Back to Journals » Clinical Ophthalmology » Volume 17
Reported Rates of Intraocular Inflammation with Intravitreal Aflibercept Administered via Pre-Filled Syringe or from Vials in Clinical Practice Between 2012 and 2022
Authors Schmidt-Ott U, Fitzpatrick S, Hasanbasic Z, Leal S, Morgan-Warren P, Zhang X, Johnson KT
Received 5 November 2022
Accepted for publication 23 December 2022
Published 26 January 2023 Volume 2023:17 Pages 385—390
DOI https://doi.org/10.2147/OPTH.S393519
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Ursula Schmidt-Ott,1 Scott Fitzpatrick,2 Zoran Hasanbasic,3 Sergio Leal,3 Peter Morgan-Warren,4 Xin Zhang,3 Kristian T Johnson5
1Bayer AG, Berlin, Germany; 2Bayer AG, Mississauga, Canada; 3Bayer Consumer Care AG, Basel, Switzerland; 4Bayer PLC, Reading, UK; 5Bayer US LLC, Cambridge, MA, USA
Correspondence: Ursula Schmidt-Ott, Bayer AG, Müllerstraße 178, Berlin, 13353, Germany, Tel +49 30 468 1111, Email [email protected]
Purpose: To determine the reported rates of intraocular inflammation (IOI) in patients treated with intravitreal aflibercept (IVT-AFL) 2 mg in routine clinical practice (ie, outside interventional studies), across all indications and within all countries (excluding the United States), with access to either the vial presentation or pre-filled syringe (PFS).
Patients and methods: A search was conducted using the Bayer EYLEA® Global Safety Pharmacovigilance Database for reported cases of IOI and IVT-AFL use between October 2012 and March 31, 2022.
Results: With more than 10 years of post-marketing experience with the IVT-AFL vial presentation (> 25 million sold units), and over 2 years of experience with the PFS of IVT-AFL (> 6.7 million sold units) the rate of any IOI, including endophthalmitis, outside the United States was 0.3 events per 10,000 units for the PFS and 1.2 events per 10,000 units for the vial presentation. The event rates specifically for endophthalmitis were 0.1 per 10,000 units for the IVT-AFL PFS and 0.6 per 10,000 units for the IVT-AFL vial presentation.
Conclusion: In patients with retinal diseases treated in routine clinical practice with IVT-AFL either from a vial or the PFS, medically important adverse events of IOI, and in particular, endophthalmitis, are infrequently reported events. Numerically, reported rates of IOI and endophthalmitis are low for the vial presentation and even lower for the PFS.
Keywords: anti-VEGF, eye, injection, IOI, drug-related side effects and adverse reactions
Introduction
The benefits of anti-vascular endothelial growth factor (VEGF) agents for various retinal diseases, including neovascular age-related macular degeneration (nAMD), diabetic macular edema (DME), and retinal vein occlusion (RVO), are well established.1–8 Severe intraocular inflammation (IOI) and endophthalmitis are well-known, albeit rare, adverse events associated with any intraocular procedure, including intravitreal injection(s) of anti-VEGF drugs or ophthalmic surgery.9,10 IOI can be classified as infectious or non-infectious in etiology, ie, “sterile”. It can be challenging to distinguish sterile uveitis/endophthalmitis from infectious endophthalmitis due to overlapping clinical characteristics; however, severe pain, hypopyon, and hyperemia are more common with infectious endophthalmitis.11,12
Sterile IOI is characterized by acute-onset IOI without infection that resolves without anti-infective treatment.12 Acute sterile inflammation can occasionally be more severe, presenting as a sterile uveitis/endophthalmitis,11 and various etiologies have been proposed. These include silicone oil used in the manufacture of disposable pre-filled syringes (PFS),13 and/or the presence of anti-drug antibodies, which can also occur with any anti-VEGF agent.11,14 In particular, specific IOI safety concerns relating to retinal vasculitis with or without retinal vascular occlusion and an associated risk of visual acuity loss have emerged with brolucizumab.15 This particular complication does, however, appear to be product-specific, and has not been reported with the other currently available commercial anti-VEGF agents.16,17
Administered via intravitreal injection, the anti-VEGF agent, aflibercept (IVT-AFL) 2 mg, was approved outside the United States in 2012, initially for the management of patients with nAMD. Additional indications for IVT-AFL subsequently included visual impairment due to DME and macular edema secondary to central and branch RVO, as well as myopic choroidal neovascularization and neovascular glaucoma in Japan.18,19 At the time of launch, IVT-AFL was distributed in a vial for single-dose intravitreal administration of 2 mg (0.05 mL) per eye.13 In 2020, the aflibercept PFS became available for use in Europe. As of the end of March 2022, the IVT-AFL vial presentation is approved in approximately 100 countries globally and the PFS in over 30 countries.
The aim of this analysis was to determine the reported rate of IOI in patients treated with IVT-AFL in routine clinical practice (ie, outside interventional clinical trials) since launch, for the vial presentation and PFS across all indications and within all countries (excluding the United States).
Methods
A search was conducted using Bayer’s Global Safety Pharmacovigilance Database for EYLEA® cases of IOI and IVT-AFL use outside the United States between October 2012 and March 31, 2022, according to 42 MedDRA (Medical Dictionary for Regulatory Affairs) Version 25.0 Preferred Terms (Box 1). The company`s Global Safety Pharmacovigilance Database captures adverse events related to a Bayer product received from various sources. The database included reports of IOI from cases spontaneously reported to Bayer; from published literature; observational studies; compassionate use, patient support programs, reimbursement programs; and market research. For cases reported to Bayer, there were various mechanisms to report a side effect experienced with EYLEA®, including through patients’ healthcare professionals, via national competent authority reporting mechanisms, or self-reported by the patient direct to Bayer through the global safety portal.
 |
Box 1 Search Terms Used to Identify Cases of Intraocular Inflammation in the Bayer EYLEA® Global Drug Safety Pharmacovigilance Database |
The most commonly used database entry terms for IOI were identified, demographic details were summarized, and outcome data (where provided) were extracted. The analysis reported all events of IOI cases and IVT-AFL administration from either the vial or PFS from October 2012 (initial IVT-AFL availability via vial) to March 31, 2022 (overall and relative to the number of units sold). All data were summarized descriptively, and no statistical hypothesis testing was undertaken.
Results
Between October 2012 and March 31, 2022, more than 25 million vials and over 6.7 million PFS of IVT-AFL had been sold outside the United States. Over this ~10-year period, the IOI reported rate was 0.3 events per 10,000 units sold for the IVT-AFL PFS and 1.2 events per 10,000 units sold for the IVT-AFL vial presentation. Over 80% of cases were spontaneously reported to Bayer by patients, caregivers or healthcare professionals. Forty-three percent of cases occurred in females and 33% in males (Table 1). In those cases where age was recorded, the mean age of patients experiencing IOI events was 74 years (range, 22–98 years). Patient sex and age were similar for vial cases and PFS cases (Table 1). IOI outcome was not reported or unknown for >50% of cases; where status was provided, 60% of cases were recovering/resolving at the time of reporting.
 |
Table 1 Age and Sex of IOI Patient Cases in the Bayer EYLEA® Global Safety Pharmacovigilance Database and Ten Most Commonly Reported IOI Terms |
The most commonly reported terms related to IOI events are shown in Figure 1 and Table 1. Endophthalmitis was the most commonly used term to report IOI-related adverse events for the vial and PFS. The reported rate of endophthalmitis was 0.1 per 10,000 units for the IVT-AFL PFS and 0.6 per 10,000 units for the vial presentation. The reported rate of retinal or ocular vasculitis was 0.003 events per 10,000 units for the IVT-AFL PFS and 0.006 events per 10,000 units for the vial presentation.
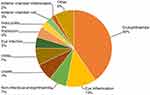 |
Figure 1 Ten most commonly reported terms related to intraocular inflammatory events with the intravitreal aflibercept vial or prefilled syringe between October 2012 and March 31, 2022. |
Discussion
This is the first report of the rates of IOI and endophthalmitis with IVT-AFL administered via the PFS and vial presentation from the Bayer EYLEA® Pharmacovigilance Safety Database. These known adverse events are rarely reported outside clinical trials with either the IVT-AFL vial presentation or PFS, and the overall rate of any IOI, and of endophthalmitis specifically, appear to be lower with the PFS than when IVT-AFL is administered from the vial presentation.
Substances injected into the eye should be sterile and remain sterile when they enter the vitreous cavity, and staff performing intravitreal injections should be well trained and employ practices known to reduce the risks associated with any intraocular procedure.9 Administration of IVT-AFL via the PFS requires fewer preparation steps for the administering healthcare professional compared to the vial presentation,18 and therefore, there is a lower risk of contamination. Additionally, although a formal time-and-motion study has not been conducted for preparation and injection of IVT-AFL, administration via the PFS is also expected to take less time than with the vial, as has been observed with other anti-VEGF agents.20,21 A reduction in time spent in the outpatient clinic for intravitreal injections is of benefit for patients as well as for healthcare professionals.22
Adverse event reports from post-marketing data often lack comprehensive medical information, including full assessments on time to onset, duration of events, culture results, and treatments. The reported rate of endophthalmitis here does not, therefore, differentiate between infectious and non-infectious cases. The database does, however, provide a comprehensive overview of reported event rates, based on adverse events collated from various sources, primarily those spontaneously provided to the company (eg, by healthcare professionals or the patients themselves). Using the number of sold units as a surrogate for the number of injections given, in our analysis, the rate of reported endophthalmitis in clinical practice was equivalent to approximately 1 in 16,000 injections when IVT-AFL was administered from the vial and was approximately 1 in 100,000 intravitreal injections when administered via the PFS.
Reporting rates of endophthalmitis depend on various factors including the anti-VEGF agent and the setting; therefore, comparisons across different treatments, outside head-to-head clinical studies, and across different populations should only be made with the appropriate caveats. A recent review described post intravitreal injection rates of 0.005‒4.4% for sterile uveitis/endophthalmitis and 0.02–0.14% for infectious endophthalmitis depending on the anti-VEGF agent and setting.11 Of particular concern recently has been the emergence of a confirmed safety signal for brolucizumab of retinal vasculitis and/or retinal vascular occlusion that may result in severe visual acuity loss, which typically occur in the presence of IOI. Latest data from the brolucizumab manufacturer provides rates of 5.6 per 10,000 injections for retinal vasculitis and 3.1 per 10,000 injections for retinal vascular occlusion.23 In contrast, a previous published analysis of the global EYLEA® pharmacovigilance database found IOI with retinal artery occlusion or retinal/ocular vasculitis was reported at a rate of approximately 1 every 6 million IVT-AFL injection vials sold (<0.00002%), and most cases were associated with endophthalmitis.17 In our analysis of cases outside the United States, the reported rate of retinal or ocular vasculitis was 0.003 events per 10,000 units for the IVT-AFL PFS and 0.006 events per 10,000 units for the vial presentation.
We should acknowledge that post-marketing data relies heavily on voluntary healthcare professional and patient reporting. It is generally recognized that there is therefore an underreporting of adverse events within existing post-marketing pharmacovigilance reporting systems. Another challenge associated with analyses based on information from mainly spontaneous post-marketing safety reporting is that they depend heavily on the completeness and quality of the individual reports, which may not include details that would be standard in clinical study reporting such as age, sex, symptom onset/resolution dates, etc. Such limitations impact our particular analysis. We should also acknowledge that the noted differences in reported rates of IOI and endophthalmitis with IVT-AFL administered via the vial and PFS are numerical and no formal significance testing was conducted.
Following mandatory adverse-event reporting in clinical trials (where injections were largely administered from vials), according to the summary of product characteristics, endophthalmitis occurred in <1 in 1900 injections of IVT-AFL.18 Although our findings will reflect underreporting associated with voluntary reporting systems, they also reflect routine clinical practice with IVT-AFL from a large multinational data-set. As discussed, rates of IOI differ across the anti-VEGF class and the results with IVT-AFL cannot be extrapolated to other anti-agents.
Conclusion
In summary, according to data from the Bayer EYLEA® Pharmacovigilance Safety Database, adverse events of IOI (and in particular, endophthalmitis) are rarely reported in patients with retinal diseases treated in routine clinical practice with IVT-AFL either from a vial or a PFS. Reported rates of IOI and endophthalmitis are low for the vial presentation and, numerically, are lower for the PFS. It is important to reduce preparation steps to the minimum required for safe administration of IVT-AFL, in accordance with the approved label and we encourage physicians to refer to the full summary of product characteristics for all anti-VEGF agents and report all drug-related adverse events directly to the manufacturers, as well as through any locally required reporting mechanisms.
Research Ethics
This data review does not originate from a dedicated investigational study to analyze this topic. The article summarizes anonymized, post-marketing, case data from Bayer’s Global Safety Database for EYLEA®, which is maintained in accordance with EU law requiring marketing authorization holders to operate a pharmacovigilance system. As such, this analysis is outside the scope of the Declaration of Helsinki and ethics committee approval was not applicable. In accordance with Article 9(2)(i) of the General Data Protection Regulation (GDPR), which recognizes that processing of health data without informed consent is necessary to fulfill pharmacovigilance legislation, informed consent was not required.
Acknowledgments
Medical writing and editorial support for the preparation of this manuscript, under the direction of the authors, was provided by Sarah Feeny, BMedSci, ApotheCom (London), and was funded by Bayer Consumer Care AG, Basel, Switzerland, in accordance with Good Publication Practice (GPP3) guidance (Ann Intern Med. 2015;163:461–464).
Disclosure
All authors are salaried employees of, and hold stock options or own stock in, Bayer companies. The authors report no other conflicts of interest in this work.
References
1. Schmidt-Erfurth U, Kaiser PK, Korobelnik JF, et al. Intravitreal aflibercept injection for neovascular age-related macular degeneration: ninety-six-week results of the VIEW studies. Ophthalmology. 2014;121:193–201. doi:10.1016/j.ophtha.2013.08.011
2. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–2254. doi:10.1016/j.ophtha.2014.05.006
3. Clark WL, Boyer DS, Heier JS, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion: 52-week results of the VIBRANT study. Ophthalmology. 2016;123:330–336. doi:10.1016/j.ophtha.2015.09.035
4. Korobelnik JF, Holz FG, Roider J, et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: one-year results of the Phase 3 GALILEO study. Ophthalmology. 2014;121:202–208. doi:10.1016/j.ophtha.2013.08.012
5. Brown DM, Heier JS, Clark WL, et al. Intravitreal aflibercept injection for macular edema secondary to central retinal vein occlusion: 1-year results from the phase 3 COPERNICUS study. Am J Ophthalmol. 2013;155():429–437.e7. doi:10.1016/j.ajo.2012.09.026
6. Schmidt-Erfurth U, Garcia-Arumi J, Bandello F, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2017;237:185–222. doi:10.1159/000458539
7. Schmidt-Erfurth U, Garcia-Arumi J, Gerendas BS, et al. Guidelines for the management of retinal vein occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica. 2019;242:123–162. doi:10.1159/000502041
8. Schmidt-Erfurth U, Chong V, Loewenstein A, et al. Guidelines for the management of neovascular age-related macular degeneration by the European Society of Retina Specialists (EURETINA). Br J Ophthalmol. 2014;98:1144–1167. doi:10.1136/bjophthalmol-2014-305702
9. Royal College of Ophthalmology. Ophthalmic Service Guidance. Intravitreal Injection Therapy. London, UK: Royal College of Ophthalmology; 2018.
10. Royal College of Ophthalmology. Ophthalmic Service Guidance. Managing an Outbreak of Postoperative Endophthalmitis. London, UK: Royal College of Ophthalmology; 2022.
11. Anderson WJ, da Cruz NFS, Lima LH, Emerson GG, Rodrigues EB, Melo GB. Mechanisms of sterile inflammation after intravitreal injection of antiangiogenic drugs: a narrative review. Int J Retina Vitr. 2021;7:1–12. doi:10.1186/s40942-021-00307-7
12. Cox JT, Eliott D, Sobrin L, Eli I, Winocur E, Sarig R. Inflammatory complications of intravitreal anti-VEGF injections. J Clin Med. 2021;11:10. doi:10.3390/jcm10050981
13. Joh NH, Thomas L, Christian TR, et al. Silicone oil particles in prefilled syringes with human monoclonal antibody, representative of real-world drug products, did not increase immunogenicity in in vivo and in vitro model systems. J Pharm Sci. 2020;109:845–853. doi:10.1016/j.xphs.2019.09.026
14. European Medicines Agency. Beovu European public assessment report; 2019. Available from: https://www.ema.europa.eu/en/documents/assessment-report/beovu-epar-public-assessment-report_en.pdf.
15. Monés J, Srivastava SK, Jaffe GJ, et al. Risk of inflammation, retinal vasculitis, and retinal occlusion–related events with brolucizumab: post hoc review of HAWK and HARRIER. Ophthalmology. 2021;128:1050–1059. doi:10.1016/j.ophtha.2020.11.011
16. Baumal CR, Spaide RF, Vajzovic L, et al. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127:1345–1359. doi:10.1016/j.ophtha.2020.04.017
17. Schmidt-Ott U, Hughes D, Chu K, et al. Differing risks of occlusive retinal vasculitis with concurrent intraocular inflammation among intravitreal antivascular endothelial growth factor therapies. Retina. 2021;41:669–670. doi:10.1097/IAE.0000000000003015
18. Bayer. Eylea (aflibercept) summary of product characteristics; 2018. Available from: https://www.ema.europa.eu/documents/product-information/eylea-epar-product-information_en.pdf.
19. Bayer. Intravitreal VEGF inhibitor EYLEA® approved as a treatment of neovascular glaucoma (NVG), its fifth indication. Available from: https://moneyworld.jp/discl-pdf/tdnet/2020032548418601GENERAL.pdf.
20. Sassalos TM, Paulus YM. Prefilled syringes for intravitreal drug delivery. Clin Ophthalmol. 2019;13:701–706. doi:10.2147/OPTH.S169044
21. Souied E, Nghiem-Buffet S, Leteneux C, et al. Ranibizumab prefilled syringes: benefits of reduced syringe preparation times and less complex preparation procedures. Eur J Ophthalmol. 2015;25:529–534. doi:10.5301/ejo.5000629
22. Denys P, Miere A, Colantuono D, Jung C, Souied EH. Intravitreal injections during COVID-19 outbreak: protective measures, total duration of care and perceived quality of care in a tertiary retina center. Eur J Ophthalmol. 2022;32:372–376. doi:10.1177/11206721211003488
23. Novartis. Post-marketing data in patients with wet AMD and DME. Available from: https://www.brolucizumab.info/post-marketing-data.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
