Back to Journals » International Journal of Nanomedicine » Volume 18
Research Progress of Polydopamine Hydrogel in the Prevention and Treatment of Oral Diseases
Authors Zhou Y , Yang Y, Liu R, Zhou Q, Lu H, Zhang W
Received 18 February 2023
Accepted for publication 12 April 2023
Published 16 May 2023 Volume 2023:18 Pages 2623—2645
DOI https://doi.org/10.2147/IJN.S407044
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Yuqi Zhou,1,* Yuanmeng Yang,2,* Rongpu Liu,1,* Qin Zhou,3 Haixia Lu,2 Wenjie Zhang1
1Department of Prosthodontics, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 2Department of Preventive Dentistry, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China; 3Department of Oral Surgery, Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Wenjie Zhang, Department of Prosthodontics, Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, 200011, People’s Republic of China, Email [email protected] Haixia Lu, Department of Preventive Dentistry, Ninth People’s Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, 200011, People’s Republic of China, Email [email protected]
Abstract: Oral diseases represent one of the most prevalent diseases globally and are associated with serious health and economic burdens, greatly altering the quality of life of affected individuals. Various biomaterials play important roles in the treatment of oral diseases. To some extent, the development of biomaterials has promoted progress in clinically available oral medicines. Hydrogels have unique tunable advantages that make them useful in the next generation of regenerative strategies and have been widely applied in both oral soft and hard tissues repair. However, most hydrogels lack self-adhesive properties, which may result in low repair efficacy. Polydopamine (PDA), the primary adhesive component, has attracted increasing attention in recent years. PDA-modified hydrogels exhibit reliable and suitable adherence to tissues and easily integrate into tissues to promote repair efficiency. This paper reviews the latest research progress on PDA hydrogels and elaborates on the mechanism of the reaction between PDA functional groups and hydrogels, and summarizes the biological properties and the applications of PDA hydrogels in the prevention and treatment of the field of oral diseases. It is also proposed that in future research we should simulate the complex microenvironment of the oral cavity as much as possible, coordinate and plan various biological events rationally, and realize the translation from scientific research to clinical practice.
Keywords: polydopamine, hydrogel, catechol groups, adhesive properties, prevention and treatment, oral diseases
Graphical Abstract:
Introduction
As an integral part of the maxillofacial region, the oral cavity is involved in essential functions, including eating, expression, and speech. However, oral diseases are among the most prevalent diseases globally and have caused severe health and economic burdens to the public.1 The oral mucosa is the soft tissue covering the entire inner part of the oral cavity. The different degrees of mucosal tissue defects severely affect the quality of life of ordinary people and even cause irreversible loss of function. The cause of these defects is multifactorial and typically associated with infections, trauma, maxillofacial tumors, and congenital malformations. Recently, applying different types of hydrogels in oral tissues to promote local defect regeneration has attracted considerable attention.2,3 Many studies have already reported the promising applications of hydrogels in the treatment of oral diseases. Hydrogels have the advantages of few systemic side effects, direct action in the area of inflammation, convenient use, and high local drug concentrations. For example, given their excellent mechanical strength and superior local adhesion, gelatin methacrylamide (Gel-MA) hydrogels are typically used as bionic restorative materials to attach to the damaged surface of the oral mucosa.4,5 Moreover, the use of polyethylene glycol (PEG) hydrogel in combination with calcium phosphate and recombinant human cementum protein1 can accelerate periodontal tissue regeneration in a rat periodontitis model.6
Hydrogels, are a kind of hydrophilic materials composed of 3D polymeric networks comprising synthetic and natural polymers and their derivatives with a strong ability to absorb large quantities of water.7 The abundance of water in hydrogels makes them highly porous and permeable, allowing small molecules, such as oxygen and nutrients, to diffuse quickly, thus providing various types of cells with living support.8 In addition, hydrogel networks can be interwoven together by physical or covalent interactions. The complex interactions between the polymer network structure determine the distinct mechanical performance of these crosslinked networks.9,10 Hydrogels have recently been increasingly modified through wet, dry, or cryogenic conditions to fabricate direct structures with increasingly sensitive responses to stimuli, swelling, and degradation.11 Hydrogels are also the most commonly applied biological materials in tissue engineering and regenerative medicine.12–14 However, the traditional hydrogels used for tissue engineering and delivery exhibit a few limitations: On the one hand, the application of hydrogels is highly dependent on the intrinsic material properties, thus requiring modification of more efficient active surface groups to tune the reactivity and versatility of hydrogels.15 On the other hand, water molecules are considered as an adverse barrier to robust adhesion of biomaterials given that water molecules interfere with the adhesion of hydrogels to target tissues and organs, leading to weaker adhesive binding and even ultimately bonding failure.16 Therefore, one of the current most significant challenges involves the development of hydrogel materials that maintain excellent biocompatibility in a moist environment while still possessing muscular tissues and cells adhesive properties.
In recent years, PDA-based biomaterials with mussel-inspired wet adhesion have emerged and promoted the development of biomedical engineering.17 PDA is a naturally occurring bionic synthetic analog of melanin similar to mussel foot protein five that contains catechol, imines, amine functional groups, and π-conjugated structures.18 PDA exhibits the ability to adhere to almost any material surface due to the enrichment in catecholamine functional groups.19,20 Due to its extraordinarily robust surface adhesion and various reactive functional groups, PDA has been widely applied for surface functional modification by different synthetic and natural polymers to enhance adhesion properties.21 PDA and its derivatives are gaining more and more substantial attention as tools for strengthening the biomechanical properties of biomaterials. This role is mainly attributed to its favorable physicochemical properties, including chemical reactivity, powerful adhesion, biodegradability, photothermal conversion, and excellent biocompatibility.22,23
Therefore, the hydrogel network interacts with PDA through covalent and noncovalent interactions (such as Michael addition reaction and Schiff base reaction) and effectively immobilize functional nanoparticles into the hydrogel network. Here, PDA acts as a nanoscale cross-linking agent that adheres to extracellular matrix (ECM)-derived hydrogel surfaces to enhance the adhesion strength of cells to the hydrogel significantly.24 Additionally, PDA can further endow hydrogels with various properties by modifying functional biomaterials. On the one hand, PDA allows cells to adhere directly to the inner wall of the hydrogel by immobilizing some peptide molecules on the hydrogel.25,26 Conversely, the porous structure of PDA-modified hydrogels can improve the microenvironment for cell adhesion and growth.27 In summary, these PDA-modified hydrogel materials, which simulate the natural ECM structure and function, could provide a biomimetic microenvironment to enhance tissue and cell biocompatibility and supply biological cues, promoting cell survival, proliferation, adhesion, and tissue formation. Such PDA-based modified hydrogels not only possess ascendant biocompatibility, bonding, and mechanical properties, but also own a wide range of development prospects in antibacterial, and anti-inflammatory applications, drug delivery and tissue regeneration, and wound healing.28 For example, a mussel bionic modification method employing a PDA self-polymerization reaction plays an essential role in surface modification, multifunctional material design, and biomedical applications. In this review, we describe the properties of PDA hydrogels and their applications for treating oral soft and hard tissues diseases, and based on the unique properties of PDA materials, and provide a basis for subsequent studies of other clinical applications.
Mechanism of Dopamine-Based Modified Hydrogels
Given its excellent biological properties, dopamine (DA), an essential derivative of 3,4-dihydroxyphenylalanine (DOPA), has attracted widespread attention from researchers. DA undergoes oxidative self-polymerization in an alkaline environment, forming a layer of PDA on the functional material, thereby achieving strong adhesion to various materials (Figure 1).16
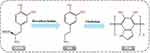 |
Figure 1 The scheme of DOPA, DA, PDA. |
Some phenolic hydroxyl groups in DA are oxidized to form quinone under conditions with an alkaline pH.29 The solid adhesive properties of PDA originate from the combination of catechol functional groups and amino groups that can form covalent or noncovalent bond on the surface of bonding material.18 Most recently, Priemel et al found that metal ions stored intracellularly in mussels reinforced the mechanical strength of the adhesive byssus fibers by protein-metal coordination mediated by DOPA.30 Given its extraordinary surface adhesion and various reactive functional groups, PDA has been widely applied for the functional modification of surfaces of various synthetic and natural polymers, thereby enhancing the adhesion properties.21 Among these polymers, hydrogels are a standard formulation adapted to biological, medical applications, given their unique properties of high water-restoring capacity and shape preservation.31 Various polymer hydrogels, including agarose, hyaluronic acid (HA), PEG, and alginate have been modified with functional groups of dopamine to enhance their adhesion properties and biological activities, including osteogenic and antibacterial properties (Figure 2).
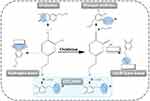 |
Figure 2 The mechanism of Dopamine-Based Modified Hydrogels. |
Catechol-and Quinone-Based Hydrogel
The catechol groups of PDA also play an essential role in its biological behaviors. The multiple redox states of catechol and its derivatives contribute to the formation of catechol-based hydrogels, endowing the hydrogel with enhanced adhesive, osteogenic, and antibacterial properties. Catechol-based hydrogels often exhibit good wet adhesiveness to the tissue surface based on the presence of PDA with two phenolic hydroxyl groups crosslinked with a hydrogel, which can form hydrogen bonds between the hydrophilic tissue surface and catechol groups to impart adhesive properties.32 Furthermore, the π-π interactions and hydrogen bonds between the PDA-catechol of hydrogel and the osteoinductive drug dexamethasone can absorb and retain the drug on the hydrogel surface to regulate osteogenic stem-cell differentiation.11 Hydrogels modified by catechol groups can chelate metal ions (such as Zn2+ and Cu2+) with enhanced photothermal performance and antibacterial properties (Figure 3).33,34
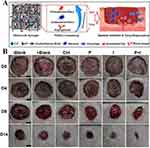 |
Figure 3 (A) Design and application of the PDA/Cu-CS composite hydrogel. (B) Wound closure photographs of the wounds with different treatments. The wounds were treated with the PDA/Cu-CS hydrogel with laser irradiation (P+I), PDA/Cu-CS hydrogel without laser irradiation (I), PDA hydrogel with laser irradiation (P), Ctrl hydrogel (Ctrl), Ciprofloxacin Hydrochloride Ointment (+Blank), and nothing (-Blank), respectively. (Scale bar = 5 mm). Reprinted with permission from Xu Q, Chang M, Zhang Y, et al. PDA/Cu bioactive hydrogel with “hot ions effect” for inhibition of drug-resistant bacteria and enhancement of infectious skin wound healing. ACS Appl Mater Interfaces. 2020;12:31255–31269. doi:10.1021/acsami.0c08890. Copyright 2020, American Chemical Society.33 |
In addition, the catechol groups can quickly lose electrons and be oxidized into quinone groups.35 The quinone groups exhibit high chemical reaction activity and can be grafted onto the chains of other polymers participating in the 3D network of hydrogel together. Thus, quinone-based hydrogels have been endowed with enhanced mechanical strength, biocompatibility, and antibacterial properties. The quinone groups from the PDA coating of the implant surface form covalent bonds with the amine groups of gelatin via Michael-type addition, enhancing the binding force between implant materials and the gelatin hydrogel.36 Su et al constructed quinone-based agarose polydopamine hydrogel (APG), improving the mechanical strength of the hydrogel and cell adhesiveness and biocompatibility by creating a double network on the hydrogel backbone based on crosslinking with PDA-quinone.37 Additionally, gentamicin can be effectively immobilized on the modified curdlan hydrogel through the quinone domain in PDA, providing curdlan hydrogels with excellent and long-lasting antibacterial properties.38
Amino-Enhanced Hydrogels
The amino groups on the PDA coating react with bioactive molecules introducing polymerized groups or functional polymers to the surface of the PDA-modified material and providing a reaction platform for further modifications of the material surface. On the one hand, the amino groups can react with carboxyl groups activated by 1-[3-(dimethylamino)propyl]-3-ethylcarbodiimide hydrochloride/N-hydroxy-succinimide (EDC/NHS) by coupling chemistry to form amide bonds to fabricate amino enhanced hydrogels.39 Using this method, Liang et al grafted DA-amino groups to HA, endowing the composite hydrogel with enhanced tissues adhesion properties, antibacterial properties, and good antioxidant ability.40 Moreover, grafting acrylate or acrylamide-based molecules via amine groups in PDA facilitate the incorporation of antimicrobial components on PDA coatings to construct multifunctional hydrogels.41 On the other hand, hydrogels can also be modified with enhanced adhesion via the PDA-amino groups cross-linked with the aldehyde groups. In the biocompatible chitosan-PDA hydrogel complex, a covalent bond network is formed between the amino group of PDA and the aldehyde group of oxidized polymethyl methacrylate (PMMA) by a Schiff base reaction, and the hydrogel exhibits optimal adhesive strength as well as cytocompatibility with the aid of PMMA.42 These studies provide new avenues for grafting strategies using engineered PDA and functional hydrogel systems in a matrix-independent manner.
Multigroup-Modified Hydrogels
The multifunctional groups confer PDA with the ability to bind to hydrogels and the surrounding medium, further enhancing the mechanical and biological properties of the composites. On the one hand, dopamine can be grafted onto the alginate hydrogel backbone via the EDC/NHS coupling chemistry method. The amine groups and sulfhydryl on the tissue surface reacted with the catechol and amino groups on the composite hydrogel to form the covalently crosslinked networks enhancing the hydrophilic and adhesive properties of the hydrogel.43 Similarly, the dopamine-amino group was attached to the HA hydrogel backbone via the amide bond catalyzed by EDC/NHS. In contrast, free dopamine molecules can be interlinked through covalent bonding to form a PDA structure. This structure provides ample catechol groups for the cross-linking of the HA-dopamine-PEG (HD-PEG) network of hydrogels, enhancing the mechanical properties of hydrogels.44 On the other hand, a new double network hydrogel based on the PDA-grafted sodium alginate (OSA-PDA), and polyacrylamide (PAM) formed dynamic covalent crosslinks through the aldehyde group of the OSA-PDA chain with the amino group of the PAM chain. In contrast, the reversible hydrogen bonds were formed between the catechol groups on the OSA-PDA chain. This reversible interaction and the dynamic cross-linking allow the hydrogel to repair itself effectively without external stimuli.45
Therefore, various synergistic interactions can be formed between the highly reactive groups in PDA stacked on top of each other and the hydrogel molecular network to achieve reversible covalent or noncovalent bond reconstruction on the fracture surface, conferring excellent mechanical and biological properties to PDA hydrogels.
The Biological Performance of PDA Hydrogels
In previous studies, hydrogels were employed as a promising option in the biomedical field due to their ECM-like properties and tunable physical and chemical properties.46,47 However, these hydrogels often lack tissue adhesion and cell affinity. PDA can be successfully deposited on the surface of various hydrogel dressings to form a modified coating, and the modified hydrogels exhibit excellent tissue adhesion and cell affinity.48,49 On the one hand, PDA can covalently bind to the hydrogel through the phenolic hydroxyl groups in PDA that can undergo Michael addition or Schiff-base reaction with the substrate surface under alkaline conditions.50 On the other hand, PDA can also non-covalently couple with the hydrogel surface due to the modification of the substrate surface achieved by the metal coordination or chelation between PDA and phenolic hydroxyl groups, hydrogen bonding, and π-π stacking, thus conferring good wet adhesion to the hydrogel system.51,52 PDA-modified hydrogels exhibit good adhesion properties, providing an opportunity for hydrogels to modulate drug release behavior. The excellent adhesion properties of PDA-hydrogel systems support their use as drug carriers to load a variety of agents, such as antibiotics,53 DNA/RNA,54 and proteins.55 These hydrogel systems can improve the encapsulation rate of drugs, exhibit good structural stability, effectively avoid the sudden release of drugs, and release drugs in a controlled and localized manner, thus achieving a delayed release effect (Figure 4).
 |
Figure 4 (A) Schematic illustration of PDA-coated nucleic acid nanogel with PEGylated surface (PEG-PDA-Nanogel). (B) The mechanism of siRNA-mediated low-temperature photothermal therapy induced by PEG-PDA-Nanogel in vivo. (C) The cellular uptake behaviour and gene silencing of PP-NGs in vitro. (c1) In vitro cellular uptake behaviour of FAM-labelled PP-NGs analyzed by flow cytometry (with equivalent FAM concentration of 1 μM). (c2) Endolysosomal escape of PP-NGs after laser treatment. Red: endolysosome stained with LysoTracker Red DND-99; green: PP-NG-siRNA labelled with FAM; yellow: colocalization of PP-NGs and endolysosome. The inset bars represent 25 μm. Reprinted from Biomaterials, 245, Ding F, Gao X, Huang X, et al. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy Copyright 2020, with permission from Elsevier.54 |
The PDA hydrogel system exhibits excellent adhesion properties that not only promote the adhesion of osteoblasts on the surface of PDA hydrogel material, but also further promote the proliferation and differentiation of specific types of cells, thus promoting new bone formation.56 Moreover, the excellent adhesion property also shows better drug-carrying ability.57 The PDA hydrogel system can be used as a carrier for osteogenic growth factors or antibacterial drugs, which effectively delaying the release of factors or drugs. This feature not only can strengthen the system’s ability to promote osteogenesis but also can achieve antibacterial function after loading antibacterial drugs. These drugs prevent bacteria adhesion and kill bacteria for a more extended period, thereby avoiding the occurrence of infection.52,53 Therefore, the PDA hydrogel system is a biological material with specific application prospects that not only exhibits excellent tissue and cell adhesion to withstand and transfer loads but also mimics the physiological environment of natural bone ECM and carries corresponding drugs or growth factors for different bone defect conditions, to achieve effective osteogenesis along with antibacterial and anti-inflammatory effects (Figure 5).
 |
Figure 5 The biological performance of PDA hydrogels. |
Osteogenic Performance of PDA Hydrogel
Bone defects are the most frequent injuries and can be caused by various events, including trauma and tumors. Minor bone abnormalities can be mended using the capability of the tissues to repair itself without the generation of scar tissues. Bone defects however may eventually contribute to slow healing or nonunion fractures in complex or multiple conditions. Currently, bone autografts and allografts are considered the “gold standard” for the treatment of patients with slow or incomplete bone healing.58,59 These treatments, however have some disadvantages. The amount/volume of bone taken limits the autografted bone, resulting in severe lesions at the donor site.60 The application of allogeneic bone is hampered by the difficulty of embedding between allogeneic bone and host tissues.61 More dependable and consistent treatments are urgently needed to overcome the challenges of regenerating critical-size bone lesions.
In recent years, innovative bone regeneration strategies for the treatment of bone defects through minimally invasive surgery combined with local injection of bone repair materials have gained widespread interest, and hydrogel networks connected with cells and bioactive molecules can be used as an “osteogenic” alternative. DA was used to decorate nanohydroxyapatite (nHA) to form PDA-decorated nHA (PHA). PHA was then introduced into a Schiff base reaction of sodium oxidized alginate (OSA) and gelatin to create a novel injectable bone repair hydrogel that simulates adhesion, proliferation, and differentiation of bone marrow-derived mesenchymal stem cells (BMSCs), as well as the repair effect of bone tissue.62 PDA treatment was used as a binding platform for bioactive compounds to create versatile gelatin–alginate (Gel–Alg) hydrogels for tissue engineering applications. Specifically, PDA was used to modify the surface properties of the hydrogel and better control the adhesion and osteogenic differentiation of human adipose-derived stem cells (hASCs). Compared to non-PDA-treated hydrogels, PDA not only adsorbs different types of bioactive molecules, such as model osteoinductive drugs (dexamethasone), but also effectively controls cytoskeletal remodeling of hASCs and promotes osteogenic differentiation without the introduction of any osteogenic factors in the culture medium.63 In addition, a mussel-inspired bilayer hydrogel was developed for osteochondral defect repair. In this hydrogel, chondrogenic factor-transforming growth factor β3 (TGF-β3) was immobilized in the upper layer, and osteogenic factor-bone morphogenetic protein 2 (BMP-2) was immobilized in the lower layer. Based on the excellent adhesion of PDA, this system was developed to synergistically promote osteochondral defect repair by increasing the load of factors and proteins and slowing the release of TGF-β3 and BMP-2 from the GelMA-PDA hydrogel.55
Antibacterial Performance of PDA Hydrogel
Rapid osseointegration of the biomaterial with the bone tissue is essential for successful implant placement in the bone defect area, and osseointegration is also important to prevent bacterial adhesion and colonization. Studies have shown that small amounts of bacteria adhering to the implant surface can rapidly increase and then form a dense bacterial film that prevents the formation of new bone tissue on the implant surface, thereby triggering infection.64 Additionally, if bacterial adhesion occurs before tissue repair, the host immune system will not be able to target bacteria on the implant surface to prevent colonization and biofilm formation. Therefore, it is necessary to establish a second line of defense to kill adherent bacteria on the surface to achieve a long-term antimicrobial effect.
The hydrogel platform activated by adhesive PDA allows us to destroy bacteria more effectively and resist infections caused by bacteria more permanently, which will give rise to the development of novel antibacterial agents. The mechanism underlying the antibacterial activity of PDA is the ability of PDA to efficiently capture bacteria, not only because it has superior absorption properties but also because it has a positive charge under acidic conditions involving biofilms at the site of bacterial infection. After capturing microorganisms, PDA physically communicates with the bacterial cell surface, interacting with the proteins bacteria secrete and impeding the metabolisms of bacteria.65 To enhance wound healing through this antioxidant and antibacterial strategy, oxidized dextran/chitosan hybrid hydrogels with excellent antioxidative and antibacterial properties of PDA nanoparticles(PDA NPs) were fabricated. The hybrid hydrogel underwent a rapid formation process of covalent Schiff base cross-linking between oxidized dextran and chitosan. In addition, the PDA in the hydrogel was pretreated with ascorbic acid, which not only provided a highly biocompatible and suitable gel matrix but also improved the free radical scavenging ability and antibacterial capacity.66 By optimizing the dose of PDA/ε-PL in the agarose network structure, hybrid agarose/PDA/ε-PL hydrogel (ADPH) with stable photothermal function and desirable physicochemical properties was obtained. ADPH possessed a satisfactory bactericidal effect in vivo and demonstrated efficacy in a bacterially infected wound model by successfully inhibiting inflammation, accelerating collagen deposition, and promoting angiogenesis, which enables rapid healing of bacterially infected skin wounds.67 In addition, another potential antimicrobial mechanism of PDA may be that the photothermal conversion efficiency (PCE) under near-infrared light radiation (NIR) confers photothermal antimicrobial capacity to PDA, which can disrupt the matrix of biofilms.68 To enhance the PCE of PDA NPs and achieve an efficient bacterial trapping/killing platform, the CG/PDA@Ag platform is synthesized by encapsulating PDA@Ag nanoparticles in a cationic guar gum (CG) hydrogel network. The formulated CG/PDA@Ag hydrogels possess not only high PCE, but also have many functional groups (such as hydroxyl and quaternary ammonium groups) and interact with bacteria through electrostatic forces, van der Waals forces, and hydrophobic interactions. These forces can effectively trap and kill some Gram-positive and Gram-negative bacteria and thus facilitate the antibacterial effect.69
Although bacterial adhesion can be significantly reduced by an antibacterial adhesion coating, it is difficult to eliminate bacteria. As long as there is bacterial colonization, the biofilm will implant on the implant surface, reducing the ability of the immune system to clear the bacteria and eventually leading to implant infection. Antibiotic drugs have long been used to treat illnesses and can effectively kill bacteria. PDA is uniquely positioned to be combined with antibiotics to provide a nanocomposite hydrogel that allows the slow release of drugs with low burst release. For example, a novel MPDA@GO/CNF composite hydrogel for controlled drug release was prepared by physically cross-linking mesoporous polydopamine (MPDA) nanoparticles encapsulated with graphene oxide (GO) in a cellulose nanofiber (CNF) hydrogel.70 MPDA NPs exhibited up to 35 wt % drug loading on tetracycline hydrochloride (TH). The MPDA@GO/CNF composite hydrogel not only utilizes GO to encapsulate MPDA NPs to prolong drug release and reduce the rate of drug burst release but also shields the toxicity of the CNF hydrogel through the use of nanocellulose to enhance the antibacterial properties of the synthetic hydrogel.
Anti-Inflammatory Performance of PDA Hydrogel
Bacterial infection and biofilm formation frequently occur in living tissues and various medical facilities, accompanied by numerous inflammatory responses. Notably, mature biofilms release free bacteria and promote infections, which further activate the immune system and produce an inflammatory response. The persistent and prolonged inflammatory response eventually leads to apoptosis and tissues necrosis, triggering inflammation or damage to the surrounding soft and bone tissues.71,72 Bone coating with anti-inflammatory properties based on PDA substrate represents effective surface modification methods that regulate the local immune microenvironment of implants based on the excellent biological properties of the substrate, thereby promoting osseointegration of the biomaterial and optimizing bone repair. As emerging drug carriers, PDA NPs possess perfect antioxidant activity in addition to sustained drug release capacity due to nanosizing.73–75 The PEG-DA/PDA/PUE/FA hydrogel network was formed by incorporating puerarin (PUE) and ferulic acid (FA) into PEG diacrylate hydrogels via PDA NPs. Due to the presence of PDA NPs, this hydrogel can be loaded with natural antioxidant drugs and stably preserved in the gel network for a long time. Under oxidative stress conditions, this hydrogel increases the activity of superoxide dismutase and glutathione peroxidase. It decreases the levels of ROS and malondialdehyde, thus promoting wound healing, collagen accumulation, and tissue regeneration.76 Through CO gas-enhanced photothermal therapy (PTT) combined with DNase I treatment, near-infrared-triggered phototherapeutic DNase-CO@mesoporous PDA NPs efficiently eliminate MRSA biofilms and simultaneously release CO gas to promote antibiofilm and anti-inflammatory effects, thus achieving rapid healing of MRSA biofilm-infected wounds.77 Cationic polyelectrolyte brushes grafted by bacterial cellulose (BC) nanofibers were introduced into the PDA/polyacrylamide hydrogel, which, in addition to exhibiting efficient antibacterial properties, the hydrogels exhibited a reduced inflammatory response and faster wound healing in rats and served as a good dressing for wound healing.78 The PDA hydrogel system can also be loaded with antibiotics to achieve practical anti-inflammation effects or to stop the onset of infection. A nanocomposite hydrogel platform consisting of PDA NPs and a multifunctional myristyl-Phe-Phe-OH peptide gel agent was used to enhance rifampicin loading and control the drug release rate, reducing the risk of infection.25
Biomedical Applications of PDA Hydrogel in Oral Tissues
Application of PDA Hydrogel in the Treatment of Oral and Maxillofacial Hard Tissues Diseases
Oral and maxillofacial hard tissues mainly include teeth and maxillofacial bone tissues. These tissues are closely related to each other and support the maxillofacial structure and function basis of the oromandibular system.79 Once a defect occurs, it not only affects the patient’s physiological functions, such as chewing and pronunciation, but also has a serious impact on the patient’s psychological health. However, the self-repair ability of oral and maxillofacial hard tissues is limited, and repair of these hard tissues mainly relies on synthetic materials for alternative repair of the defective area. Although clinically applied synthetic materials are widely available, their physicochemical properties and structural characteristics differ from those of human hard tissues. And there are various problems, such as slow material degradation and immune rejection, make the repair of defective areas extremely poor. The underlying cause is the inferior bionic technology of these materials, which leads to low tissues integration performance.80 Therefore, developing bionic materials that mimic the micro-and macrostructures of dental and skeletal tissues is an important method to treat oral and maxillofacial hard tissues defects (Table 1).
 |
Table 1 PDA Hydrogel Properties are Crucial for the Prevention and Treatment of Oral Soft and Hard Tissue Diseases |
Application of PDA Hydrogel Material in Caries Treatment
Caries is a dental hard tissue disease caused by bacterial infection.81,82 The cycle of bacterial infection and dental hard tissue demineralization is the main cause of caries progression. Caries can be classified into enamel and dentin caries based on the tooth tissue structure. Currently, the application of PDA hydrogels for caries prevention mainly focuses on two aspects bacterial inhibition and induction of dentin tissue remineralization.
Based on the good antibacterial and drug-carrying properties of PDA-hydrogel, it was applied to the prevention and treatment of early enamel caries research, and used in constructing highly compatible bionanocomposites. Enamel is a highly mineralized hard tissues characterized by needle-like bundles of hydroxyapatite crystals arranged in a highly ordered manner.83 PDA hydrogels can induce enamel remineralization by simulating the hydroxyapatite crystal structure, and this approach has become an essential strategy for enamel bionic restoration. The bionic anodic aluminum oxide (AAO)-assisted bilayer gel system allows minerals to form on the PDA-modified surface by introducing PDA into the hydrogel (Figure 6). Through the synergistic effect of the bilayer gel and AAO film, mineralized ions are carefully regulated to form a highly oriented and aligned mineral crystal structure, which helps better to mimic the structure and function of tooth enamel and prevent the development of early caries.84
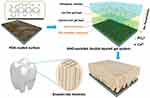 |
Figure 6 Schematic illusion of the AAO-assisted double-layer gel system (ADGS). AAO membrane, a calcium-containing gelatin hydrogel and an ion-free gelatin hydrogel were sequentially coated to PDA-modified substrates, from bottom to up. Phosphate solution was added to the top for mineralization through ion diffusion. Used with permission of Royal Society of Chemistry from Bioinspired enamel-like oriented minerals on general surfaces: towards improved mechanical properties. Chen Z, Miao Z, Zhang P, et al, J Mater Chem B. copyright 2019;7:5237–5244; permission conveyed through Copyright Clearance Center, Inc.84 |
When caries develop in the dentin layer, food residues, bacteria, and metabolites will enter the exposed dentin, and patients will experience significant clinical symptoms. PDA hydrogel-based nanocomposites exhibit a combination of good antibacterial and pro-mineralization properties and are applied to treat dentin caries. PDA hydrogels inhibit bacterial adhesion and biofilm formation, on the one hand, promote intrafibrillar mineralization and induce remineralization of dentin tissue. Regarding a potential mechanism, the catecholamine groups within PDA attract calcium ions through static interactions and promote the formation of nanohydroxyapatite crystals that precipitate along the c-axis.83 Induction of dentin remineralization by the biomolecule PDA is also an effective method to protect collagen from degradation. After the dentin is exposed, its internal collagen fibers are easily degraded by matrix metalloproteinase, further leading to restoration failure.85,86 Before remineralization, the PDA-modified repaired dentin attained mechanical properties that simulated those of natural dentin. By establishing a model of the intrafibrillar mineralization of PDA-modified type I collagen, it was found that PDA can enhance collagen mineralization by modifying collagen as well as the solid-liquid interface to reduce the heterogeneous nucleation barrier of hydroxyapatite, which further solves the challenge of fibrous degradation in the clinical treatment of dentin caries (Figure 7).87
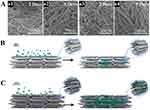 |
Figure 7 (A) SEM images of the remineralized dentin ultrastructure. The SEM images of dentin without (a1, a2) and with PDA pretreatment (a3, a4). (B) Schematic of the PDA treatment of the mineralization of collagen fibrils. Collagen fibrils without treatment. (C) Collagen fibrils pretreated with PDA. Triangles represent parts of CaP transformed into HAP. Reprinted with permission from Qu Y, Gu T, Du Q, et al. Polydopamine promotes dentin remineralization via interfacial control. ACS Biomater Sci Eng. 2020;6:3327–3334. doi:10.1021/acsbiomaterials.0c00035, Copyright 2020, American Chemical Society.87 |
Application of PDA Hydrogel Material in the Repair of Maxillofacial Bone Defects
Oral and maxillofacial bone tissues exhibit essential physiological functions and constitute the scaffolding of facial appearance. However, inflammation, trauma, tumors, and other factors often lead to bone defects. Due to the unique nature of the oral environment, traditional bone repair materials are associated with risks of bacterial infection and poor mechanical properties, making it difficult to obtain satisfactory clinical results.88,89 Therefore, the development of a bionic bone repair material similar to natural bone in structure and function represents an urgent challenge in the field of bone defect repair. The PDA hydrogel system is widely used in oral and maxillofacial bone tissue engineering, given its similar structure to natural bone tissues. The application of PDA to synthesize silver nanoparticles (AgNPs) in situ and cross-linking them with PEG, not only promoted MC3T3-E1 cells osteogenesis and enhanced the healing of maxillary defects in vivo but also provided antibacterial effects given that the AgNPs were encapsulated on the surface of DA (Figure 8).90
 |
Figure 8 (A) The scheme of the preparation of AgNPs/PDA gel. (B) SEM images of PEGda gel without any coating on surface (b1), dopamine-coated PEGda gel (b2), and AgNPs/PDA gel (b3 and b4); The bars of (b1–b3) = 20 μm, the bar of (b4) = 10 μm, the bar of (b5) = 1 μm, the bar of (b6) = 200 nm. (C) The CT photos of the mice treated with AgNPs/PDA gel. The blue dots were the bone defects. Bar = 10 mm. Reprinted from Materials Science and Engineering: C, 90, Xu H, Zhang G, Xu K, et al, Mussel-inspired dual-functional PEG hydrogel inducing mineralization and inhibiting infection in maxillary bone reconstruction, 379–386, Copyright 2018, with permission from Elsevier.90 |
In addition, biodegradable PDA synthetic scaffolds exhibit for a wide range of promising applications in oral and maxillofacial guided bone tissue regeneration. As a new biomimetic scaffold material, the silver/collagen-coated PLGA/PCL scaffold makes full use of the PLGA/PCL electrospun scaffold as a delivery system, and PDA-coating technology, as well as AgNPs in situ reduction technology, are employed to attain antibacterial and osteogenic properties and maintain the three-dimensional interfibrillar structure of the scaffold (Figure 9).91
 |
Figure 9 (A) Schematic illustration of the preparation procedure of PP-pDA-Ag-COL scaffolds. Surface topography the PP-pDA, PP-pDA-Ag, and PP-pDA-Ag-COL scaffolds incubated with S. aureus (B), S. mutans (C) were observed by FESEM. Scale bars = 2 μm. (D) μCT 3D reconstruction of the left maxillary mouse molars after 6 week implantation of PP, PP-pDA-Ag, and PP-pDA-Ag-COL scaffolds. The periodontitis mouse model is the control. Reprinted with permission from Qian Y, Zhou X, Zhang F, et al. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl Mater Interfaces. 2019;11:37381–37396. doi:10.1021/acsami.9b07053. Copyright 2019, American Chemical Society.91 |
The essence of periodontal bone tissue regeneration involves the repair or reconstruction of periodontal supporting tissues lost due to periodontitis, resulting in the formation of new bone and alveolar bone.92,93 Periodontitis is an inflammatory disease caused by host immune dysregulation due to plaque biofilms. Therefore, inhibition of plaque formation and the local inflammatory response is a prerequisite for treating periodontal bone defects.94,95 OSA-GelDA@ACP/DA/Ag, a hydrogel with antibacterial properties, was modified by grafting the gel with PDA, which facilitated the formation of a Schiff base reaction between the aldehyde group on OSA and the amino group on GelDA (Figure 10).96 This hydrogel not only exhibits good wet adhesion and antibacterial ability but is also expected to be used as an injectable periodontal bone restoration material in clinical treatment. In addition, 4D printing can be applied to fabricate 4D-PDA membranes based on the shape memory properties of PGS and PCL under thermal stimulation. Here, 4D-PDA membranes with PGS/PCL hydrogel structures are particularly useful for treating peri-implant alveolar bone defects and can simulate complex natural bone tissues without filling with bone substitutes to maximize the biological properties of the hydrogel.97
 |
Figure 10 (A) Schematic diagram of OSA-GelDA@ACP/DA/Ag adhesive antibacterial hydrogel. (B) The adhesion of OSA-GelDA hydrogel to porcine tissues. Reprinted from Materials Science and Engineering: C, 118, Zhong W, Xiong Y, Wang X, et al. Synthesis and characterization of multifunctional organic-inorganic composite hydrogel formed with tissue-adhesive property and inhibiting infection. Mater Sci Eng C Mater Biol Appl. Copyright 2021, with permission from Elsevier.96 |
Application of PDA Hydrogels in the Treatment of Oral Soft Tissue Diseases
Application of PDA Hydrogels in the Treatment of Oral Ulcers
Oral mucosal ulcer is a soft tissue disease caused by a dysbiosis of the microflora in the oral mucosa and saliva, which affects the balance of the immune response and epithelial barrier function, leading to the development and progression of oral mucosal disease.98,99 Hydrogel coatings represent a better treatment strategy for oral mucosal ulcer, given their advantages of being able to absorb wound-secreted exudates and maintain a stable microenvironment. Specifically, hydrogels offer a hydrated environment for ulcer, essential for mucosal healing and tissue regeneration. However, most of the reported hydrogels lack antibacterial properties, leading to the proliferation of bacteria around the mucosa (Figure 11).100
 |
Figure 11 The Schematic diagram of antimicrobial lipopeptide gel formation in methanol through covalent photo polymerization under UV irradiation. Reproduced with permission from De Zoysa GH, Wang K, Lu J, et al. Covalently immobilized battacin lipopeptide gels with activity against bacterial biofilms. Molecules. 2020;25:5945. doi:10.3390/molecules25245945. http://creativecommons.org/licenses/by/4.0/.100 |
The PDA-modified hydrogel system not only effectively prevents bacterial proliferation and creates a healthy oral microenvironment but also treats wound healing. For example, a new hydrogel composed of PDA, silver, and GO is used as a wound dressing for mouth ulcer. The hydrogel can produce muscular contractility after irradiation by NIR light, which can accelerate wound healing in a short period (Figure 12).101 An H et al developed a mussel-inspired Janus gelatin-polydopamine nanoclay (GPC) hydrogel with controlled viscosity and toughness through the synergistic physical and chemical interactions of gelatin (Gel), nanoclay, and dopamine (DA). The matrix dissipative layer of the hydrogel is achieved by physical cross-linking of gelatin, chemical cross-linking of gelatin with polydopamine (Michael addition and Schiff base formation), and nanoclay-induced molecular chain restriction, which enhances the intrinsic strength of the hydrogel. This hydrogel has excellent cell affinity and promotes cell adhesion and proliferation. Moreover, the immediacy and strong adhesion of this hydrogel provide a long-lasting therapeutic effect of the drug, thus enhancing the healing of mouth ulcer. Therefore, mussel-inspired wet mucoadhesive hydrogels can be used as a platform for mucosal dressings and drug delivery systems.102
 |
Figure 12 (A) Schematic illustration of PDA@Ag5GO hydrogel in the treatment of skin wound. (B) The optical image of the wound treated with different materials after 0, 5, 10 and 15 days of healing. Reprinted from Materials Science and Engineering: C, 129, Huang H, He D, Liao X, et al. An excellent antibacterial and high self-adhesive hydrogel can promote wound fully healing driven by its shrinkage under NIR. Copyright 2021, with permission from Elsevier.101 |
Application of PDA Hydrogel in the Treatment of Peri-Implantitis
The long-term stability of the soft tissues surrounding the implant is essential for implant success and survival.103 When dental implants do not tightly integrate with the soft tissues of the gingiva, the remaining gap is exposed to food debris and bacterial infection, which can cause severe peri-implantitis.104,105 One approach to improve peri-implant soft tissues bonding is the application of a biodegradable adhesive at the tissue-implant interface, which can serve as a temporary matrix to bind the tissue to the implant, thus facilitate wound healing. However, the ultrahigh requirement for biodegradability significantly reduces the choice of adhesives, so identifying a bond that both induces osseointegration and promotes stable peri-implant soft tissue bonding is very challenging. The study proposes a method of combining microbial transglutaminase (mTG) cross-linked gelatin hydrogel with PDA coating, which significantly improves the adhesion between the implants and tissues. In addition, this new means can also be applied to the interface between the implant and the surrounding soft tissue to enhance soft tissue binding.36 Clinical implantation of photothermal PDA nanoparticle coatings onto the surface of materials requiring tissue integration can be achieved by precisely controlling the duration of NIR irradiation to reduce bacterial adhesion to the material surface, ultimately providing optimal long-term protection for biomaterial implants.106
Application of PDA Hydrogel in the Repair of Oral and Maxillofacial Skin Defects
The maxillofacial skin is easily damaged by trauma and mechanical accidents, causing severe defects, peptic ulceration, and chronic infectious wounds. These conditions not only cause incalculable damage to the patient’s physiology and psychology but also represent a major challenge in clinical treatment. PDA hydrogels can effectively inhibit bacterial growth and can promote rapid healing of infected wounds, which is important for the treatment of maxillofacial skin tissues defects. The PDA/Cu-CS composite hydrogel has a unique “hot ion effect” that rapidly and effectively removes bacteria from infected wound areas and inhibits bacterial growth and adhesion for a long time, enhancing antibacterial properties and thus accelerating the wound healing process. In addition, the hydrogel significantly promotes angiogenesis and collagen deposition during the healing process of infected skin wounds and reduces the formation of scar tissue.107 The strong stability and degradability of PDA hydrogel materials are beneficial to the repair of maxillofacial trauma tissue and can induce tissue regeneration. However, these materials are mainly applied in the regenerative repair of bone tissues, and research related to soft tissues repair in the oral and maxillofacial areas is relatively limited.
Application of PDA Hydrogel in the Treatment of Oral Malignancies
Oral cancer is one of the most common malignant tumors of the head and neck, with a high degree of malignancy and high rates of metastasis and recurrence.33 Traditional treatment methods also produce some adverse effects. For example, after surgical resection, patients’ physiological functions, such as pronunciation and chewing, are impaired.108 Radiotherapy may cause some adverse effects, such as salivary gland dysfunction.19 Chemotherapy may cause toxic reactions to surrounding normal tissues due to the nontargeting nature of drugs.109 PTT has been widely applied in the treatment of oral cancer. For oral tongue squamous carcinoma, NIR-responsive PTT offers several advantages, such as high selectivity, tissue penetration, minimal noninvasiveness, and long-term biosafety.110,111 However, conventional PTT by intravenous injection of photothermal agents (PTAs) only acts at the site of photosensitizer enrichment and light irradiation and shows low biocompatibility, which inevitably causes side effects in normal tissues.112 Therefore, it is crucial to construct a novel NIR photoresponsive and peritumor injectable hydrogel that exhibits the following features: useful in photothermal therapy, nontoxic and nonharmful to surrounding normal tissues, inhibits tumor recurrence, and widely applicable in the clinical treatment of oral cancer.
It is of great significance to design and fabricate PDA responsive self-healing hydrogels as an intelligent drug carrier, and to achieve controlled drug release through the hydrophobic transformation of PDA hydrogel. This type of material is essential because PDA NPs not only exhibit high adhesion properties, but also can be used as excellent photothermal therapeutic agents, and the morphology of the nanoparticles dispersed in hydrogels changes with temperature.113–116 Upon incorporating of PDA NPs into injectable nanocomposite hydrogel SP (DMAEMA-co-HEMA-AA)/PEI, the photothermal effect of PDA NPs significantly enhanced the synergistic impact of efficient term chemotherapy under near-infrared laser irradiation, resulting in a hydrophilic-hydrophobic transition of the hydrogel. In addition, improved drug utilization and precise treatment were achieved, reducing drug side effects (Figure 13).117 Nanohydrogels for chemotherapy-photothermal therapy encapsulating the near-infrared (NIR) photothermal agent PDA and anticancer drug doxorubicin (DOX) on a terbium-doped hydroxyapatite (HATb) platform showed not only NIR-responsive release properties but also better antitumor effects and minimal side effects in OSCC cells.118
 |
Figure 13 (A) (a1) Synthesis of the SP (DMAEMA-co-HEMA-AA) Star-Shaped Copolymer; (a2) Preparation Process of the Injectable Self-Healing Hydrogel by the Reaction of SP (DMAEMA-co-HEMA-AA) with PEI (Formation of the Dynamic Covalent Enamine Bond), Loading PDA NPs and DOX; and (a3) Schematic Process of the Synergistic Thermochemotherapy under NIR. (B) (b1) TEM image of PDA NPs. (b2) SEM image of the SP (DMAEMA-co-HEMA-AA)/PEI hydrogel. (b3) SEM image of the SP (DMAEMAco-HEMA-AA)/PEI/PDA-NP nanocomposite hydrogel. (b4) SEM image of the SP (DMAEMA-co-HEMA-AA)/PEI/PDA-NP nanocomposite hydrogel after NIR irradiation for 5 min (808 nm, 1 W/cm2). (b5) Top and front views and the temperature distribution, captured by an infrared camera, of SP (DMAEMA-co-HEMA-AA)/PEI/PDA-NP nanocomposite hydrogel columnar samples (diameter: 1 cm) before and after NIR irradiation for 5 min (808 nm, 1 W/cm2) (scale bar: 1 cm). (C) Different retention effects of hydrogels containing the fluorescent dye Cy5.5 and PBS containing Cy5.5 at different times. Reprinted with permission from Wang C, Zhao N, Yuan W. NIR/thermoresponsive injectable self-healing hydrogels containing polydopamine nanoparticles for efficient synergistic cancer thermochemotherapy. ACS Appl Mater Interfaces. 2020;12:9118–9131. doi:10.1021/acsami.9b23536. Copyright 2020, American Chemical Society.117 |
Furthermore, the generation of ROS by NIR treatment led to increased apoptosis and synergistically increased cell death in vitro. The researchers loaded nucleic acid nanogels with anti-Hsp70 siRNA (siHsp70) and then coated the nanogels with a layer of PDA.54 PDA, an excellent biodegradable photothermal conversion agent for PTT, not only provides a source of photothermal conversion but also protects the embedded siRNA. The mechanical stability of the nanocomplexes is further enhanced by surface PEGylation, providing a superior gene silencing effect and significantly increasing the ability to ablate oral malignancies effectively.54
Conclusion and Outlook
The oral cavity is an integral part of the human body, and oral health is also an important aspect of human physical and mental health. The introduction of PDA hydrogels into the studies of oral disease diagnosis and treatment and tissue regeneration materials to take advantage of multi-functionalization is of great significance in promoting oral health. As biomaterials, such as implant materials or medical excipients, conventional self-healing hydrogels still lack properties such as biocompatibility and tissue adhesion that are compatible with the human environment. Given their advantages of good biocompatibility and strong adhesion, PDA hydrogels not only exhibit important mechanical properties but also have other properties that allow them to adapt to the human environment. On the one hand, covalent or noncovalent synergies can be formed between the highly reactive molecular groups in PDA and the hydrogel network, conferring various of biological properties (osteogenic properties, antimicrobial properties, drug release, anti-inflammation) to the hydrogel system. PDA-based hydrogel systems are helpful in a wide range of applications for biomedical engineering as a more advanced delivery system. On the other hand, the osteogenic, antibacterial, drug-carrying, and anti-inflammatory properties of PDA hydrogels make them promising candidates for use in a wide range of applications in the prevention and treatment of caries, periodontal disease, oral mucosal disease, oral and maxillofacial trauma, and tumors. Specifically, these hydrogels are not only valid for the repair of soft and hard tissues but also exhibit efficacy when used in combination with bioactive molecules to exert synergistic antibacterial and anti-inflammatory effects. Although the performance of PDA hydrogels has been thoroughly investigated, further studies regarding the drug delivery performance of PDA hydrogels, specifically their interaction with human cells, biodegradability, and in vivo clearance, are needed. Future research may focus on the clinical application of PDA hydrogels in the prevention and treatment of oral diseases and realize the transformation from in vitro biomaterials to in vivo biomaterials.
Caries is a destructive disease that relies on bacterial biofilm. When cariogenic bacteria become the dominant flora in the oral cavity and metabolize carbohydrates through fermentation, demineralization of dental hard tissues (including enamel and dentin) occurs, which leads to continuous acid production and caries by bacteria within the biofilm. Therefore, the effective removal of dental plaque biofilm is essential for treating of dental caries. Previously, Xu et al reported a detachable photothermal antimicrobial nanoagent using PDA loaded with Ag+ on the surface of Fe3O4 nanoparticles and subsequently grafted with glycolic chitosan (GCS), which can use PDA to control the release of Ag+ and reduce tissues damage, and also use GCS to drive strong adhesion to the target carious bacteria to achieve effective targeting of pathological bacteria under NIR conditions. Although biomaterials based on PDA coating can effectively resist the proliferation of caries causing bacteria, little research has been conducted on constructing PDA hydrogel networks.119 In this case, the PDA hydrogel network system needs to be designed centrally to realize the PDA hydrogel system to target the caries causing bacteria in pathological environment, protect the ecological balance of multi-colonies in the oral cavity, and avoid causing damage to other tissues and organs of the human body.
Periodontal disease is a highly prevalent inflammatory disease caused by infection with oral pathogens. Once infected, the host will develop a defensive innate immune response that destroys periodontal supporting tissues such as gingiva, periodontal membrane, and alveolar bone. Among them, excess reactive oxygen species (ROS) cause impaired tissues and bone healing by inhibiting antioxidant enzyme activity, exacerbating local inflammation, and degrading host tissues, thereby creating a pro-inflammatory microenvironment at the site of periodontal tissues damage and bone defects. PDA is a highly adhesive antioxidant that promotes M2 macrophage polarization by scavenging ROS. Li et al based on a PDA-modified dual network scaffold in diabetic conditions, could accelerate periodontal bone healing with good immunomodulatory activity for bone regeneration.120 In the periodontal inflammatory microenvironment, inhibition of antioxidant systems, overproduction of ROS, and exacerbation of inflammation may become a vicious cycle. However, no hydrogel material can be released explicitly in response to periodontal disease microenvironment stimuli, allowing the material to deliver drugs, factors, or other bioactive components in a more precise and targeted manner. As the pathological environment becomes more complex, there is an increasing need to propose an effective response strategy to meet the therapeutic needs. Therefore, to regenerate bone defects in the periodontal inflammatory microenvironment, there is an urgent need to design an intelligently responsive hydrogel that is precisely coordinated and choreographed in the tissue regeneration process, which can provide targeted therapy upon receipt of appropriate stimuli and better fits the bone immune system cascade response for more efficient periodontal bone healing and inflammation control.
Oral mucosal ulcers mainly occur as localized tissue defects or depressions in the oral mucosal epithelium. Unlike maxillofacial skin defects, contain a large amount of saliva and are associated with involuntary swallowing and oral movements. Inspired by the strong adhesive properties of mussels, a large amount of research work has focused on the development of hydrogel network systems of catechol modified polymers for the treatment of maxillofacial skin defects. In contrast, the effect of hydrogel systems with solid adhesion and drug bioavailability in the humid oral environment remains a challenge. The study reported by Hu et al proposed a buccal tissues mucoadhesive film containing PDA, which not only has strong adhesion to the wet buccal mucosa, but also can control and prolong drug release across the epithelial barrier and improve drug transport efficiency.121 Also, many studies have reported the ability of PDA hydrogel delivering systems to deliver high doses of drug therapy and controlled drug release in oral and maxillofacial tumor tissue sites, capable of reducing side effects in other tissues and organs in vivo. Nevertheless, hydrogel systems that can balance drug release and enhance immune modulation according to the complex microenvironment of the oral cavity are relatively rare. Therefore, this inspired us to focus our future work on the intelligent design of highly adhesive hydrogel materials to achieve smart regulation of drug release while activating the immune microenvironment to enhance immune regulation and provide new targets for the treatment of oral diseases.
This review provides a summary of the different properties of PDA hydrogels and their therapeutic applications in various soft and hard tissues of the oral cavity. Despite the great potential of PDA hydrogels in future tissue engineering research, the oral cavity is a complex microbial environment filled with various microbial flora, cells, and tissues rich in biological signals, and it is still a difficult task to achieve precise controlled, and on-demand release of drugs and bioactive components in a specific time and space, and to rationally coordinate and program various biological events in response to changes in the surrounding environment. Therefore, in future research, we should simulate the oral microenvironment as much as possible, establish the communication between the PDA hydrogel network system and cells and environment, and explore more meaningful intelligent biomaterials for treating oral diseases.
Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (Grant Nos. 82022015 and 32071361), Shanghai Rising-Star Program (Program No. 20QA1405700) and Shanghai “Rising Stars of Medical Talent” Youth Development Program.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Peres MA, Macpherson LMD, Weyant RJ, et al. Oral diseases: a global public health challenge. Lancet. 2019;394:249–260. doi:10.1016/S0140-6736(19)31146-8
2. Zhang W, Bao B, Jiang F, et al. Promoting oral mucosal wound healing with a hydrogel adhesive based on a phototriggered S-nitrosylation coupling reaction. Adv Mater. 2021;33:e2105667. doi:10.1002/adma.202105667
3. Lei L, Liu Z, Yuan P, et al. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J Mater Chem B. 2019;7:2722–2735. doi:10.1039/C9TB00025A
4. Tabatabaei F, Moharamzadeh K, Tayebi L. Fibroblast encapsulation in gelatin methacryloyl (GelMA) versus collagen hydrogel as substrates for oral mucosa tissue engineering. J Oral Biol Craniofac Res. 2020;10:573–577. doi:10.1016/j.jobcr.2020.08.015
5. Vila A, Torras N, Castaño AG, et al. Hydrogel co-networks of gelatine methacrylate and poly(ethylene glycol) diacrylate sustain 3D functional in vitro models of intestinal mucosa. Biofabrication. 2020;12:025008. doi:10.1088/1758-5090/ab5f50
6. Chen X, Liu Y, Miao L, et al. Controlled release of recombinant human cementum protein 1 from electrospun multiphasic scaffold for cementum regeneration. Int J Nanomedicine. 2016;11:3145–3158. doi:10.2147/IJN.S104324
7. Shi L, Ding P, Wang Y, et al. Self-healing polymeric hydrogel formed by metal-ligand coordination assembly: design, fabrication, and biomedical applications. Macromol Rapid Commun. 2019;40:e1800837. doi:10.1002/marc.201800837
8. Sharma S, Tiwari S. A review on biomacromolecular hydrogel classification and its applications. Int J Biol Macromol. 2020;162:737–747. doi:10.1016/j.ijbiomac.2020.06.110
9. Bovone G, Dudaryeva OY, Marco-Dufort B, et al. Engineering hydrogel adhesion for biomedical applications via chemical design of the junction. ACS Biomater Sci Eng. 2021;7:4048–4076. doi:10.1021/acsbiomaterials.0c01677
10. Li Z, Li G, Xu J, et al. Hydrogel transformed from nanoparticles for prevention of tissue injury and treatment of inflammatory diseases. Adv Mater. 2022;34:e2109178. doi:10.1002/adma.202109178
11. Pacelli S, Rampetsreiter K, Modaresi S, et al. Fabrication of a double-cross-linked interpenetrating polymeric network (IPN) hydrogel surface modified with polydopamine to modulate the osteogenic differentiation of adipose-derived stem cells. ACS Appl Mater Interfaces. 2018;10:24955–24962. doi:10.1021/acsami.8b05200
12. Neves SC, Moroni L, Barrias CC, et al. Leveling up hydrogels: hybrid systems in tissue engineering. Trends Biotechnol. 2020;38:292–315. doi:10.1016/j.tibtech.2019.09.004
13. Tayler IM, Stowers RS. Engineering hydrogels for personalized disease modeling and regenerative medicine. Acta Biomater. 2021;132:4–22. doi:10.1016/j.actbio.2021.04.020
14. Wang X, Yang Y, Shi Y, et al. Editorial: smart hydrogels in tissue engineering and regenerative medicine. Front Chem. 2020;8:245. doi:10.3389/fchem.2020.00245
15. Correa S, Grosskopf AK, Lopez Hernandez H, et al. Translational applications of hydrogels. Chem Rev. 2021;121:11385–11457. doi:10.1021/acs.chemrev.0c01177
16. Guo Q, Chen J, Wang J, et al. Recent progress in synthesis and application of mussel-inspired adhesives. Nanoscale. 2020;12:1307–1324. doi:10.1039/C9NR09780E
17. Heidarian P, Kouzani AZ, Kaynak A, et al. Rational design of mussel-inspired hydrogels with dynamic catecholato-metal coordination bonds. Macromol Rapid Commun. 2020;41:e2000439. doi:10.1002/marc.202000439
18. Barros NR, Chen Y, Hosseini V, et al. Recent developments in mussel-inspired materials for biomedical applications. Biomater Sci. 2021;9:6653–6672. doi:10.1039/D1BM01126J
19. He H, Zhao K, Xiao L, et al. Detection and chiral recognition of α-hydroxyl acid through 1 H and CEST NMR spectroscopy using a ytterbium macrocyclic complex. Angew Chem Int Ed Engl. 2019;58:18286–18289. doi:10.1002/anie.201912072
20. Davidsen MB, Teixeira JFL, Dehli J, et al. Post-treatments of polydopamine coatings influence cellular response. Colloids Surf B Biointerfaces. 2021;207:111972. doi:10.1016/j.colsurfb.2021.111972
21. Qiu WZ, Yang HC, Xu ZK. Dopamine-assisted co-deposition: an emerging and promising strategy for surface modification. Adv Colloid Interface Sci. 2018;256:111–125. doi:10.1016/j.cis.2018.04.011
22. Hu J, Yang L, Yang P, et al. Polydopamine free radical scavengers. Biomater Sci. 2020;8:4940–4950. doi:10.1039/D0BM01070G
23. Xie X, Tang J, Xing Y, et al. Intervention of polydopamine assembly and adhesion on nanoscale interfaces: state-of-the-art designs and biomedical applications. Adv Healthc Mater. 2021;10:e2002138. doi:10.1002/adhm.202002138
24. Lee HA, Park E, Lee H. Polydopamine and its derivative surface chemistry in material science: a focused review for studies at KAIST. Adv Mater. 2020;32:e1907505. doi:10.1002/adma.201907505
25. Falcone N, Andoy NMO, Sullan RMA, Kraatz HB. Peptide-polydopamine nanocomposite hydrogel for a laser-controlled hydrophobic drug delivery. ACS Appl Bio Mater. 2021;4:6652–6657. doi:10.1021/acsabm.1c00699
26. Wu Y, Yu C, Xing M, Wang L, Guan G. Surface modification of polyvinyl alcohol (PVA)/polyacrylamide (PAAm) hydrogels with polydopamine and REDV for improved applicability. J Biomed Mater Res B Appl Biomater. 2020;108:117–127. doi:10.1002/jbm.b.34371
27. Chen L, Lin Z, Liu L, et al. Fe2+/Fe3+ ions chelated with ultrasmall polydopamine nanoparticles induce ferroptosis for cancer therapy. ACS Biomater Sci Eng. 2019;5:4861–4869. doi:10.1021/acsbiomaterials.9b00461
28. Park SE, Georgescu A, Oh JM, Kwon KW, Huh D. Polydopamine-based interfacial engineering of extracellular matrix hydrogels for the construction and long-term maintenance of living three-dimensional tissues. ACS Appl Mater Interfaces. 2019;11:23919–23925. doi:10.1021/acsami.9b07912
29. Huang L, Liu M, Huang H, et al. Recent advances and progress on melanin-like materials and their biomedical applications. Biomacromolecules. 2018;19:1858–1868. doi:10.1021/acs.biomac.8b00437
30. Priemel T, Palia G, Förste F, et al. Microfluidic-like fabrication of metal ion-cured bioadhesives by mussels. Science. 2021;374:206–211. doi:10.1126/science.abi9702
31. Rastogi P, Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11:042001. doi:10.1088/1758-5090/ab331e
32. Feng L, Shi W, Chen Q, et al. Smart asymmetric hydrogel with integrated multi-functions of NIR-triggered tunable adhesion, self-deformation, and bacterial eradication. Adv Healthc Mater. 2021;10:e2100784. doi:10.1002/adhm.202100784
33. Xu Q, Chang M, Zhang Y, et al. PDA/Cu bioactive hydrogel with “hot ions effect” for inhibition of drug-resistant bacteria and enhancement of infectious skin wound healing. ACS Appl Mater Interfaces. 2020;12:31255–31269. doi:10.1021/acsami.0c08890
34. Xiang Y, Mao C, Liu X, et al. Rapid and superior bacteria killing of carbon quantum Dots/ZnO decorated injectable folic acid-conjugated PDA hydrogel through dual-light triggered ROS and membrane permeability. Small. 2019;15:e1900322. doi:10.1002/smll.201900322
35. Guo Y, Baschieri A, Mollica F, et al. Hydrogen atom transfer from HOO. to ortho-quinones explains the antioxidant activity of polydopamine. Angew Chem Int Ed Engl. 2021;60:15220–15224. doi:10.1002/anie.202101033
36. Dinh TN, Hou S, Park S, et al. Gelatin hydrogel combined with polydopamine coating to enhance tissue integration of medical implants. ACS Biomater Sci Eng. 2018;4:3471–3477. doi:10.1021/acsbiomaterials.8b00886
37. Su T, Zhang M, Zeng Q, et al. Mussel-inspired agarose hydrogel scaffolds for skin tissue engineering. Bioact Mater. 2020;6:579–588. doi:10.1016/j.bioactmat.2020.09.004
38. Michalicha A, Pałka K, Roguska A, et al. Polydopamine-coated curdlan hydrogel as a potential carrier of free amino group-containing molecules. Carbohydr Polym. 2021;256:117524. doi:10.1016/j.carbpol.2020.117524
39. Skopinska-Wisniewska J, Tuszynska M, Olewnik-Kruszkowska E. Comparative study of gelatin hydrogels modified by various cross-linking agents. Materials. 2021;14:396. doi:10.3390/ma14020396
40. Liang Y, Zhao X, Hu T, et al. Adhesive hemostatic conducting injectable composite hydrogels with sustained drug release and photothermal antibacterial activity to promote full-thickness skin regeneration during wound healing. Small. 2019;15:e1900046. doi:10.1002/smll.201900046
41. Liu CY, Huang CJ. Functionalization of polydopamine via the aza-michael reaction for antimicrobial interfaces. Langmuir. 2016;32:5019–5028. doi:10.1021/acs.langmuir.6b00990
42. Trinh KTL, Le NXT, Lee NY. Chitosan-polydopamine hydrogel complex: a novel green adhesion agent for reversibly bonding thermoplastic microdevice and its application for cell-friendly microfluidic 3D cell culture. Lab Chip. 2020;20:3524–3534. doi:10.1039/D0LC00621A
43. Zhang FX, Liu P, Ding W, et al. Injectable Mussel-Inspired highly adhesive hydrogel with exosomes for endogenous cell recruitment and cartilage defect regeneration. Biomaterials. 2021;278:121169. doi:10.1016/j.biomaterials.2021.121169
44. Hwang C, Lee SY, Kim HJ, et al. Polypseudorotaxane and polydopamine linkage-based hyaluronic acid hydrogel network with a single syringe injection for sustained drug delivery. Carbohydr Polym. 2021;266:118104. doi:10.1016/j.carbpol.2021.118104
45. Chen T, Chen Y, Rehman HU, et al. Ultratough, self-healing, and tissue-adhesive hydrogel for wound dressing. ACS Appl Mater Interfaces. 2018;10:33523–33531. doi:10.1021/acsami.8b10064
46. Wolf MT, Daly KA, Brennan-Pierce EP, et al. A hydrogel derived from decellularized dermal extracellular matrix. Biomaterials. 2012;33:7028–7038. doi:10.1016/j.biomaterials.2012.06.051
47. Abdallah M, Martin M, El Tahchi MR, et al. Influence of hydrolyzed polyacrylamide hydrogel stiffness on podocyte morphology, phenotype, and mechanical properties. ACS Appl Mater Interfaces. 2019;11:32623–32632. doi:10.1021/acsami.9b09337
48. Xue G, Zhang Y, Xie T, et al. Cell adhesion-mediated piezoelectric self-stimulation on polydopamine-modified poly(vinylidene fluoride) membranes. ACS Appl Mater Interfaces. 2021;13:17361–17371. doi:10.1021/acsami.1c02457
49. Yan J, Wu R, Liao S, et al. Applications of polydopamine-modified scaffolds in the peripheral nerve tissue engineering. Front Bioeng Biotechnol. 2020;8:590998. doi:10.3389/fbioe.2020.590998
50. Yu QH, Zhang CM, Jiang ZW, et al. Mussel-inspired adhesive polydopamine-functionalized hyaluronic acid hydrogel with potential bacterial inhibition. Glob Chall. 2019;4:1900068. doi:10.1002/gch2.201900068
51. Zhao Z, Li L, Geleta GS, et al. Polyacrylamide-phytic acid-polydopamine conducting porous hydrogel for efficient removal of water-soluble dyes. Sci Rep. 2017;7:7878. doi:10.1038/s41598-017-08220-6
52. Gao G, Jiang YW, Jia HR, et al. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials. 2019;188:83–95. doi:10.1016/j.biomaterials.2018.09.045
53. Zhang M, Huang Y, Pan W, et al. Polydopamine-incorporated dextran hydrogel drug carrier with tailorable structure for wound healing. Carbohydr Polym. 2021;253:117213. doi:10.1016/j.carbpol.2020.117213
54. Ding F, Gao X, Huang X, et al. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy. Biomaterials. 2020;245:119976. doi:10.1016/j.biomaterials.2020.119976
55. Gan D, Wang Z, Xie C, et al. Mussel-inspired tough hydrogel with in situ nanohydroxyapatite mineralization for osteochondral defect repair. Adv Healthc Mater. 2019;8:e1901103. doi:10.1002/adhm.201901103
56. Xu Y, Zhao S, Weng Z, et al. Jelly-inspired injectable guided tissue regeneration strategy with shape auto-matched and dual-light-defined antibacterial/osteogenic pattern switch properties. ACS Appl Mater Interfaces. 2020;12:54497–54506. doi:10.1021/acsami.0c18070
57. Li H, Yin D, Li W, et al. Polydopamine-based nanomaterials and their potentials in advanced drug delivery and therapy. Colloids Surf B Biointerfaces. 2021;199:111502. doi:10.1016/j.colsurfb.2020.111502
58. Annamalai RT, Hong X, Schott NG, et al. Injectable osteogenic microtissues containing mesenchymal stromal cells conformally fill and repair critical-size defects. Biomaterials. 2019;208:32–44. doi:10.1016/j.biomaterials.2019.04.001
59. Aghali A. Craniofacial bone tissue engineering: current approaches and potential therapy. Cells. 2021;10:2993. doi:10.3390/cells10112993
60. Bhumiratana S, Bernhard JC, Alfi DM, et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci Transl Med. 2016;8:343ra83. doi:10.1126/scitranslmed.aad5904
61. Xie C, Ye J, Liang R, et al. Advanced strategies of biomimetic tissue-engineered grafts for bone regeneration. Adv Healthc Mater. 2021;10:e2100408. doi:10.1002/adhm.202100408
62. Liu C, Wu J, Gan D, et al. The characteristics of mussel-inspired nHA/OSA injectable hydrogel and repaired bone defect in rabbit. J Biomed Mater Res B Appl Biomater. 2020;108:1814–1825. doi:10.1002/jbm.b.34524
63. Pacelli S, Chakravarti AR, Modaresi S, et al. Investigation of human adipose-derived stem-cell behavior using a cell-instructive polydopamine-coated gelatin-alginate hydrogel. J Biomed Mater Res A. 2021;109:2597–2610. doi:10.1002/jbm.a.37253
64. Wei PF, Yuan ZY, Jing W, et al. Regenerating infected bone defects with osteocompatible microspheres possessing antibacterial activity. Biomater Sci. 2018;7:272–286. doi:10.1039/C8BM00903A
65. Adnan NNM, Sadrearhami Z, Bagheri A, et al. Exploiting the versatility of polydopamine-coated nanoparticles to deliver nitric oxide and combat bacterial biofilm. Macromol Rapid Commun. 2018;39:e1800159. doi:10.1002/marc.201800159
66. Fu Y, Zhang J, Wang Y, et al. Reduced polydopamine nanoparticles incorporated oxidized dextran/chitosan hybrid hydrogels with enhanced antioxidative and antibacterial properties for accelerated wound healing. Carbohydr Polym. 2021;257:117598. doi:10.1016/j.carbpol.2020.117598
67. Qi X, Pan W, Tong X, et al. ε-Polylysine-stabilized agarose/polydopamine hydrogel dressings with robust photothermal property for wound healing. Carbohydr Polym. 2021;264:118046. doi:10.1016/j.carbpol.2021.118046
68. Jin A, Wang Y, Lin K, et al. Nanoparticles modified by polydopamine: working as “drug” carriers. Bioact Mater. 2020;5:522–541. doi:10.1016/j.bioactmat.2020.04.003
69. Qi X, Huang Y, You S, et al. Engineering robust ag-decorated polydopamine nano-photothermal platforms to combat bacterial infection and prompt wound healing. Adv Sci. 2022;9:e2106015. doi:10.1002/advs.202106015
70. Liu Y, Fan Q, Huo Y, et al. Construction of a Mesoporous Polydopamine@GO/Cellulose Nanofibril Composite Hydrogel with an Encapsulation Structure for Controllable Drug Release and Toxicity Shielding. ACS Appl Mater Interfaces. 2020;12:57410–57420. doi:10.1021/acsami.0c15465
71. Puthia M, Butrym M, Petrlova J, et al. A dual-action peptide-containing hydrogel targets wound infection and inflammation. Sci Transl Med. 2020;12:eaax6601. doi:10.1126/scitranslmed.aax6601
72. Tu C, Lu H, Zhou T, et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597. doi:10.1016/j.biomaterials.2022.121597
73. Poinard B, Neo SZY, Yeo ELL, et al. Polydopamine nanoparticles enhance drug release for combined photodynamic and photothermal therapy. ACS Appl Mater Interfaces. 2018;10:21125–21136. doi:10.1021/acsami.8b04799
74. Wang Y, Ge W, Ma Z, et al. Use of mesoporous polydopamine nanoparticles as a stable drug-release system alleviates inflammation in knee osteoarthritis. APL Bioeng. 2022;6:026101. doi:10.1063/5.0088447
75. Tian Y, Lei M. Polydopamine-based composite nanoparticles with redox-labile polymer shells for controlled drug release and enhanced chemo-photothermal therapy. Nanoscale Res Lett. 2019;14:186. doi:10.1186/s11671-019-3027-6
76. Ou Q, Zhang S, Fu C, et al. More natural more better: triple natural anti-oxidant puerarin/ferulic acid/polydopamine incorporated hydrogel for wound healing. J Nanobiotechnology. 2021;19:237. doi:10.1186/s12951-021-00973-7
77. Yuan Z, Lin C, Dai L, et al. Near-infrared light-activatable dual-action nanoparticle combats the established biofilms of methicillin-resistant staphylococcus aureus and its accompanying inflammation. Small. 2021;17:e2007522. doi:10.1002/smll.202007522
78. Yang Z, Huang R, Zheng B, et al. Highly stretchable, adhesive, biocompatible, and antibacterial hydrogel dressings for wound healing. Adv Sci. 2021;8:2003627. doi:10.1002/advs.202003627
79. Liu P, Zhang Y, Ma Y, et al. Application of dental pulp stem cells in oral maxillofacial tissue engineering. Int J Med Sci. 2022;19:310–320. doi:10.7150/ijms.68494
80. Lin H, Sohn J, Shen H, et al. Bone marrow mesenchymal stem cells: aging and tissue engineering applications to enhance bone healing. Biomaterials. 2019;203:96–110. doi:10.1016/j.biomaterials.2018.06.026
81. Cui T, Luo W, Xu L, et al. Progress of antimicrobial discovery against the major cariogenic pathogen streptococcus mutans. Curr Issues Mol Biol. 2019;32:601–644. doi:10.21775/cimb.032.601
82. Iwai K, Azuma T, Yonenaga T, et al. Association between dental caries and Helicobacter pylori infection in Japanese adults: a cross-sectional study. PLoS One. 2022;17:e0271459. doi:10.1371/journal.pone.0271459
83. Sharma V, Srinivasan A, Nikolajeff F, et al. Biomineralization process in hard tissues: the interaction complexity within protein and inorganic counterparts. Acta Biomater. 2021;120:20–37. doi:10.1016/j.actbio.2020.04.049
84. Chen Z, Miao Z, Zhang P, et al. Bioinspired enamel-like oriented minerals on general surfaces: towards improved mechanical properties. J Mater Chem B. 2019;7:5237–5244. doi:10.1039/C9TB00676A
85. Ashwini A, Dineshkumar T, Rameshkumar A, et al. Dentin degradonomics - The potential role of salivary MMP-8 in dentin caries. J Clin Exp Dent. 2020;12:e108–e115. doi:10.4317/jced.56144
86. Boukpessi T, Menashi S, Camoin L, et al. The effect of stromelysin-1 (MMP-3) on non-collagenous extracellular matrix proteins of demineralized dentin and the adhesive properties of restorative resins. Biomaterials. 2008;29:4367–4373. doi:10.1016/j.biomaterials.2008.07.035
87. Qu Y, Gu T, Du Q, et al. Polydopamine promotes dentin remineralization via interfacial control. ACS Biomater Sci Eng. 2020;6:3327–3334. doi:10.1021/acsbiomaterials.0c00035
88. Lu Y, Zhang W, Wang J, et al. Recent advances in cell sheet technology for bone and cartilage regeneration: from preparation to application. Int J Oral Sci. 2019;11:17. doi:10.1038/s41368-019-0050-5
89. Yin S, Zhang W, Zhang Z, et al. Recent advances in scaffold design and material for vascularized tissue-engineered bone regeneration. Adv Healthc Mater. 2019;8:e1801433. doi:10.1002/adhm.201801433
90. Xu H, Zhang G, Xu K, et al. Mussel-inspired dual-functional PEG hydrogel inducing mineralization and inhibiting infection in maxillary bone reconstruction. Mater Sci Eng C Mater Biol Appl. 2018;90:379–386. doi:10.1016/j.msec.2018.04.066
91. Qian Y, Zhou X, Zhang F, et al. Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl Mater Interfaces. 2019;11:37381–37396. doi:10.1021/acsami.9b07053
92. Kitamura M, Mochizuki Y, Miyata Y, et al. Pathological characteristics of periodontal disease in patients with chronic kidney disease and kidney transplantation. Int J Mol Sci. 2019;20:3413. doi:10.3390/ijms20143413
93. Liu J, Ruan J, Weir MD, et al. Periodontal bone-ligament-cementum regeneration via scaffolds and stem cells. Cells. 2019;8(6):537. doi:10.3390/cells8060537
94. Gegout PY, Stutz C, Olson J, et al. Interests of exosomes in bone and periodontal regeneration: a systematic review. Adv Exp Med Biol. 2021;1341:67–87.
95. Li Q, Yang G, Li J, et al. Stem cell therapies for periodontal tissue regeneration: a network meta-analysis of preclinical studies. Stem Cell Res Ther. 2020;11:427. doi:10.1186/s13287-020-01938-7
96. Zhong W, Xiong Y, Wang X, et al. Synthesis and characterization of multifunctional organic-inorganic composite hydrogel formed with tissue-adhesive property and inhibiting infection. Mater Sci Eng C Mater Biol Appl. 2021;118:111532. doi:10.1016/j.msec.2020.111532
97. Liu X, Chen W, Shao B, et al. Mussel patterned with 4D biodegrading elastomer durably recruits regenerative macrophages to promote regeneration of craniofacial bone. Biomaterials. 2021;276:120998. doi:10.1016/j.biomaterials.2021.120998
98. Lin D, Yang L, Wen L, et al. Crosstalk between the oral microbiota, mucosal immunity, and the epithelial barrier regulates oral mucosal disease pathogenesis. Mucosal Immunol. 2021;14:1247–1258. doi:10.1038/s41385-021-00413-7
99. Surboyo MD, Ernawati DS, Budi HS. Oral mucosal lesions and oral symptoms of the SARS-CoV-2 infection. Minerva Dent Oral Sci. 2021;70:161–168. doi:10.23736/S2724-6329.21.04493-9
100. De Zoysa GH, Wang K, Lu J, et al. Covalently immobilized battacin lipopeptide gels with activity against bacterial biofilms. Molecules. 2020;25:5945. doi:10.3390/molecules25245945
101. Huang H, He D, Liao X, et al. An excellent antibacterial and high self-adhesive hydrogel can promote wound fully healing driven by its shrinkage under NIR. Mater Sci Eng C Mater Biol Appl. 2021;129:112395. doi:10.1016/j.msec.2021.112395
102. An H, Gu Z, Zhou L, et al. Janus mucosal dressing with a tough and adhesive hydrogel based on synergistic effects of gelatin, polydopamine, and nano-clay. Acta Biomater. 2022;149:126–138. doi:10.1016/j.actbio.2022.07.016
103. Raphel J, Holodniy M, Goodman SB, et al. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials. 2016;84:301–314. doi:10.1016/j.biomaterials.2016.01.016
104. Yin S, Sun N, Jiang F, et al. The translation from in vitro bioactive ion concentration screening to in vivo application for preventing peri-implantitis. ACS Appl Mater Interfaces. 2021;13:5782–5794. doi:10.1021/acsami.0c19698
105. Lopez MA, Andreasi Bassi M, Confalone L, et al. treatment of peri-implant diseases: a new approach using hybenx® as a decontaminant for implant surface and oral tissues. Oral Implantol. 2016;9:106–114.
106. Ren X, Gao R, van der Mei HC, et al. Eradicating infecting bacteria while maintaining tissue integration on photothermal nanoparticle-coated titanium surfaces. ACS Appl Mater Interfaces. 2020;12:34610–34619. doi:10.1021/acsami.0c08592
107. Yin W, Liu Y, Bian Z. MG53 inhibits the progression of tongue cancer cells through regulating PI3K-AKT signaling pathway: evidence from 3d cell culture and animal model. Small. 2019;15:e1805492. doi:10.1002/smll.201805492
108. Zhang SQ, Chen HB, Liu J, et al. Research status and prospects of acupuncture for prevention and treatment of chemo- and radiotherapy-induced salivary gland dysfunction in head and neck cancer. Anat Rec. 2021;304:2381–2396. doi:10.1002/ar.24784
109. Lai TY, Wang TH, Liu CJ, et al. Risk factors for osteonecrosis of the jaw in oral cancer patients after surgery and eventual adjuvant treatment: the potential role of chemotherapy. Radiother Oncol. 2017;123:406–411. doi:10.1016/j.radonc.2017.05.001
110. Jiang Y, Li J, Zhen X, et al. Dual-peak absorbing semiconducting copolymer nanoparticles for first and second near-infrared window photothermal therapy: a comparative study. Adv Mater. 2018;30:e1705980. doi:10.1002/adma.201705980
111. Wang Y, Zhang W, Sun P, et al. A novel multimodal NIR-II nanoprobe for the detection of metastatic lymph nodes and targeting chemo-photothermal therapy in oral squamous cell carcinoma. Theranostics. 2019;9:391–404. doi:10.7150/thno.30268
112. Hu JJ, Cheng YJ, Zhang XZ. Recent advances in nanomaterials for enhanced photothermal therapy of tumors. Nanoscale. 2018;10:22657–22672. doi:10.1039/C8NR07627H
113. Lu J, Cai L, Dai Y, et al. Polydopamine-based nanoparticles for photothermal therapy/chemotherapy and their synergistic therapy with autophagy inhibitor to promote antitumor treatment. Chem Rec. 2021;21:781–796. doi:10.1002/tcr.202000170
114. Zhang P, Li X, Xu Q, et al. Polydopamine nanoparticles with different sizes for NIR-promoted gene delivery and synergistic photothermal therapy. Colloids Surf B Biointerfaces. 2021;208:112125. doi:10.1016/j.colsurfb.2021.112125
115. Gholami Derami H, Gupta P, Weng KC, et al. Reversible photothermal modulation of electrical activity of excitable cells using polydopamine nanoparticles. Adv Mater. 2021;33:e2008809. doi:10.1002/adma.202008809
116. Cao H, Yang Y, Liang M, et al. Pt@polydopamine nanoparticles as nanozymes for enhanced photodynamic and photothermal therapy. Chem Commun. 2021;57:255–258. doi:10.1039/D0CC07355E
117. Wang C, Zhao N, Yuan W. NIR/thermoresponsive injectable self-healing hydrogels containing polydopamine nanoparticles for efficient synergistic cancer thermochemotherapy. ACS Appl Mater Interfaces. 2020;12:9118–9131. doi:10.1021/acsami.9b23536
118. Gu M, Jiang L, Hao L, et al. A novel theranostic nanoplatform for imaging-guided chemo-photothermal therapy in oral squamous cell carcinoma. J Mater Chem B. 2021;9:6006–6016. doi:10.1039/D1TB01136G
119. Xu X, Fan M, Yu Z, et al. A removable photothermal antibacterial “warm paste” target for cariogenic bacteria. Chem Eng J. 2022;429:132491. doi:10.1016/j.cej.2021.132491
120. Li Y, Yang L, Hou Y, et al. Polydopamine-mediated graphene oxide and nanohydroxyapatite-incorporated conductive scaffold with an immunomodulatory ability accelerates periodontal bone regeneration in diabetes. Bioact Mater. 2022;18:213–227. doi:10.1016/j.bioactmat.2022.03.021
121. Hu S, Pei X, Duan L, et al. A mussel-inspired film for adhesion to wet buccal tissue and efficient buccal drug delivery. Nat Commun. 2021;12:1689. doi:10.1038/s41467-021-21989-5
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
