196 eyes from 103 patients were included in the study. The others were excluded due to lack of follow-up after the LPI (15 eyes), inadequate charting (7 eyes) and age <18 years (3 eyes).
Patient demographics are shown in Table 1. Patients had a mean (±SD) age of 60.45±11.2 years, 64% were female and most subjects (66.3%) had a diagnosis of PACS. Forty-two percent of the patients had underwent bilateral LPI. The baseline mean IOP before treatment was 19.48±11.1 mmHg and at 30–45 minutes after the procedure the mean IOP was 18.30±7.8 mmHg. Approximately 25% of the procedures were performed by residents during their first-year, 33% in their second-year and 42% in the third-year. The mean total power used by all residents was 78.25±68.9 mJ per eye, which decreased significantly with increasing residency year (p=0.011) (Table 2). A subgroup analysis revealed that mean power use on LPIs decreased with increasing residency year among Caucasian subjects (p=0.006), while no significant change in power use was observed among non-Caucasian subjects (p=0.23) (Table 2). Among the various subclasses of angle closure, mean power use on LPIs performed on eyes with PAC significantly decreased with increasing residency year (p=0.0031), while mean power usage did not significantly change over the years for other subclasses of angle closure (Table 2).
  | Table 1 Baseline characteristics of subjects |
  | Table 2 Mean power use by year of training |
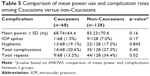
The total intraoperative complication rate was 25%, with hyphema or microhyphema occurring in 18% (35/196), IOP spikes >8 mmHg in 5% (10/196) and lasering non-iris structures in 0.005% (1/196) (Table 3). Complication rates did not differ with increased training (p=0.16). LPIs were repeated in 22.4% (44/196) of the time, and there was no significant difference in the rate of repeat procedures among resident classes (p=0.45). The greatest IOP spike observed was an increase of 17 mmHg. A total of 8 patients received 250 mg of acetazolamide 30 minutes prior to laser treatment. Only 4 of these patients had a preoperative IOP of >21 mmHg, and 2 of the 8 (25%) had post-laser IOP spikes. Pre- or perioperative acetazolamide was given based on provider preference.
Mean power and complication rates were also compared between the different types of indications for LPI. The power used in patients who were diagnosed with PACS was significantly lower than both PAC and PACG diagnoses (p=0.023), while complication rates did not differ significantly (p=0.670) (Table 4).
Additional subgroup analysis was performed comparing Caucasian and non-Caucasian subjects (Table 5). The mean power use on the Caucasian subjects did not differ significantly when compared to non-Caucasians, nor did total intraoperative complication rates. Complication rates by ethnicity did not reveal significant differences among differing levels of training (p=0.40). However, there was a higher incidence of repeat LPI among the non-Caucasian compared to the Caucasian subjects (34.4% versus 13.2%, respectively; p=0.02). Additional analysis on energy use and complication rates among uveitic versus non-uveitic eyes did not reveal significant differences (p=0.68). There was no correlation between post-LPI IOP elevation and energy use (r=0.046, p=0.90).
Discussion
This study reports the findings of a retrospective, single-center study of LPI procedures performed by resident physicians. The primary aim of the study was to evaluate the average power use on resident-performed YAG LPI and whether this changed with increasing resident experience. We found significantly less power use between the first-, second- and third-year residents with a 35% mean decrease between the first- and third-year residents.
In a study performed by Lewis et al,5 the mean energy used in YAG LPI varied from 41.0 mJ to 49.0 mJ (range 3.1–198 mJ) among different types of glaucoma. Jiang et al6 presented mean energy ranging from 146.0±185.2 mJ to 205.8±118.5 mJ in their Asian population. The study performed by Vera et al4 showed a mean total energy ranging from 41.5±48.2 mJ to 47.1±107 mJ in a mixed but predominately Caucasian and Asian population. By the third-year of residency, the mean power usage in Caucasian and non-Caucasian eyes (45±40 mJ and 79.0±61.8 mJ, respectively) was quite comparable to the reported literature for Caucasian and Asian eyes (Table 2). The amount of power used between PACS patients versus PACG patients was also significantly different with PACG patients requiring significantly more power (p=0.023). This trend was not found or examined in other studies.4–8 Ethnicity was our surrogate for iris color, since iris color was not routinely recorded. Interestingly, when examining power usage by residency year for the various subtypes of angle closure, a significant decrease in mean energy use was observed in eyes with PAC only. The cause of this significant decrease is unclear but may suggest that eyes with PAC are particularly challenging for the novice residents and may warrant additional structured teaching and supervision.
When the overall complication rates between the residents were analyzed, there was no significant difference between individual years of training. There was also no significant difference in complication rates between the Caucasian and the non-Caucasian subjects. However, there was a significant increase in repeat LPI among the non-Caucasian subjects (p=0.02). This difference may be due to the greater amount of pigment dispersed and more inflammation of darker, thicker irides among non-Caucasian eyes leading to closure of the iridotomy. Alternatively, the openings created in thicker irides may be smaller and thus more prone to closing. In this patient population, there might be an indication to pretreat with argon laser to thin and shrink the iris stroma. Although power per shot used was not consistently documented, one could argue that increased power settings may be more effective by creating a concentrated force to penetrate the iris stroma rather than multiple weak ineffective shots that just disperse pigment while minimally penetrating tissue.
The overall complication rate, which included IOP spikes, corneal burns and hyphema of any degree, was 24%. The total rate of IOP spikes was 5%, which compared favorably with what others have reported (9.8%–30%).6–8 Twenty-five out of the total 196 patients had IOPs ≥30 mmHg prior to treatment. Twenty patients had IOPs ≥40 mmHg, and 8 had IOPs ≥50 mmHg. No significant correlation was found between pre-laser IOP and total energy used (r=0.11, p=0.13). While Jiang et al reported an association between the total amount of energy use and risk of post-LPI IOP elevation, other studies did not report such an association.6 Likewise, we did not identify a correlation between post-LPI IOP elevation and energy use. The incidence of hyphema in our study was 18%, which is comparable to the 8.9%–34.6% reported in the literature.4,6–8 In our study, no subjects with hyphema had post-LPI elevation.
It is known that complications such as inflammation, hyphema, corneal decompensation, cataract formation, IOP elevation, retinal detachments and cystoid macular edema are more common with higher total Nd:YAG energy use in LPI and capsulotomy procedures.1,2,7,10–15 It is recommended that the iridotomies are created using the lowest laser energy necessary to minimize complications.
Our incidence of re-treatment (22%) was higher than rates reported in the literature, which range from <2% to 9%.5–7 A total of 44 patients underwent repeat LPIs. All repeat procedures were performed due to occluded iridotomies except for 2, which were considered inadequate in size after gonioscopic examination. The 3 patients who did not achieve patency with initial LPIs were included in the repeat group and were ultimately successful for patency. Incidence of re-treatment was not significantly different among the resident classes. As previously mentioned, the rate of re-treatment was significantly greater among non-Caucasian eyes compared to Caucasian eyes. Photocoagulative laser treatment is commonly used prior to photodisruptive Nd:YAG laser to pretreat the iris by thinning the tissue and minimizing the risk of bleeding. Of note, none of our patients received pretreatment which may have contributed to the higher re-treatment rate. Furthermore, there was no documentation as to the size of iridotomy that was created at the conclusion of the procedure. Small iridotomies are likely more susceptible to closure. As pretreatment miotics are commonly used prior to LPIs, once the miotics wear off and the pupil is no longer on tension, the iridotomies may decrease in size and become blocked or occluded from iris folds. Further resident education and standardization of iridotomy size in addition to photocoagulative pretreatment may help improve the re-treatment rate, especially in non-Caucasian eyes.
Due to the retrospective nature of this study, the location of the LPI was provider preference and was not standardized. For 63 of the 196 procedures, the location of the LPI was mentioned in the chart review. Of the 63, 37 were superior, and 26 were temporally located. Within this smaller subset, the superior site required more energy on average (82.6±10.0 mJ) than the temporal site (58.9±9.0 mJ); however, this difference did not reach statistical significance (p=0.09). None of the patients complained of dysphotopsia.
One limitation of this study is that patients were assigned to receive their LPI in a non-randomized fashion, and therefore, difficult cases may not have been evenly distributed across all resident classes. More junior residents may preferentially get assigned LPI procedures that are perceived as more straightforward, leaving the more difficult cases for the senior residents. The decrease in mean power usage across resident classes may perhaps be even more pronounced among patients randomized to residents of varying experience. The decreasing total power use among residents of increasing seniority suggests that a learning curve is present, though whether this learning curve is sufficiently aggressive remains to be seen. With proper supervision and standardized training, it might be reasonable to expect residents to be performing LPIs using total powers comparable to mean powers reported in the literature for Caucasian and non-Caucasian eyes at a much earlier stage in training. The learning curve might be shortened with standardization of power per shot depending on the thickness of the iris (based on color or ethnicity) or with a lower threshold to increase power per shot if the laser setting is not effective. Increased observation of junior residents may help them to improve laser aim and focusing to decrease rate of ineffective laser shots. Standardizing iridotomy size might also be beneficial to prevent early closure of the iridotomy or an unnecessarily large iridotomy.
Another limitation is the lack of a standardized post-LPI assessment of the efficacy of iridotomy. Ideally, an objective measure, such as anterior segment optical coherence tomography (AS-OCT) or ultrasound biomicroscopy, would demonstrate a deepening of the anterior chamber angles to confirm the success of the LPI. However, other studies have reported that up to 50%–63% of eyes remain narrow even after LPI due to plateau iris, anterior rotation of the ciliary processes or lens-induced mechanism16–18 so even using AS-OCT may not be a complete metric for success. Similarly, as previously mentioned, there was no standardization of iridotomy size. Bochmann et al19 have demonstrated that small peripheral iridotomies (PIs) (<100 μm) may not be sufficient for relieving pupillary block and that widening an existing small PI can lead to additional anterior chamber angle deepening. Fleck20 discussed that iridotomies in the range of 50–150 μm diameters may fail to prevent angle closure glaucoma based on a mathematical model suggesting a larger PI (150–200 μm) may be needed. Again, standardizing the PI size to at least 150–200 μm may improve our chances of successfully treating pupillary block in addition to decreasing our re-treatment rate.
Lastly, the laser used in this study is one of many laser machines that are currently available on the market, and the energy and power may not be extrapolated to other machines. We limited our study to one machine at one location to prevent any discrepancies.
Conclusion
Total power, used as a surrogate for procedural efficiency, decreased with increasing level of training and approached or was below values reported in the literature for attending-performed LPIs. Complication rates did not change with level of training and the incidence of the most common complications for all 3 years studied were comparable to reported attending-performed LPIs, in hyphema rates and IOP spikes. The higher incidence of repeat procedure warrants further investigation and indicates an area where additional resident training is needed.
Acknowledgment
This study was funded by Research to Prevent Blindness. The funding organization had no role in the design or conduct of this research.
Disclosure
The authors report no conflicts of interest in this work.
References
Laser peripheral iridotomy for pupillary-block glaucoma. American Academy of Ophthalmology. Ophthalmology. 1994;101(10):1749–1758. | ||
Robin AL, Pollack IP. A comparison of neodymium: YAG and argon laser iridotomies. Ophthalmology. 1984;91(9):1011–1016. | ||
Accreditation Council for Graduate Medical Education. ACGME ophthalmology surgery case logs national data report: 2014–2015. 2015. Available from: http://www.acgme.org/Portals/0/PDFs/240_National_Report_Program_Version.pdf. Accessed May 1, 2017. | ||
Vera V, Naqi A, Belovay GW, Varma DK, Ahmed II. Dysphotopsia after temporal versus superior laser peripheral iridotomy: a prospective randomized paired eye trial. Am J Ophthalmol. 2014;157(5):929–935. | ||
Lewis R, Perkins TW, Gangnon R, Kaufman PL, Heatley GA. The rarity of clinically significant rise in intraocular pressure after laser peripheral iridotomy with apraclonidine. Ophthalmology. 1998;105(12):2256–2259. | ||
Jiang Y, Chang DS, Foster PJ, et al. Immediate changes in intraocular pressure after laser peripheral iridotomy in primary angle-closure suspects. Ophthalmology. 2012;119(2):283–288. | ||
Schwartz LW, Moster MR, Spaeth GL, Wilson RP, Poryzees E. Neodymium-YAG laser iridectomies in glaucoma associated with closed or occludable angles. Am J Ophthalmol. 1986;102(1):41–44. | ||
Golan S, Levkovitch-Verbin H, Shemesh G, Kurtz S. Anterior chamber bleeding after laser peripheral iridotomy. JAMA Ophthalmol. 2013;131(5):626–629. | ||
Prum BE Jr, Herndon LW Jr, Moroi SE, et al. Primary angle closure preferred practice pattern(®) guidelines. Ophthalmology. 2016;123(1):P1–P40. | ||
Ari S, Cingü AK, Sahin A, Çinar Y, Çaça I. The effects of Nd:YAG laser posterior capsulotomy on macular thickness, intraocular pressure, and visual acuity. Ophthalmic Surg Lasers Imaging. 2012;43(5):395–400. | ||
Barnes EA, Murdoch IE, Subramaniam S, Cahill A, Kehoe B, Behrend M. Neodymium:yttrium-aluminum-garnet capsulotomy and intraocular pressure in pseudophakic patients with glaucoma. Ophthalmology. 2004;111(7):1393–1397. | ||
Alimanović-Halilović E. Komplikacije na stražnjem očnom segmentu nakon Nd-YAG laser kapsulotomije. [Complications in the posterior eye segment after Nd-YAG laser capsulotomy]. Med Arh. 2004;58(1):7–9. Bosnian [with English abstract]. | ||
Bhargava R, Kumar P, Phogat H, Chaudhary KP. Neodymium-yttrium aluminium garnet laser capsulotomy energy levels for posterior capsule opacification. J Ophthalmic Vis Res. 2015;10(1):37–42. | ||
Ranta P, Tommila P, Kivela T. Retinal breaks and detachment after neodymium: YAG laser posterior capsulotomy: five-year incidence in a prospective cohort. J Cataract Refract Surg. 2004;30(1):58–66. | ||
Wu SC, Jeng S, Huang SC, Lin SM. Corneal endothelial damage after neodymium: YAG laser iridotomy. Ophthalmic Surg Lasers. 2000;31(5):411–416. | ||
Garudadri CS, Chelerkar V, Nutheti R. An ultrasound biomicroscopic study of the anterior segment in Indian eyes with primary angle-closure glaucoma. J Glaucoma. 2002;11(6):502–507. | ||
Moghimi S, Chen R, Johari M, et al. Changes in anterior segment morphology after laser peripheral iridotomy in acute primary angle closure. Am J Ophthalmol. 2016;166:133–140. | ||
Yan YJ, Wu LL, Wang X, Xiao GG. Appositional angle closure in Chinese with primary angle closure and primary angle closure glaucoma after laser peripheral iridotomy. Invest Ophthalmol Vis Sci. 2014;55(12):8506–8512. | ||
Bochmann F, Johnson Z, Atta HR, Azuara-Blanco A. Increasing the size of a small peripheral iridotomy widens the anterior chamber angle: an ultrasound biomicroscopy study. Klin Monbl Augenheilkd. 2008;225(5):349–352. | ||
Fleck BW. How large must an iridotomy be? Br J Ophthalmol. 1990;74(10):583–588. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.