Back to Journals » Journal of Asthma and Allergy » Volume 15
Research Advances in the Treatment of Allergic Rhinitis by Probiotics
Authors Liu P, Hu T , Kang C, Liu J, Zhang J, Ran H, Zeng X, Qiu S
Received 22 July 2022
Accepted for publication 11 September 2022
Published 7 October 2022 Volume 2022:15 Pages 1413—1428
DOI https://doi.org/10.2147/JAA.S382978
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Peng Liu,1 Tianyong Hu,2 Chenglin Kang,1 Jiangqi Liu,2 Jin Zhang,1 Hong Ran,1 Xianhai Zeng,2 Shuqi Qiu2
1Department of Graduate and Scientific Research, Zunyi Medical University Zhuhai Campus, Zunyi, People’s Republic of China; 2Department of Otolaryngology, Longgang E.N.T Hospital & Shenzhen Key Laboratory of E.N.T, Institute of E.N.T Shenzhen, Shenzhen, People’s Republic of China
Correspondence: Shuqi Qiu; Xianhai Zeng, Email [email protected]; [email protected]
Abstract: Allergic rhinitis (AR) impairs the quality of life of patients and reduces the efficiency of social work, it is an increasingly serious public medical and economic problem in the world. Conventional anti-allergic drugs for the treatment of allergic rhinitis (AR) can cause certain side effects, which limit the quality of life of patients. Therefore, it makes sense to look for other forms of treatment. Several studies in recent years have shown that probiotics have shown anti-allergic effects in various mouse and human studies. For example, the application of certain probiotic strains can effectively relieve the typical nasal and ocular symptoms of allergic rhinitis in children and adults, thereby improving the quality of life and work efficiency. At the same time, previous studies in humans and mice have found that probiotics can produce multiple effects, such as reduction of Th2 cell inflammatory factors and/or increase of Th1 cell inflammatory factors, changes in allergy-related immunoglobulins and cell migration, regulate Th1/Th2 balance or restore intestinal microbiota disturbance. For patients with limited activity or allergic rhinitis with more attacks and longer attack duration, oral probiotics have positive effects. The efficacy of probiotics in the prevention and treatment of allergic rhinitis is remarkable, but its specific mechanism needs further study. This review summarizes the research progress of probiotics in the treatment of allergic rhinitis in recent years.
Keywords: allergy rhinitis, probiotics, immune tolerance, Th1/Th2 balance, Treg/Th17 balance, mucosal barrier
Introduction
AR is a global health problem, with significant burden and economic impact on various countries. The economic impact of AR is often underestimated, as indirect costs are often overlooked, and in the European Union, the impact of AR on job productivity is estimated at 30 billion to 50 billion euros per year.1,2 AR is estimated to affect approximately 10 to 30% of adults and up to 40% of children,3 and the prevalence is increasing year by year.4 AR typical symptoms can have a significant negative impact on patients’ quality of life (QoL), sleep quality, mood, learning efficiency and sexual function.5 At present, traditional drug treatments for AR mainly include antihistamines, nasal mucosa decongestants, and glucocorticoids, the only one with disease immune regulation is allergen specific immunotherapy.6 At present, the commonly used treatment of AR mainly focuses on conservative drug treatment, but drugs used to treat AR are often accompanied by adverse side effects (eg, dry mouth, drowsiness, dizziness), some of which can seriously affect quality of life, making it an urgent issue to find alternative treatments. At the same time, the use of probiotics as an alternative is increasing in the world, and consumption of probiotics is expected to modulate immune responses in AR patients, establish a more balanced gut microbiota, and make these patients more moderately responsive to inhaled allergens, and can reduce the damage caused by inflammation. Therefore, the use of probiotics is currently a popular strategy for adjuvant treatment of AR.
General Situation and Treatment of Allergic Rhinitis
Overview of Allergic Rhinitis
AR, a chronic inflammation of the nasal mucosa, is caused by a specific immunoglobulin E (IgE)-mediated response to type II helper T(Th2) cell-driven inhaled allergens and affects approximately one-sixth of the world’s One of the people.7,8 The etiology of AR is determined by multiple factors such as genetics, environment and family susceptibility, its typical symptoms include intermittent or persistent nasal itching and sneezing, rhinorrhea, nasal congestion and eyelid edema, these symptoms are often caused by seasonal or perennial allergies, it is a type I allergic disease, which affects patients’ sleep, attention, study, work and leisure activities, reduces the quality of life, and is often associated with allergic conjunctivitis and asthma.9,10
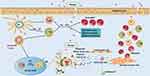
When a patient is first exposed to an allergen, the allergic immune response is in the sensitization phase. Dendritic cells (DCs) in the nasal mucosa take up allergens, process them and transport them to the draining lymph nodes, and then present the allergens to naive CD4+T cells after secondary processing by the draining lymph nodes, naive CD4+T cells differentiate into allergen-specific Th2 cells, which in turn induce B cell activation to produce plasma cells, which further differentiate to produce specific IgE, which then undergo recirculation and interaction on the surface of effector cells such as mast cells and basophils, binds to IgE receptors (FcεRI) with high affinity. These processes simultaneously lead to the formation of memory allergen-specific Th2 cells and B cells.11–16 Activation of Th2 plays an important role in the development and maintenance of AR, while mast cells, eosinophils and basophils are innate immune response cells and are considered to be the main effector cells of AR, at the same time, the reduction of basophils can also reduce the recruitment of eosinophils and reduce the Th2 response, afterwards, inflammatory mediators such as histamine, prostaglandins, leukotrienes, and tryptases are released, and most of the pathological processes in the nasal mucosa involve these mediators,9,17,18 which is a key step in the occurrence of allergy. But when the nasal mucosa is exposed to various allergens sporadically, seasonally or chronically, it will become a process of repeated exposure to allergens. When a patient previously sensitized by exposure to an allergen is re-exposed to the allergen, the allergen binds to allergen-specific IgE on mast cells of the nasal mucosa, and then IgE and FcεRI are cross-linked, causing mast cell activation and degranulation, while releasing pre-stocked and newly synthesized mediators, including histamine, sulfinyl peptide leukotrienes, prostaglandin D2 and other products.13 These mediators interact with the nasal sensory nerves, vasculature, and glands, resulting in AR symptoms (see Figure 1).
Treatment of Allergic Rhinitis
Appropriate treatment drugs are selected according to the specific severity of the disease, type of disease, and lifestyle (see Table 1).
 |
Local treatment: Topical nasal corticosteroids act rapidly, especially to relieve nasal congestion. Topical steroids bind to specific cytoplasmic glucocorticoid receptors (GRs), activate anti-inflammatory gene transcription and inhibit pro-inflammatory gene transcription, and the anti-inflammatory effects of topical steroids reduce all nasal and ocular symptoms.20 Topical steroids with combined antihistamines: MP Aze-Flu, a nasal spray consisting of azelastine hydrochloride and fluticasone propionate, was more effective in symptom scores and quality of life than placebo or fluticasone propionate alone valid.21,22 Nasal congestion reducer: Because of the rebound effect and habituation effect of the nasal mucosa, continuous use is preferably not more than 7 days.23 Most drugs can make alpha adrenergic receptors work, causing vasodilation and contraction, which can immediately relieve the symptoms of nasal congestion, mainly including pseudoephedrine, oxymetazoline, trichomazoline or phenylephrine. Nasal anticholinergics and cromolyn/mast cell stabilizers: Nasal cromolyn and anticholinergics, which primarily affect nasal secretions, have some older studies, but there is insufficient evidence to make an adequate recommendation.23 Saline irrigation: Hyde et al24 noted that increased nasal irrigation in children is beneficial compared to no nasal irrigation. It also appears to reduce nasal eosinophils and neutrophils.25
Systemic therapy: All mechanisms of systemic glucocorticoids are regulated by GR, which belongs to the ligand-regulated nuclear receptor superfamily, and the anti-inflammatory effects of steroids can be explained by three broad molecular mechanisms: decreased pro-inflammatory gene expression, anti-inflammatory increased inflammatory gene expression and non-genomic mechanisms.26 Oral antihistamines: Four histamine receptors, H1 and H2 receptors, have been identified on a variety of cells, stimulating both the early and late stages of allergic reactions. Second-generation/third-generation non-sedating H1 receptor antagonists are the antihistamines of choice for AR.27 Cetirizine has been shown to be efficacious in many studies, and cetirizine is superior to loratadine in symptom relief with a favorable safety profile.28,29 Leukotriene Receptor Antagonists (LTRA): Leukotrienes are a family of inflammatory mediators, including LTA4, LTB4, LTC4, LTD4 and LTE4, by blocking the cysteine LT1 (CysLT1) receptor, LTRAs (such as Montelu sterol) can improve the symptoms of allergic rhinitis and asthma.30 At present, the research on oral cromoglycate as a mast cell stabilizer is insufficient.
To date, allergen immunotherapy is the only immune-modifying and causal treatment currently available for patients with IgE-mediated allergic disease. The purpose of AIT is to reprogram the immune system to reduce the production of specific IgE, thereby inducing tolerance to allergens, it can be divided into subcutaneous and sublingual immunization methods through different routes of administration, patients can be desensitized by continuously increasing the allergen dose.31 Meanwhile, Liu et al32 published a population-wide study to investigate the effects of influenza vaccination and air pollution on allergic respiratory disease symptoms, and found that vaccination could improve the negative effects of long-term air pollution in allergic respiratory tract. A study by Dulny et al33 showed that preventive immunization against rubella, typhoid, and smallpox showed a lower incidence of AR, while measles vaccine showed a higher incidence of AR.
Current treatment of AR is still based on allergen avoidance, symptom-relieving drugs, anti-inflammatory therapy, and allergy immunotherapy. At this stage, there are many adverse drug reactions in the treatment of AR and cannot be cured, the symptoms are easy to repeat, and the immunotherapy course is longer and the compliance is poor,34 and at the same time reduce the quality of life. Probiotics can be used as immunomodulators and activators of the host defense pathway, in addition, oral probiotics can regulate the immune response of the respiratory system, and can prevent and treat upper respiratory diseases such as asthma, AR and other allergic diseases by modulating changes in the gut microbiota and immune response.35 However, the research and application of probiotics as an alternative treatment method in the world is increasing, and most of the studies suggest that probiotics can significantly improve the symptoms of AR patients.36,37 Probiotics can activate Th1 or inhibit Th2, causing anti-inflammatory effects, and can also stimulate the production of immune factors such as interleukin 10 (IL-10), whose main role is to suppress inflammatory responses.38,39 Probiotics have the advantages of safety and high cost performance, therefore, the basic research and clinical application of probiotics for AR treatment are increasing.
Probiotics
Introduction to Probiotics
Probiotics are active microorganisms that can improve the balance of intestinal flora in the body and have a beneficial effect on the body. The World Health Organization (WHO) defines probiotics as live microorganisms that, when administered in appropriate amounts, can have beneficial effects on the health of the host.40 The best probiotics are human-derived, safe, and free from carriers that can create antibiotic resistance and pathogenic or virulent factors. In addition, probiotics have a strong ability to survive under intestinal conditions (acidic pH, enzymes, bile salts, etc.), and at the same time, probiotics show significant beneficial effects on the body by fighting pathogens and stimulating the immune system. It is also possible to maintain probiotic activity, growth efficiency, and function through technical treatments.41,42
There are many kinds of probiotics and about 400 kinds of in the human body, according to the reported probiotics, they are roughly divided into the following five categories: Streptococcus, Lactobacillus, Bifidobacterium, Bacillus and others, the common representative strains are shown in Table 2.43–48 Streptococcus, bifidobacteria and lactobacilli can all produce lactic acid, so they can be classified into lactic acid bacteria, probiotics that do not produce lactic acid include Bacillus, propionic acid bacteria and yeast.49 At present, more than 60 species and subspecies of Streptococcus have been reported and confirmed to be classified,43 more than 50 species of Lactobacillus (of which more than 10 species are commonly used),44 and more than 30 species of Bifidobacterium (14 of which are closely related to humans),45 there are more than 150 species of Bacillus (more than 10 common species).46
 |
Table 2 CIassification of Probiotics and Common Representative Strains |
Types of Probiotics Used to Treat Allergic Rhinitis
There have been a large number of clinical studies on probiotics in the treatment of AR. Ahmed et al50 found that in the treatment of perennial AR, children taking Lactobacillus paracasei (LP-33) for 6 weeks had the same effect as taking cetirizine, and almost all children had the same effect baseline symptoms (rhinorrhea, sneezing, nasal congestion, cough, difficulty sleeping, and difficulty eating) all improved significantly. Similarly, a study evaluating the use of Lactobacillus helveticus SBT2171 (LH2171) to treat patients with mild to moderate AR for 16 weeks showed significantly improved nasal symptoms and significantly lower eosinophil counts in nasal fluid and peripheral blood in the LH2171 group in the placebo group.51
Gram-positive probiotic combinations have been extensively studied in AR. A 2017 study investigated the treatment of children with seasonal AR with a mixture of bifidobacteria (B. longum BB536, B. banfanits M-63, B. breve M-16V), symptoms and quality of life in children treated with a mixture of probiotics (QoL) was significantly improved.52 Efficacy of NVP-1703 probiotic mixture (B. longum IM55 and Lactobacillus plantarum IM76) compared with placebo for 4 weeks in a study in perennial adults with AR, treatment group TNSS and Rhinitis Control Assessment Test (RCAT) scores Significant improvement. At the same time, the level of dust mite-specific IgE was also significantly lower in the NVP-1703 group compared with the placebo group. At week 4, serum levels of IL-10 were significantly increased in the NVP-1703-treated group compared with the placebo group.53 Another study included 250 AR-affected children aged 6 to 17, randomly assigned to intervention (150) or placebo (100), in addition to usual care (topical glucocorticoids and/or oral antihistamines) in addition to the drug), the intervention group took a mixture containing two Bifidobacterium strains (Lactobacillus BB12 DSM 15954 and Enterococcus faecium L3 LMG P-27496), and the results showed that the NSS of the intervention group was significantly reduced.54 A preliminary study of 20 adult patients (18–65 years old) with allergic rhinitis caused by house dust mite allergy showed that adding five natural, non-genetically modified probiotic strains to bed sheets reduced symptoms, improve the quality of life.55 At the same time, a study on compound lactic acid bacteria solid drinks (Lactobacillus paracasei GM-080TM, Lactobacillus acidophilus, Lactobacillus fermentum GM-090TM, Lactobacillus paracasei GMNL-133) can improve the quality of life of children with AR, the results suggest that the modified Rhinoconjunctivitis Quality of Life Questionnaire (PRQLQ) score improved significantly.56 Another clinical study on Clostridium butyric live capsules showed that the proportion of factors that can inhibit inflammation (IL-10, transforming growth factor-β1) in the serum of AR patients was significantly increased, and various scoring scales were significantly higher improvement.57
Probiotic-assisted combination therapy is also an important area of focus. In a study using a Gram-positive oral probiotic formulation (Familact capsules) in combination with budesonide, Jalali et al58 found that the addition of probiotics significantly improved the quality of life of AR patients compared with budesonide alone (according to SNOT-22 and control test scores for allergic rhinitis and asthma). The benefits of combined treatment with probiotics and AIT have also been studied. One study compared four groups of placebo, dust mite-specific SCIT, C. butyricum, and C. butyricum-containing SCIT for the treatment of house dust mite-induced AR. Nasal symptoms were significantly reduced in the C. butyricum group and C. butyricum-containing SCIT group compared with the placebo group. Furthermore, combination therapy enhanced SCIT efficacy by improving nasal symptom scores and reducing specific IgE and TH2 cytokines.59 The combined treatment of SLIT and probiotics has also been studied, and it has been suggested that probiotics combined with SLIT are effective in improving AR symptoms in children.60 A 5-month randomized, controlled trial in 100 children (ages 5–12 years) to assess the efficacy of SLIT in combination with vitamin D, placebo, and Lactobacillus rhamnosus without SLIT in the control group. They observed a decrease in symptom drug scores in all groups treated with SLIT and found a significant increase in CD4+CD25+Fox3+ cells in children treated with SLIT and Lactobacillus rhamnosus compared with children treated with SLIT and vitamin D.61 As an add-on therapy that can effectively treat AR, probiotics are a valuable treatment option in the management of AR patients (see Table 3). Future studies will need to use validated AR models to evaluate probiotic therapy.
 |
Table 3 Recent Investigations into the Use of Probiotics for Allergic Rhinitis |
Mechanisms of Probiotic Treatment of Allergic Rhinitis
Effect of Probiotics on Serum Inflammatory Factors
A study in perennial adult AR evaluated the efficacy and safety of NVP-1703 probiotic mixture (B. longum IM55 and Lactobacillus plantarum IM76) intervention for 4 weeks, NVP-1703 group compared with placebo group, IL-4 The serum level of Dermatophagoides was not significantly changed, but the level of D. dust mite-specific IgE was significantly decreased in the NVP-1703 group. At week 4, the serum levels of IL-5 and IL-13 were decreased in the NVP-1703 group compared with the placebo group, while the serum levels of IL-10 were significantly increased.53 In an earlier study, 60 AR patients were randomly divided into a Broncho-vaxom (BV) group and a control group. After BV treatment, the drug score of the treatment group was significantly lower than that of the control group, and both individual and total nasal symptom scores were significantly lower significantly decreased. The levels of IL-4 and IL-13 in the nasal lavage fluid of the BV group were significantly decreased, while the level of interferon gamma (INF-γ) was significantly increased, which made the ratio of IL-4/INF-γ significantly decreased, and eosinophils Cells were also significantly reduced, and the BV-induced benefits persisted for a longer period of time.62 The increased rate/severity of respiratory viral infections in children with AR may be caused by multiple mechanisms, but IFN-γ deficiency may be one of them,63,64 and probiotics can improve respiratory viral infections by raising IFN-γ levels.
Choi et al65 found that oral administration of Lactobacillus plantarum reduced the number of infiltrating cells in the nasal cavity and lungs in an AR mouse model, while bronchoalveolar lavage fluid and draining lymph node samples showed decreased immune cell counts, IL-4, IL- 5, the levels of IL-13, serum IgE and specific serum IgG1 were decreased, while the secretion of IFN-γ and specific serum IgG2a were increased to improve allergic rhinitis. In addition, the study on the efficacy of oral Clostridium butyricum on ovalbumin-induced allergic airway inflammation in mice found that the Clostridium butyricum group significantly reduced lung resistance, pulmonary airway inflammation, mast cell degranulation, airway inflammation in mice remodeling and OVA-specific IgE/G1 expression. At the same time, it also reversed the Th1/Th2 imbalance and increased the anti-inflammatory serum factor IL-10.66 Probiotics were used to ferment chemically transformed red ginseng (RG), in this study, the effect of probiotic-fermented RG (FRG) on ovalbumin (OVA)-induced allergic rhinitis model in mice was found to be FRG, it reduced IL-4 and IgE levels in bronchoalveolar lavage fluid, nasal fluid, and serum more effectively than RG, suggesting that FRG has a better immunomodulatory effect than RG. FRG also down-regulated immune cell levels (eosinophils, basophils) compared to RG, overall, the results suggest that FRG treatment reduces inflammation.67 Another study demonstrated that Lactobacillus helveticus SBT2171 (LH2171) could induce cytokine production in naive mouse splenocytes stimulated by antigen in vitro, which could inhibit the production of IL-4 and IL-13, and increase IFN-γ and IL-10 generation.68
Combined with the study of probiotics in human and animal models of AR, most serum inflammatory factors have decreased to varying degrees, such as IL-4, IL-5, IL-13, IgE, specific serum IgG1, eosinophils and the level of basophils decreased, but some anti-inflammatory factors increased, such as IL-10, IFN-γ and specific serum IgG2a secretion increased.Therefore, probiotics can alleviate the inflammatory response of AR patients by improving the level of inflammatory factors in the serum, thereby alleviating their clinical symptoms.
Balance of Probiotics Against Allergic Rhinitis Th1/Th2
AR is a type I allergic disease, which is mainly caused by IL-4, IL-5 and IL-13 produced by Th2 cells, causing the Th1/Th2 balance to tilt towards Th2, thereby producing specific IgE, while mast cells release histamine and Leukotrienes.69 On the other hand, Th1 suppresses the Th2 immune response by producing anti-inflammatory factors such as IFN-γ and IL10,70 thereby alleviating AR symptoms.
Yang et al71 found that Lactobacillus plantarum (NR16) extracted from fermented Korean kimchi was a powerful Th1 inducer, and when NR16 was co-cultured with immune cells, it could produce a large amount of IFN-γ and IL-12, and at the same time oral administration of NR16 reduces airway hyperresponsiveness and leukocyte infiltration in mice. Furthermore, oral administration of NR16 may alleviate AR symptoms by inducing a Th1 immune response, which in turn may rebalance the Th1/Th2 ratio by reducing the production of Th2 cytokines in specific mucosal lesions. Choi et al65 found that Lactobacillus plantarum can increase the production of Th1-type cytokines (IFN-γ, specific serum IgG2a) in AR mouse model, Th2-type cytokines (IL-4, IL-5, IL-13) decreased and reached the balance of Th1/Th2.
Another randomized, controlled study showed that after Broncho-vaxom (BV) treatment, compared with the control group, the contents of IL-4 and IL-13 in the nasal lavage fluid of the BV group were significantly reduced, while the content of INF-γ was significantly increased high, which resulted in a marked decrease in the ratio of IL-4/INF-γ, and BV could modulate the Th1/Th2 cytokine balance as a potential cell signaling mechanism to improve overall mucosal immunity.62 Ren et al72 confirmed that oral administration of Bifidobacterium breve can inhibit Th2 response and induce CD4+CD25+Tregs activity, but does not cause Th1 response, but can regulate Th1/Th2 balance and has anti-allergic effect. Second, high doses of Bifidobacterium breve can significantly reduce the frequency of sneezing, while reducing serum IL-4 and specific IgE levels, increasing the number of CD4+CD25+ Tregs in the spleen, and significantly reducing the allergic reaction of nasal mucosa epithelial, low doses of Bifidobacterium breve provide only mild relief from allergic reactions.
Effects of Treg/Th17 Cell Balance
Treg acts as an immunosuppressive CD4+ T cell, while Th17 acts as an inflammatory CD4+ T cell, the balance between the two is a key condition for maintaining the stability of the human immune system.73 In recent years, we have found in some studies on autoimmune diseases that the occurrence and development of diseases are often accompanied by an increase in the number and function of Th17. A study in patients with allergic fungal sinusitis showed that the secretion of IL-1, IL-17, IL-21 and TGF-β in serum all increased to varying degrees, leading to a shift in the Th17/Treg balance Th17 direction is inclined.74 Research data confirmed that the secretion of inflammatory factors such as IL-17, IL-35, and Th17 in the peripheral blood of AR patients increased,75 and the increase in inflammatory factors caused Treg/Th17 imbalance, which in turn led to Th1/Th2 imbalance, resulting in a series of AR typical clinical symptoms and nasal mucociliary destruction, nasal gland hyperplasia and inflammatory cell infiltration.
Probiotics can modulate autoimmunity by affecting the balance of Treg and Th17. Fu et al76 found that the induction of CD4+FoxP3+Treg cells by Clostridium spores could inhibit the pro-inflammatory response of Th17 cells. Ekmekciu et al77 used the probiotic mixture VSL#3 to induce the proliferation of Treg cells. Cell experiments by Johansson et al78 showed that the supernatant of lactic acid bacteria can reduce the activation of CD4+ T cells, CD8+ T cells, mucosa-associated constant T cells, etc., and the products of lactic acid bacteria can inhibit the proliferation and degranulation of these cells. Other studies have shown that changes in T cell metabolism caused by inflammation can affect the immune function of Treg cells. For example, enolase during glycolysis can regulate the binding variant of FoxP3 in exons, and changes in Treg metabolism caused by stress state, it is an important part of triggering an autoimmune response.79 Wang et al80 used Lactobacillus casei as an intervention control, and the results showed that the percentage of CD4+CD25+Foxp3+Tregs in the spleen of the intervention group increased, while the percentage of CD4+IL-17A+Th17 cells decreased, regulates the imbalance of Treg/Th17 cell ratio. Another study showed that Lactobacillus rhamnosus GG (LGG) extract could maintain Treg/Th17 homeostasis by reducing the ratio of IL-17+Th17 and increasing the ratio of CD25+Foxp3+Treg via the Toll receptor (TLR2) pathway.81
Probiotics can improve the immune regulation of allergy and immune diseases by regulating the balance of Treg/Th17, and have produced some targeted treatment methods with considerable results. Further exploration of the Treg/Th17 balance mechanism and its influencing factors will provide a more comprehensive understanding of the human body’s autoimmune regulation mechanism, or provide theoretical support for the treatment of AR and the development of new drugs.
Influence on the Activity of Tolerant Dendritic Cells (Intestinal Immune Tolerance)
Dendritic cells (DCs) are the most efficient antigen-presenting cells (APCs) in the body, which can effectively induce antigen-specific immune responses by regulating tolerance and immunity to microbial antigens.82 Tolerogenic DCs (TDCs) play a key role in regulating immune tolerance and are characterized by a semi-mature phenotype that expresses co-stimulatory molecules (CD80/CD86), which can be activated by TLR ligands or by exposure to specific cells, differentiation in a factor environment.83 In addition, they also express immunoregulatory molecules and produce immunosuppressive factors, and semi-mature, co-stimulatory CD80/CD86 signaling affects the activation of Treg on T cells through the action of CD28 molecules, which in turn induces immune tolerance.84 Currently, several clinical trials are underway to explore the effectiveness of TDC as an alternative treatment option for immune-mediated diseases.85 These TDCs have a semi-mature phenotype, exhibit low levels of T-cell co-stimulatory properties, and a reduced ability to produce pro-inflammatory cytokines compared to anti-inflammatory molecules, especially through the expansion of regulatory T cells (Tregs) and/or or induction.86 Other studies have also shown that TDCs secrete anti-inflammatory cytokines and regulate T cells to promote Foxp3+ Treg development in the mouse and human gut.87,88 Globally, these data suggest that the DC/Treg/B regulatory axis plays a central role in the gut by re-establishing tolerance and regulating Tregs.
Recent evidence suggests that probiotics may affect immune regulation in vitro and in vivo by modulating DC maturation and TDC production, thereby suppressing inflammation.89 The immunomodulatory effects of probiotics arise from the interaction of immune cells with intestinal DCs, thereby regulating the innate and adaptive immune systems.90 Studies have shown that probiotics are able to react with pattern recognition receptors (PRRs) on DCs, which detect distinct evolutionarily conserved structures (pathogen-associated molecular patterns, PAMPs) on pathogens, or by producing soluble compounds, thereby inducing TDCs.91 Different species and strains of probiotics may directly affect the maturation of DCs, and probiotics may regulate the levels of anti-inflammatory cytokines, such as transforming growth factor beta (TGF-β), IL-10, and induce Treg. A study targeting four strains of probiotics (including Lactobacillus salivarius, Bifidobacterium, Bacillus coagulans, and Bacillus subtilis natto) all induced stimulation of DC production of IL-10 and TGF-β, Bifidobacterium and Bifidobacterium coagulans exhibited a stronger ability to induce IL-10 and TGF-β. Therefore, probiotic-induced DC activity to produce anti-inflammatory cytokines plays a key role in immunomodulatory functions.92 TDCs are induced to produce TGF-β, IL-10, and stimulate Treg production, suppress effector T cell responses, and suppress allergic airway inflammation in mouse models, whereas depletion or blockade of CD25+ cells and TGF-β, IL-10 signaling can abolish this inhibitory effect, indicating that Treg is involved in the regulation of anti-inflammatory activity of TDC.93 In conclusion, probiotics are potential targets for AR treatment by regulating TDC activity.
Stimulation of Toll Like Receptors?
Toll-like receptors (TLRs), as one of the main components of the body’s immunity, are recognition receptors expressed on the surface of intestinal mucosal lymphocytes and epithelial cells, providing a defense barrier against invading pathogens and inflammatory responses. TLRs are located in the cytoplasmic membrane and also in intracellular endosomes, and can detect a series of pathogenic molecular patterns of bacteria, viruses and fungi, and TLR activation in dendritic cells can affect the adaptive immune response.94
Many microbial infections can activate TLR4 signaling, and probiotics, as part of the commensal gut microbiota, can affect TLRs, especially TLR4.95,96 Probiotic-derived polysaccharide capsules can play a key role in controlling immune responses by modulating Th1/Th2 balance, inducing T regulatory cell differentiation, and activating DCs, which then interact with gut microbiota through TLRs.97 In a study of probiotics (Lactobacillus rhamnosus GG) combined with sublingual immunotherapy (SLIT), the between-group analysis showed that the induction rate of CD4+CD25+Foxp3+ was significantly increased in the SLIT probiotic group, compared with the SLIT vitamin D group, in contrast, the percentage reduction in the TLR-positive cell group was higher.61 The study by Marschan et al98 showed that the transient protein produced by probiotics can induce TLR production, and this protein can alleviate allergic reactions caused by specific IgE. In addition, some TLRs can stimulate DC activation, which in turn leads to increased Treg cell production. Previous studies have pointed out that TLR may be a potential target for probiotics to affect the proliferation and differentiation of Treg cells.
In the study of Wu et al,99 the regulatory effect of probiotics on the TLR4/NF-kB pathway in the regulation of host defense against lipopolysaccharide ovalbumin (LPS OVA)-induced lung injury and airway inflammation was elucidated. Allergic infantile asthma and TLRs have effects. Similarly, Li et al100 studied the in vitro macrophage model, with live and inactive Lactobacillus acidophilus (La KLDS 1.0738) strains and TLR4 inhibitors, miR-146a inhibitors were treated with β Milk protein (β-Lg)-induced macrophages. The results showed that β-Lg stimulation caused increased transduction of the TLR4/NF-κB signaling pathway in macrophages. Similar to the TLR4 signaling pathway inhibitor, La KLDS 1.0738 intervention significantly reduced allergic inflammation by inhibiting the TLR4 pathway, which was superior to the control group, especially the live Lactobacillus acidophilus treatment group. In addition, La KLDS 1.0738 strain could significantly reduce TLR4 transduction and inflammatory cytokine production, which were closely associated with upregulation of miR-146a levels. Taken together, these observations suggest that probiotics can modulate allergic inflammation dependent on the TLR4/NF-κB pathway.
Probiotics Affect Type 2 Innate Lymphocytes
Innate lymphocytes (ILCs) are innate immune cells that are difficult to identify, and are divided into five subtypes, NK cells correspond to CD8+ T cells, and Th1, Th2, and Th17 cells correspond to ILC1s, ILC2s, and ILC3s, respectively. Related ILC and T cell subsets have similar functions and similarly similar regulatory pathways.101 Type 2 innate lymphocytes (ILC2s) correspond to Th2 cells in adaptive immunity and are closely related to allergic disease development and systemic immune regulation.102 Th2 cells and ILC2s play a role in the development of type II immune responses by releasing cytokines such as IL-4, IL-5, IL-9, and IL-13.103 Although the number of ILC2s is small in various diseases, they are indispensable for various allergic diseases, in-depth study of the impact of ILC2s on different allergic diseases will help to better understand the relationship between allergic response and immune system, the relationship between them is important.
AR is an IgE-mediated inflammation that results in increased numbers of Th2 cells and type II cytokines in the nasal mucosa.104 Peng et al105 identified the distribution of ILC2s on the nasal mucosa by immunohistochemistry and found that the number of ILC2s in the nasal mucosa was positively correlated with AR clinical visual analog scale (VAS) scores. Multiple lipid receptors have been reported to be upregulated in AR patients, including CysL1R (LTD4 ligand) and PGD2. Although LTD4 was shown to activate IL-4 production in ILC2s, IL-4 levels in nasal secretions of AR patients were not significantly changed.106 Ozone aggravates AR symptoms by inducing the release of IL-5 and IL-13 from ILC2s.107 Children with HDM-AR had significantly higher levels of ILC2 in peripheral blood than children without HDM-AR.108 All these findings suggest that ILC2s play an important role in regulation in AR. Meanwhile, in a study of papain-induced BL6 mice, treatment with the probiotic Escherichia coli strain Nissle 1917 (ECN) resulted in a smaller decrease in IL-5, a significant decrease in IL-13, and a significant decrease in IL-33 levels. ECN-treated mice had significantly lower CD3+CD4+IL5+ and IL13+ cell frequencies compared to untreated controls. Data suggest that ECN is able to inhibit the activation of Th2 and ILC2s and the production of prototypical sensitizing IL-5 and IL-13.109 Therefore, probiotics can control the occurrence and development of AR by inhibiting the activation of ILC2s, but the current research is relatively limited, and more basic and clinical studies are needed to evaluate the long-term therapeutic effect in the future.
Regulation of the Gut Microflora (Regulation of Metabolism?)
As the largest digestive organ in the human body, the gut contains hundreds of microbiota.110 The microbiota in the human gut is closely related to the physiological functions of the body, is an important part of human life activities, and is mostly considered to be beneficial to the human body, the microbiota can not only improve the efficiency of life activities related to energy metabolism, but also participate in human immunity, system activation, while maintaining the homeostasis of human immunity.111,112 Under normal circumstances, the interaction between the microbiota and the body is the basis for determining the health of the body, and if one of these links is damaged, it may cause intestinal flora imbalance.113 Dysregulation of the gut microbiota significantly affects the metabolism between the microbiota and the host, and suppresses the host immune system.114,115 Most allergic diseases are associated with an imbalance of gut microbiota, such as AR.116,117 Alterations in microbial diversity in early infancy relative to school age (6–8 years) predispose to the development of AR and asthma. Elevated serum IgE levels are a risk factor for allergen sensitization in children, and some studies have found that decreased gut microbiota diversity may be closely related to increased serum IgE levels.118 In summary, the imbalance of the body’s microbiota may be beneficial to the occurrence and development of AR.
As an important means to regulate the balance of intestinal flora, probiotics include a wide variety of bacteria, and their main role is to promote the production of pro-anti-inflammatory factors, maintain the balance of the immune system, improve the structure of the flora, restore the balance of the flora, and at the same time, it can alleviate the local mucosal inflammatory response in the intestinal tract, restore the mucosal barrier, and block the invasion of foreign pathogens.119 Studies have shown that the addition of probiotics can modulate the immune response of AR by restoring intestinal flora disturbances.120 Another study pointed out that after treatment with probiotic fermented milk, the serum-specific IgE in patients with hay fever was significantly reduced, the immune function was significantly improved, the structure of intestinal flora in the body was improved, and the balance of intestinal flora was restored, symptoms were also significantly relieved. As an adjuvant therapy for AR, probiotics can not only restore the intestinal microbiota disturbance of the body from a deeper level and relieve the typical symptoms of nasal allergy, but also have the advantages of high cost performance and low risk.121 In a study on the efficacy of probiotics in the treatment of AR, Schaefer et al122 found that the allergic symptoms of patients were not significantly relieved, but the nasal mucosa microenvironment of some patients was improved compared with before treatment. Kim et al123 found that AR treatment with a probiotic mixture (PM) of Bifidobacterium longum and Lactobacillus plantarum isolated from human feces and kimchi could alleviate AR by controlling intestinal flora disturbance (significantly inhibited deformation bacteria, increasing the composition of Bacteroides and Actinomyces). The results of Hu et al124 showed that probiotics and L-glutamine can effectively regulate the level of gastrointestinal peptides in the treatment of children with AR, restore the balance of intestinal microflora, and restore the barrier function of the intestinal mucosa for the purpose of treatment.
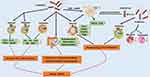
Based on the above research results, it can be seen that probiotics can regulate and restore intestinal microbiota disorders to treat AR. Currently, as a new direction of clinical allergic disease research, it is expected to become a potential new target for AR control and treatment. The possible mechanisms of probiotics for AR treatment is shown in Figure 2.
Conclusion
Probiotics have clear benefits for clinical AR patients and can represent an aspect of future management of AR therapy.Probiotics have excellent immune regulation effects. A large number of research data show that probiotics play a vital role in regulating the immune system of the body, and also have an impact on various stages of autoimmune-related diseases, it has great potential in related diseases, especially for the treatment of AR. At present, clinicians have an increasing understanding of how probiotics can affect the immune regulation of the body, and the research on the basic mechanism of using probiotics to treat AR is increasing, mainly focusing on how probiotics regulate the balance and influence of Th1/Th2 and Treg/Th17 cells Tolerance dendritic cell activity, stimulation of Toll-like receptors, regulation affecting type 2 innate lymphocytes and gut microbiota, and associated immune cells and immune factors. At the same time, probiotic fusion proteins may be a new way to improve the therapeutic effect of AR. The optimal strain, dosage and duration of probiotics need to be further explored, and further research should clarify the clinical efficacy of probiotics, the selection scheme, the design of appropriate study populations, and the safety of using probiotics. Fundamental research in this area is underway and will hopefully provide better insights into how probiotics can help treat AR and even allergy-related diseases.
Funding
This work was supported by grants Natural Science Foundation of China (No. 82004046, No. 81700888), Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515010592, No. 2021A1515010971), Shenzhen Science and Technology Program (No. JCYJ20210324142207019), Shenzhen Key Medical Discipline Construction Fund (No. SZXK039), and Science and Technology Development Special Fund of Shenzhen Longgang District (No. LGKCYLWS2019000864, LGKCZSYS2019000046).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vandenplas O, Vinnikov D, Blanc PD, et al. Impact of rhinitis on work productivity: a systematic review. J Allergy Clin Immunol Pract. 2018;6(4):1274–1286.e9. doi:10.1016/j.jaip.2017.09.002
2. Colás C, Brosa M, Antón E, et al. Estimate of the total costs of allergic rhinitis in specialized care based on real-world data: the FERIN Study. Allergy. 2017;72(6):959–966. doi:10.1111/all.13099
3. Meltzer EO. Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin North Am. 2016;36(2):235–248. doi:10.1016/j.iac.2015.12.002
4. Small P, Keith PK, Kim H. Allergic rhinitis. Allergy Asthma Clin Immunol. 2018;14(Suppl 2):51. doi:10.1186/s13223-018-0280-7
5. Bousquet J, Anto JM, Bachert C, et al. Allergic rhinitis. Nat Rev Dis Primers. 2020;6(1):95. doi:10.1038/s41572-020-00227-0
6. Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136(3):556–568. doi:10.1016/j.jaci.2015.04.047
7. Yang Q, Wang F, Li B, et al. The efficacy and safety of ciclesonide for the treatment of perennial allergic rhinitis: a systematic review and meta-analysis. Braz J Otorhinolaryngol. 2019;85(3):371–378. doi:10.1016/j.bjorl.2018.10.008
8. Wheatley LM, Togias A. Clinical practice. Allergic rhinitis. N Engl J Med. 2015;372(5):456–463. doi:10.1056/NEJMcp1412282
9. Kiricsi Á, Tiszlavicz L, Rázga Z, et al. Prospective, multicenter, randomized clinical study to evaluate the clinical efficacy and tolerability of long term mixed ultraviolet and visible light phototherapy in eosinophil nasal polyps. J Photochem Photobiol B. 2017;176:118–123. doi:10.1016/j.jphotobiol.2017.09.022
10. Okubo K, Kurono Y, Ichimura K, et al. Japanese guidelines for allergic rhinitis 2020. Allergol Int. 2020;69(3):331–345. doi:10.1016/j.alit.2020.04.001
11. Humbert M, Bousquet J, Bachert C, et al. IgE-mediated multimorbidities in allergic asthma and the potential for omalizumab therapy. J Allergy Clin Immunol Pract. 2019;7(5):1418–1429. doi:10.1016/j.jaip.2019.02.030
12. Palomares O, Akdis M, Martín-Fontecha M, et al. Mechanisms of immune regulation in allergic diseases: the role of regulatory T and B cells. Immunol Rev. 2017;278(1):219–236. doi:10.1111/imr.12555
13. Palomares Ó, Sánchez-Ramón S, Dávila I, et al. dIvergEnt: how IgE axis contributes to the continuum of allergic asthma and anti-IgE therapies. Int J Mol Sci. 2017;18(6):1328. doi:10.3390/ijms18061328
14. Ihara F, Sakurai D, Yonekura S, et al. Identification of specifically reduced Th2 cell subsets in allergic rhinitis patients after sublingual immunotherapy. Allergy. 2018;73(9):1823–1832. doi:10.1111/all.13436
15. Iinuma T, Okamoto Y, Morimoto Y, et al. Pathogenicity of memory Th2 cells is linked to stage of allergic rhinitis. Allergy. 2018;73(2):479–489. doi:10.1111/all.13295
16. Nakayama T, Hirahara K, Onodera A, et al. Th2 cells in health and disease. Annu Rev Immunol. 2017;35:53–84. doi:10.1146/annurev-immunol-051116-052350
17. Poddighe D, Mathias CB, Brambilla I, Marseglia GL, Oettgen HC. Importance of basophils in eosinophilic asthma: the murine counterpart. J Biol Regul Homeost Agents. 2018;32(2):335–339.
18. Gelardi M, Giancaspro R, Cassano M, Ribatti D. The underestimated role of mast cells in the pathogenesis of rhinopathies. Int Arch Allergy Immunol. 2022;183(2):153–159. doi:10.1159/000518924
19. Department of Rhinology, Editorial Committee of Chinese Journal of Otolaryngology Head and Neck Surgery, Department of Rhinology, Otolaryngology Head and Neck Surgery Credit Association of Chinese Medical Association. Chinese guidelines for the diagnosis and treatment of allergic rhinitis (2022, Revised). Chin J Otolaryngol Head Neck Surg. 2022;57(02):106–129.
20. Khattiyawittayakun L, Seresirikachorn K, Chitsuthipakorn W, et al. Effects of double-dose intranasal corticosteroid for allergic rhinitis: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2019;9(1):72–78. doi:10.1002/alr.22204
21. Berger W, Bousquet J, Fox AT, et al. MP-AzeFlu is more effective than fluticasone propionate for the treatment of allergic rhinitis in children. Allergy. 2016;71(8):1219–1222. doi:10.1111/all.12903
22. Berger W, Meltzer EO, Amar N, et al. Efficacy of MP-AzeFlu in children with seasonal allergic rhinitis: importance of paediatric symptom assessment. Pediatr Allergy Immunol. 2016;27(2):126–133. doi:10.1111/pai.12540
23. Roberts G, Xatzipsalti M, Borrego LM, et al. Paediatric rhinitis: position paper of the European Academy of Allergy and Clinical Immunology. Allergy. 2013;68(9):1102–1116. doi:10.1111/all.12235
24. Head K, Snidvongs K, Glew S, et al. Saline irrigation for allergic rhinitis. Cochrane Database Syst Rev. 2018;6(6):CD012597. doi:10.1002/14651858.CD012597.pub2
25. Malizia V, Fasola S, Ferrante G, et al. Efficacy of buffered hypertonic saline nasal irrigation for nasal symptoms in children with seasonal allergic rhinitis: a randomized controlled trial. Int Arch Allergy Immunol. 2017;174(2):97–103. doi:10.1159/000481093
26. Hox V, Lourijsen E, Jordens A, et al. Benefits and harm of systemic steroids for short- and long-term use in rhinitis and rhinosinusitis: an EAACI position paper. Clin Transl Allergy. 2020;10:1. doi:10.1186/s13601-019-0303-6
27. Parisi GF, Licari A, Papale M, et al. Antihistamines: ABC for the pediatricians. Pediatr Allergy Immunol. 2020;31(Suppl 24):34–36. doi:10.1111/pai.13152
28. Nayak AS, Berger WE, LaForce CF, et al. Randomized, placebo-controlled study of cetirizine and loratadine in children with seasonal allergic rhinitis. Allergy Asthma Proc. 2017;38(3):222–230. doi:10.2500/aap.2017.38.4050
29. Parisi GF, Leonardi S, Ciprandi G, et al. Cetirizine use in childhood: an update of a friendly 30-year drug. Clin Mol Allergy. 2020;18:2. doi:10.1186/s12948-020-00118-5
30. Cingi C, Muluk NB, Ipci K, et al. Antileukotrienes in upper airway inflammatory diseases. Curr Allergy Asthma Rep. 2015;15(11):64. doi:10.1007/s11882-015-0564-7
31. Şahin E, Bafaqeeh SA, Güven SG, et al. Mechanism of action of allergen immunotherapy. Am J Rhinol Allergy. 2016;30(5):1–3. doi:10.2500/ajra.2016.30.4367
32. Liu K, Li S, Qian ZM, et al. Benefits of influenza vaccination on the associations between ambient air pollution and allergic respiratory diseases in children and adolescents: new insights from the Seven Northeastern Cities study in China. Environ Pollut. 2020;256:113434. doi:10.1016/j.envpol.2019.113434
33. Dulny G, Sybilski AJ, Zalewska M, et al. The effect of preventive immunization on the incidence of allergic conditions. Iran J Allergy Asthma Immunol. 2015;14(4):402–409.
34. Juniper EF, Ståhl E, Doty RL, et al. Clinical outcomes and adverse effect monitoring in allergic rhinitis. J Allergy Clin Immunol. 2005;115(3 Suppl 1):S390–413. doi:10.1016/j.jaci.2004.12.014
35. Huang J, Zhang J, Wang X, et al. Effect of probiotics on respiratory tract allergic disease and gut microbiota. Front Nutr. 2022;9:821900. doi:10.3389/fnut.2022.821900
36. Yan S, Ai S, Huang L, et al. Systematic review and meta-analysis of probiotics in the treatment of allergic rhinitis. Allergol Immunopathol. 2022;50(3):24–37. doi:10.15586/aei.v50i3.507
37. Farahmandi K, Mohr AE, McFarland LV. Effects of probiotics on allergic rhinitis: a systematic review and meta-analysis of randomized clinical trials. Am J Rhinol Allergy. 2022;19458924211073550. doi:10.1177/19458924211073550
38. Gourbeyre P, Denery S, Bodinier M. Probiotics, prebiotics, and synbiotics: impact on the gut immune system and allergic reactions. J Leukoc Biol. 2011;89(5):685–695. doi:10.1189/jlb.1109753
39. Hoyte FCL, Nelson HS. Recent advances in allergic rhinitis. F1000Res. 2018;7:F1000 Faculty Rev–1333. doi:10.12688/f1000research.15367.1
40. Na D, Xiao Z, Yanhui T, et al. Approach probiotics. Biol Teach. 2013;38(9):79.
41. Plaza-Díaz J, Ruiz-Ojeda FJ, Gil-Campos M, et al. Immune-mediated mechanisms of action of probiotics and synbiotics in treating pediatric intestinal diseases. Nutrients. 2018;10(1):42. doi:10.3390/nu10010042
42. Plaza-Díaz J, Robles-Sánchez C, Abadía-Molina F, et al. Gene expression profiling in the intestinal mucosa of obese rats administered probiotic bacteria. Sci Data. 2017;4:170186. doi:10.1038/sdata.2017.186
43. Zhong J. New progress in the classification and identification of Streptococcus. J Clin Lab. 2006;24(5):39l–393.
44. Limin X, Dan L, Xiufang C, et al. Study on the types of probiotics and their application in human nutrition and health care. Anhui Agric Sci. 2013;4L(17):7694–7695.
45. Xianfu L, Qin N, Shulin Q, et al. Research progress on classification, physiological function and application of bifidobacteria. Biotechnology. 2017;2017(3):100–105.
46. Xiao C, Yanxia X, Junjie L, et al. Application of Bacillus in agricultural production. Soil Crops. 2019;8(1):32–42.
47. Hai NV. Application of probiotics in aquaculture. Biotechnol World. 2015;(4):30–33.
48. Feifei A, Qihui D, Rong W, et al. Research progress of traditional fermented food and its fermentation microorganisms in different countries and regions. Food Sci. 2020;41(21):1–12.
49. Yinhong C. Research progress on action mechanism and function of probiotics. Biotechnol World. 2015;2015(5):39–45.
50. Ahmed M, Billoo AG, Iqbal K. Efficacy of probiotic in perennial allergic rhinitis under five year children: a randomized controlled trial. Pak J Med Sci. 2019;35(6):1538–1543. doi:10.12669/pjms.35.6.744
51. Yamashita M, Miyoshi M, Iwai M, et al. Lactobacillus helveticus SBT2171 alleviates perennial allergic rhinitis in Japanese adults by suppressing eosinophils: a randomized, double-blind, placebo-controlled study. Nutrients. 2020;12(12):3620. doi:10.3390/nu12123620
52. Miraglia Del Giudice M, Indolfi C, Capasso M, et al. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr. 2017;43(1):25. doi:10.1186/s13052-017-0340-5
53. Kang MG, Han SW, Kang HR, et al. Probiotic NVP-1703 alleviates allergic rhinitis by inducing IL-10 expression: a four-week clinical trial. Nutrients. 2020;12(5):1427. doi:10.3390/nu12051427
54. Anania C, Di Marino VP, Olivero F, et al. Treatment with a probiotic mixture containing Bifidobacterium animalis Subsp. Lactis BB12 and Enterococcus faecium L3 for the prevention of allergic rhinitis symptoms in children: a randomized controlled trial. Nutrients. 2021;13(4):1315. doi:10.3390/nu13041315
55. Berings M, Jult A, Vermeulen H, et al. Probiotics-impregnated bedding covers for house dust mite allergic rhinitis: a pilot randomized clinical trial. Clin Exp Allergy. 2017;47(8):1092–1096. doi:10.1111/cea.12937
56. Zhiling C. Efficacy of probiotics in the treatment of allergic rhinitis in children. Chin J Integr Otolaryngol. 2021;29(06):446–449.
57. Qiao Y, Liu T, Zhang K, et al. Efficacy of Clostridium butyrates dipolympic bacteria capsules in the treatment of allergic rhinitis. J Clin Otolaryngol Head Neck Surg. 2017;31(17):1315–1321.
58. Jalali MM, Soleimani R, Alavi Foumani A, et al. Add-on probiotics in patients with persistent allergic rhinitis: a randomized crossover clinical trial. Laryngoscope. 2019;129(8):1744–1750. doi:10.1002/lary.27858
59. Xu LZ, Yang LT, Qiu SQ, et al. Combination of specific allergen and probiotics induces specific regulatory B cells and enhances specific immunotherapy effect on allergic rhinitis. Oncotarget. 2016;7(34):54360–54369. doi:10.18632/oncotarget.10946
60. Nabil F, Elbehedy EM, Sedeek R, et al. Clinical efficacy of combined probiotics and immunotherapy in childhood allergic rhinitis. Egypt J Immunol. 2020;27(2):73–79.
61. Jerzynska J, Stelmach W, Balcerak J, et al. Effect of Lactobacillus rhamnosus GG and vitamin D supplementation on the immunologic effectiveness of grass-specific sublingual immunotherapy in children with allergy. Allergy Asthma Proc. 2016;37(4):324–334. doi:10.2500/aap.2016.37.3958
62. Meng Q, Li P, Li Y, et al. Broncho-vaxom alleviates persistent allergic rhinitis in patients by improving Th1/Th2 cytokine balance of nasal mucosa. Rhinology. 2019;57(6):451–459. doi:10.4193/Rhin19.161
63. Santillan Salas CF, Mehra S, Pardo Crespo MR, Juhn YJ. Atopic conditions other than asthma and risk of the 2009 novel H1N1 infection in children: a case-control study. Allergy Asthma Proc. 2013;34(5):459–466. doi:10.2500/aap.2013.34.3686
64. Saad K, Abdelmoghny A, Abdel-Raheem YF, Gad EF, Elhoufey A. Prevalence and associated risk factors of recurrent otitis media with effusion in children in Upper Egypt. World J Otorhinolaryngol Head Neck Surg. 2020;7(4):280–284. doi:10.1016/j.wjorl.2020.08.002
65. Choi SP, Oh HN, Choi CY, et al. Oral administration of Lactobacillus plantarum CJLP133 and CJLP243 alleviates birch pollen-induced allergic rhinitis in mice. J Appl Microbiol. 2018;124(3):821–828. doi:10.1111/jam.13635
66. Juan Z, Zhao-Ling S, Ming-Hua Z, et al. Oral administration of Clostridium butyricum CGMCC0313-1 reduces ovalbumin-induced allergic airway inflammation in mice. Respirology. 2017;22(5):898–904. doi:10.1111/resp.12985
67. Bae CH, Kim J, Nam W, et al. Fermented red ginseng alleviates ovalbumin-induced inflammation in mice by suppressing interleukin-4 and immunoglobulin E expression. J Med Food. 2021;24(6):569–576. doi:10.1089/jmf.2020.4854
68. Makino T, Yamashita M, Takeuchi N, et al. Lactobacillus helveticus SBT2171 alleviates allergic symptoms in a murine model for pollen allergy. Biosci Biotechnol Biochem. 2019;83(12):2298–2306. doi:10.1080/09168451.2019.1654847
69. Pawankar R, Mori S, Ozu C, et al. Overview on the pathomechanisms of allergic rhinitis. Asia Pac Allergy. 2011;1(3):157–167. doi:10.5415/apallergy.2011.1.3.157
70. Takahashi N, Kitazawa H, Iwabuchi N, et al. Immunostimulatory oligodeoxynucleotide from Bifidobacterium longum suppresses Th2 immune responses in a murine model. Clin Exp Immunol. 2006;145(1):130–138. doi:10.1111/j.1365-2249.2006.03111.x
71. Yang J, Bae J, Choi CY, et al. Oral administration of Lactiplantibacillus plantarum NR16 isolated from Kimchi ameliorates murine allergic rhinitis. Lett Appl Microbiol. 2022;75:152–160. doi:10.1111/lam.13716
72. Ren J, Zhao Y, Huang S, et al. Immunomodulatory effect of Bifidobacterium breve on experimental allergic rhinitis in BALB/c mice. Exp Ther Med. 2018;16(5):3996–4004. doi:10.3892/etm.2018.6704
73. Zhang LL, Chen X, Zheng PY, et al. Oral Bifidobacterium modulates intestinal immune inflammation in mice with food allergy.J. Gastroenterol Hepatol. 2010;25(5):928–934. doi:10.1111/j.1440-1746.2009.06193.x
74. Rai G, Das S, Ansari MA, et al. Phenotypic and functional profile of Th17 and Treg cells in allergic fungal sinusitis. Int Immunopharmacol. 2018;57:55–61. doi:10.1016/j.intimp.2018.02.009
75. Liu CM, Ren XM, Yin X, et al. Effects of sublingual specific immunotherapy on expression levels of IL-17 and IL-35 and Treg/Th17 cell balance in patients with dust mite-induced allergic rhinitis. J Clin Otolaryngol Head Neck Surg. 2016;30(17):1372–1375, 1380.
76. Fu L, Peng J, Zhao S, et al. Lactic acid bacteria-specific induction of CD4+Foxp3+T cells ameliorates shrimp tropomyosin-induced allergic response in mice via suppression of mTOR signaling. Sci Rep. 2017;7(1):1987. doi:10.1038/s41598-017-02260-8
77. Ekmekciu I, von Klitzing E, Fiebiger U, et al. The probiotic compound VSL#3 modulates mucosal, peripheral, and systemic immunity following murine broad-spectrum antibiotic treatment. Front Cell Infect Microbiol. 2017;7:167. doi:10.3389/fcimb.2017.00167
78. Johansson MA, Björkander S, Mata Forsberg M, et al. Probiotic lactobacilli modulate Staphylococcus aureus-induced activation of conventional and unconventional T cells and NK cells. Front Immunol. 2016;7:273. doi:10.3389/fimmu.2016.00273
79. Binger KJ, Côrte-Real BF, Kleinewietfeld M. Immunometabolic regulation of interleukin-17-producing T helper cells: uncoupling new targets for autoimmunity. Front Immunol. 2017;8:311. doi:10.3389/fimmu.2017.00311
80. Wang K, Dong H, Qi Y, et al. Lactobacillus casei regulates differentiation of Th17/Treg cells to reduce intestinal inflammation in mice. Can J Vet Res. 2017;81(2):122–128.
81. Jia L, Wu R, Han N, et al. Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin Transl Immunol. 2020;9(11):e1213. doi:10.1002/cti2.1213
82. Audiger C, Rahman MJ, Yun TJ, et al. The importance of dendritic cells in maintaining immune tolerance. J Immunol. 2017;198(6):2223–2231. doi:10.4049/jimmunol.1601629
83. Hasegawa H, Matsumoto T. Mechanisms of tolerance induction by dendritic cells in vivo. Front Immunol. 2018;9:350. doi:10.3389/fimmu.2018.00350
84. Devi KS, Anandasabapathy N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin Immunopathol. 2017;39(2):137–152. doi:10.1007/s00281-016-0602-0
85. Phillips BE, Garciafigueroa Y, Trucco M, et al. Clinical tolerogenic dendritic cells: exploring therapeutic impact on human autoimmune disease. Front Immunol. 2017;8:1279. doi:10.3389/fimmu.2017.01279
86. Flórez-Grau G, Zubizarreta I, Cabezón R, et al. Tolerogenic dendritic cells as a promising antigen-specific therapy in the treatment of multiple sclerosis and neuromyelitis optica from preclinical to clinical trials. Front Immunol. 2018;9:1169. doi:10.3389/fimmu.2018.01169
87. Coombes JL, Siddiqui KR, Arancibia-Cárcamo CV, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757–1764. doi:10.1084/jem.20070590
88. Matisz CE, Geuking MB, Lopes F, et al. Helminth antigen-conditioned dendritic cells generate anti-inflammatory Cd4 T cells independent of antigen presentation via major histocompatibility complex class II. Am J Pathol. 2018;188(11):2589–2604. doi:10.1016/j.ajpath.2018.07.008
89. Baradaran Ghavami S, Asadzadeh Aghdaei H, Sorrentino D, et al. Probiotic-induced tolerogenic dendritic cells: a novel therapy for inflammatory bowel disease? Int J Mol Sci. 2021;22(15):8274. doi:10.3390/ijms22158274
90. Foligné B, Dewulf J, Breton J, et al. Probiotic properties of non-conventional lactic acid bacteria: immunomodulation by Oenococcus oeni. Int J Food Microbiol. 2010;140(2–3):136–145. doi:10.1016/j.ijfoodmicro.2010.04.007
91. Ludwig IS, Broere F, Manurung S, et al. Lactobacillus rhamnosus GG-derived soluble mediators modulate adaptive immune cells. Front Immunol. 2018;9:1546. doi:10.3389/fimmu.2018.01546
92. Ghavami SB, Yadegar A, Aghdaei HA, et al. Immunomodulation and generation of tolerogenic dendritic cells by probiotic bacteria in patients with inflammatory bowel disease. Int J Mol Sci. 2020;21(17):6266. doi:10.3390/ijms21176266
93. Sun W, Wei JW, Li H, et al. Adoptive cell therapy of tolerogenic dendritic cells as inducer of regulatory T cells in allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(11):1291–1299. doi:10.1002/alr.22217
94. Kirtland ME, Tsitoura DC, Durham SR, et al. Toll-like receptor agonists as adjuvants for allergen immunotherapy. Front Immunol. 2020;11:599083. doi:10.3389/fimmu.2020.599083
95. Mohammed SK, Magdy YM, El-Waseef DA, et al. Modulation of hippocampal TLR4/BDNF signal pathway using probiotics is a step closer towards treating cognitive impairment in NASH model. Physiol Behav. 2020;214:112762. doi:10.1016/j.physbeh.2019.112762
96. Grylls A, Seidler K, Neil J. Link between microbiota and hypertension: focus on LPS/TLR4 pathway in endothelial dysfunction and vascular inflammation, and therapeutic implication of probiotics. Biomed Pharmacother. 2021;137:111334. doi:10.1016/j.biopha.2021.111334
97. Yang J, Kuang H, Li N, et al. The modulation and mechanism of probiotic-derived polysaccharide capsules on the immune response in allergic diseases. Crit Rev Food Sci Nutr. 2022;2022:1–13.
98. Marschan E, Kuitunen M, Kukkonen K, et al. Probiotics in infancy induce protective immune profiles that are characteristic for chronic low-grade inflammation. Clin Exp Allergy. 2008;38(4):611–618. doi:10.1111/j.1365-2222.2008.02942.x
99. Wu Z, Mehrabi Nasab E, Arora P, et al. Study effect of probiotics and prebiotics on treatment of OVA-LPS-induced of allergic asthma inflammation and pneumonia by regulating the TLR4/NF-kB signaling pathway. J Transl Med. 2022;20(1):130. doi:10.1186/s12967-022-03337-3
100. Li A, Yang J, Zhang C, et al. Lactobacillus acidophilus KLDS 1.0738 inhibits TLR4/NF-κB inflammatory pathway in β-lactoglobulin-induced macrophages via modulating miR-146a. J Food Biochem. 2021;45(10):e13662. doi:10.1111/jfbc.13662
101. Vivier E, Artis D, Colonna M, et al. Innate lymphoid cells: 10 years on. Cell. 2018;174(5):1054–1066. doi:10.1016/j.cell.2018.07.017
102. Klose CS, Artis D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat Immunol. 2016;17(7):765–774. doi:10.1038/ni.3489
103. Lloyd CM, Snelgrove RJ. Type 2 immunity: expanding our view. Sci Immunol. 2018;3(25):eaat1604. doi:10.1126/sciimmunol.aat1604
104. Wise SK, Lin SY, Toskala E, et al. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8(2):108–352. doi:10.1002/alr.22073
105. Peng YQ, Qin ZL, Fang SB, et al. Effects of myeloid and plasmacytoid dendritic cells on ILC2s in patients with allergic rhinitis. J Allergy Clin Immunol. 2020;145(3):855–867.e8. doi:10.1016/j.jaci.2019.11.029
106. Qin ZL, Peng YQ, Fang SB, et al. CysLT1R expression on ILC2s and effects of CysLT1R antagonist on ILC2 activity in patients with allergic rhinitis. Allergy. 2020;75(4):977–981. doi:10.1111/all.14117
107. Yang Q, Ge MQ, Kokalari B, et al. Group 2 innate lymphoid cells mediate ozone-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2016;137(2):571–578. doi:10.1016/j.jaci.2015.06.037
108. Sun R, Yang Y, Huo Q, et al. Increased expression of type 2 innate lymphoid cells in pediatric patients with allergic rhinitis. Exp Ther Med. 2020;19(1):735–740. doi:10.3892/etm.2019.8235
109. Secher T, Maillet I, Mackowiak C, et al. The probiotic strain Escherichia coli Nissle 1917 prevents papain-induced respiratory barrier injury and severe allergic inflammation in mice. Sci Rep. 2018;8(1):11245. doi:10.1038/s41598-018-29689-9
110. Fengyi G, Xiao Y, Tianshu G. Research progress of intestinal flora in autoimmune diseases. Int J Immunol. 2021;44(1):91–96.
111. Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65(2):330–339. doi:10.1136/gutjnl-2015-309990
112. Li Q, Ma L, Shen S, et al. Intestinal dysbacteriosis-induced IL-25 promotes development of HCC via alternative activation of macrophages in tumor microenvironment. J Exp Clin Cancer Res. 2019;38(1):303. doi:10.1186/s13046-019-1271-3
113. Luo X, Xu B, Xiong T, et al. Hepatic dysfunction induced by intestinal dysbacteriosis mainly manifests as immunologic abnormity in mice. Pathog Dis. 2020;78(6):ftaa041. doi:10.1093/femspd/ftaa041
114. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535(7610):75–84. doi:10.1038/nature18848
115. Spencer SP, Fragiadakis GK, Sonnenburg JL. Pursuing human-relevant gut microbiota-immune interactions. Immunity. 2019;51(2):225–239. doi:10.1016/j.immuni.2019.08.002
116. Zhu L, Xu F, Wan W, et al. Gut microbial characteristics of adult patients with allergy rhinitis. Microb Cell Fact. 2020;19(1):171. doi:10.1186/s12934-020-01430-0
117. Chiu CY, Chan YL, Tsai MH, et al. Gut microbial dysbiosis is associated with allergen-specific IgE responses in young children with airway allergies. World Allergy Organ J. 2019;12(3):100021. doi:10.1016/j.waojou.2019.100021
118. Brożek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
119. Park E, Kim KT, Choi M, et al. In vivo evaluation of immune-enhancing activity of red gamju fermented by probiotic Levilactobacillus brevis KU15154 in mice. Foods. 2021;10(2):253. doi:10.3390/foods10020253
120. Salgaço MK, Perina NP, Tomé TM, et al. Probiotic infant cereal improves children’s gut microbiota: insights using the Simulator of Human Intestinal Microbial Ecosystem (SHIME®). Food Res Int. 2021;143:110292. doi:10.1016/j.foodres.2021.110292
121. Harata G, Kumar H, He F, et al. Probiotics modulate gut microbiota and health status in Japanese cedar pollinosis patients during the pollen season. Eur J Nutr. 2017;56(7):2245–2253. doi:10.1007/s00394-016-1264-3
122. Schaefer M, Enck P. Effects of a probiotic treatment (Enterococcus faecalis) and open-label placebo on symptoms of allergic rhinitis: study protocol for a randomised controlled trial. BMJ Open. 2019;9(10):e031339. doi:10.1136/bmjopen-2019-031339
123. Kim WG, Kang GD, Kim HI, et al. Bifidobacterium longum IM55 and Lactobacillus plantarum IM76 alleviate allergic rhinitis in mice by restoring Th2/Treg imbalance and gut microbiota disturbance. Benef Microbes. 2019;10(1):55–67. doi:10.3920/BM2017.0146
124. Hu B, Kuang Y, Jing Y, et al. Pediatric allergic rhinitis with functional gastrointestinal disease: associations with the intestinal microbiota and gastrointestinal peptides and therapeutic effects of interventions. Hum Exp Toxicol. 2021;40(11):2012–2021. doi:10.1177/09603271211017325
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.